Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of Temperature, Solvent and pH on the Selective Extraction of Total Phenolic Compounds (TPC) and Trolox Equivalent Antioxidant Capacity (TEAC) from Tiger Nuts by-Products
2.2. Impact of Temperature, Solvent and pH on the Selective Extraction of Individual Phenolic Compounds from Tiger Nuts by-Products
2.3. Optimization and Validation of the Extraction Conditions
2.4. Comparison of Hydroethanolic Extraction of Individual Phenolics Compounds from Horchata by-Products with Those of Folchand Supercritical-CO2 Extraction
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Samples
3.3. Extraction at Different Temperatures, Ethanol:Water Mixtures and pH
3.4. Total Antioxidant Capacity
3.5. Determination of Total Phenolic Content (TPC)
3.6. Triple TOF–LC–MS–MS Characterization of Phenolic Compounds
3.7. Experimental Design and Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roselló-Soto, E.; Poojary, M.M.; Barba, F.J.; Koubaa, M.; Lorenzo, J.M.; Mañes, J.; Moltó, J.C. Thermal and non-thermal preservation techniques of tiger nuts’ beverage “horchata de chufa”. Implications for food safety, nutritional and quality properties. Food Res. Int. 2018, 105, 945–951. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Poojary, M.M.; Barba, F.J.; Lorenzo, J.M.; Mañes, J.; Moltó, J.C. Tiger nut and its by-products valorization: From extraction of oil and valuable compounds to development of new healthy products. Innov. Food Sci. Emerg. Technol. 2018, 45, 306–312. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Fernández-López, J.; Angel Pérez-Alvarez, J. Tiger nut (Cyperus esculentus) commercialization: Health aspects, composition, properties, and food applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Díaz-Vela, J.; Pérez-Chabela, M.L.; Pérez-Alvarez, J.A.; Fernández-López, J. Evaluation of the effect of tiger nut fibre as a carrier of unsaturated fatty acids rich oil on the quality of dry-cured sausages. Food Bioprocess Technol. 2013, 6, 1181–1190. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Fuentes-Zaragoza, E.; Viuda-Martos, M.; Fernández-López, J.; Sendra, E.; Sayas, E.; Pérez-Alvarez, J.A. Reclaim of the by-products from “Horchata” elaboration process. Food Bioprocess Technol. 2012, 5, 954–963. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Zunino, V.; Pérez-Alvarez, J.A.; Fernández-López, J. Effect of tiger nut fibre addition on the quality and safety of a dry-cured pork sausage “Chorizo” during the dry-curing process. Meat Sci. 2013, 95, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, O.; Niranjan, K.; Gordon, M.H. Effect of enzyme pre-treatments on bioactive compounds in extracted tiger nut oil and sugars in residual meals. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Lorenzo, J.M.; Cantavella-Ferrero, Y. Enhancing bioactive antioxidants’ extraction from “Horchata de Chufa” by-products. Foods 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, L.; Fan, L.; Ding, Y.; Zhao, S.; Liu, Y.; Ma, C. Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crop. Prod. 2013, 42, 587–594. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, I.; Adeyemi Bilikis, A.; Aluko Olanrewaju, G. Chemical compositions, antioxidant capacity of Tigernut (Cyperus esculentus) and potential health benefits. Eur. Sci. J. 2015, 11. [Google Scholar]
- Oladele, A.; Adebowale, J.; Bamidele, O. Phenolic Profile and Antioxidant Activity of Brown and Yellow Varieties of Tigernut (Cyperus esculentus L.). Niger. Food J. 2017, 35, 51–59. [Google Scholar]
- Koubaa, M.; Barba, F.J.; Mhemdi, H.; Grimi, N.; Koubaa, W.; Vorobiev, E. Gas assisted mechanical expression (GAME) as a promising technology for oil and phenolic compound recovery from tiger nuts. Innov. Food Sci. Emerg. Technol. 2015, 32, 172–180. [Google Scholar] [CrossRef]
- Parker, M.L.; Ng, A.; Smith, A.C.; Waldron, K.W. Esterified phenolics of the cell walls of chufa (Cyperus esculentus L.) tubers and their role in texture. J. Agric. Food Chem. 2000, 48, 6284–6291. [Google Scholar] [CrossRef] [PubMed]
- Badejo, A.A.; Damilare, A.; Ojuade, T.D. Processing effects on the antioxidant activities of beverage blends developed from Cyperus esculentus, Hibiscus sabdariffa, and Moringa oleifera extracts. Prev. Nutr. Food Sci. 2014, 19, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J.-G.; Li, W.-F.; Chen, J.; Wang, D.-Y.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crop. Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Vatai, T.; Škerget, M.; Knez, Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lin, S.J.; Zhang, J.J.; Zhao, C.N.; Li, H. Bin Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: Optimization and identification of phenolic compounds. Molecules 2017, 22, 1481. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.-Y.; Zhou, C.-S.; Tang, J.-P. Microwave assisted-semi bionic extraction of lignan compounds from Fructus Forsythiae by orthogonal design. J. Cent. South Univ. Technol. 2008, 15, 59–63. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Dragović-Uzelac, V.; Režek Jambrak, A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R.; Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The application of supercritical carbon dioxide and ethanol for the extraction of phenolic compounds from chokeberry pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Wan Aida, W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427–1435. [Google Scholar]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Influence of ethanol/water ratio in ultrasound and high-pressure/high-temperature phenolic compound extraction from agri-food waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef]
- Radnai, B.; Tucsek, Z.; Bognar, Z.; Antus, C.; Mark, L.; Berente, Z.; Gallyas, F.; Sumegi, B.; Veres, B. Ferulaldehyde, a water-soluble degradation product of polyphenols, inhibits the lipopolysaccharide-induced inflammatory response in mice. J. Nutr. 2009, 139, 291–297. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Moltó, J.C. Phenolic profile of oils obtained from “horchata” by-products assisted by supercritical-CO2 and its relationship with antioxidant and lipid oxidation parameters: Triple TOF-LC-MS-MS characterization. Food Chem. 2019, 274, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Rodríguez-Amado, I.; Agregán, R.; Munekata, P.E.S.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT 2018, 88, 1–8. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioprocess Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Oxidants and Antioxidants Part A Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
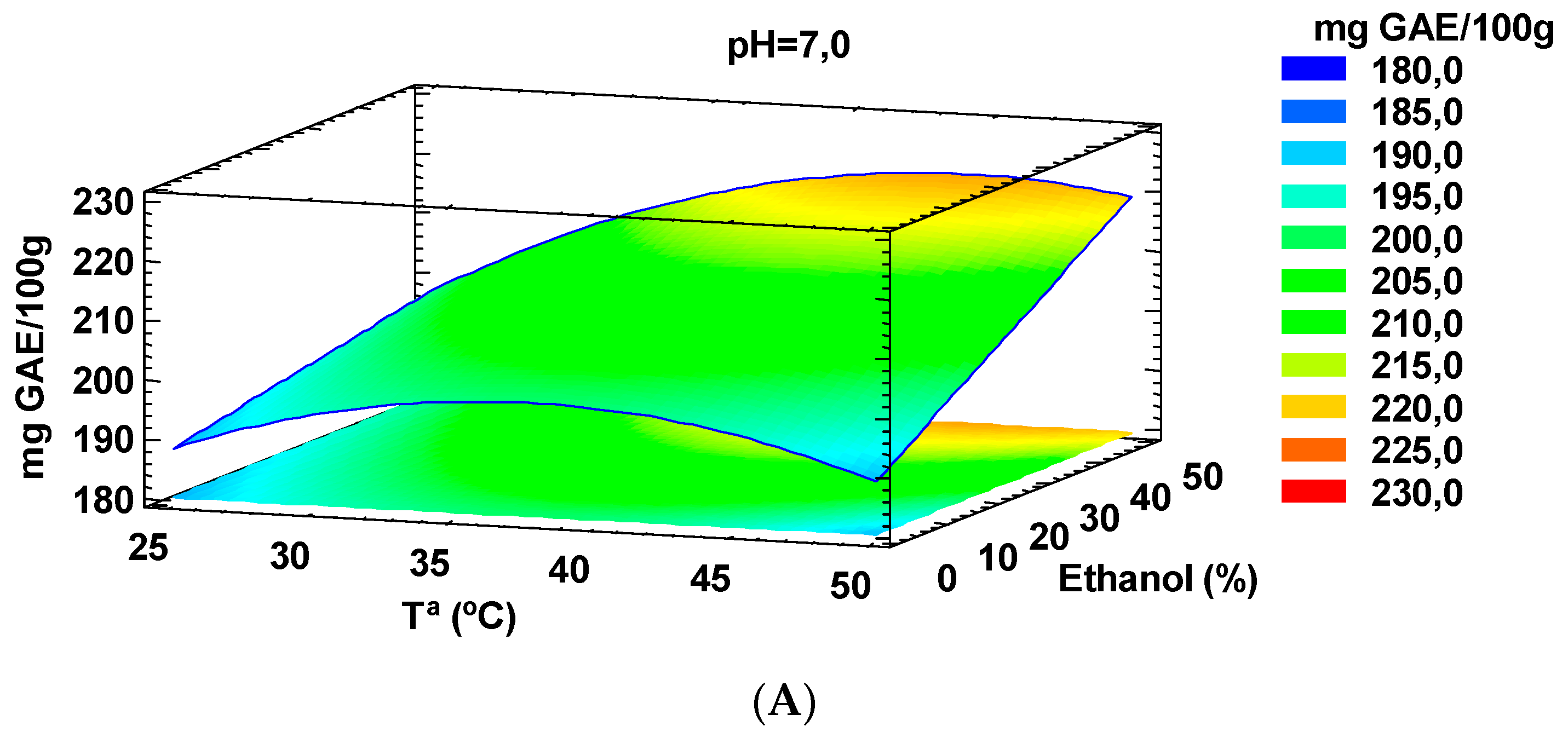
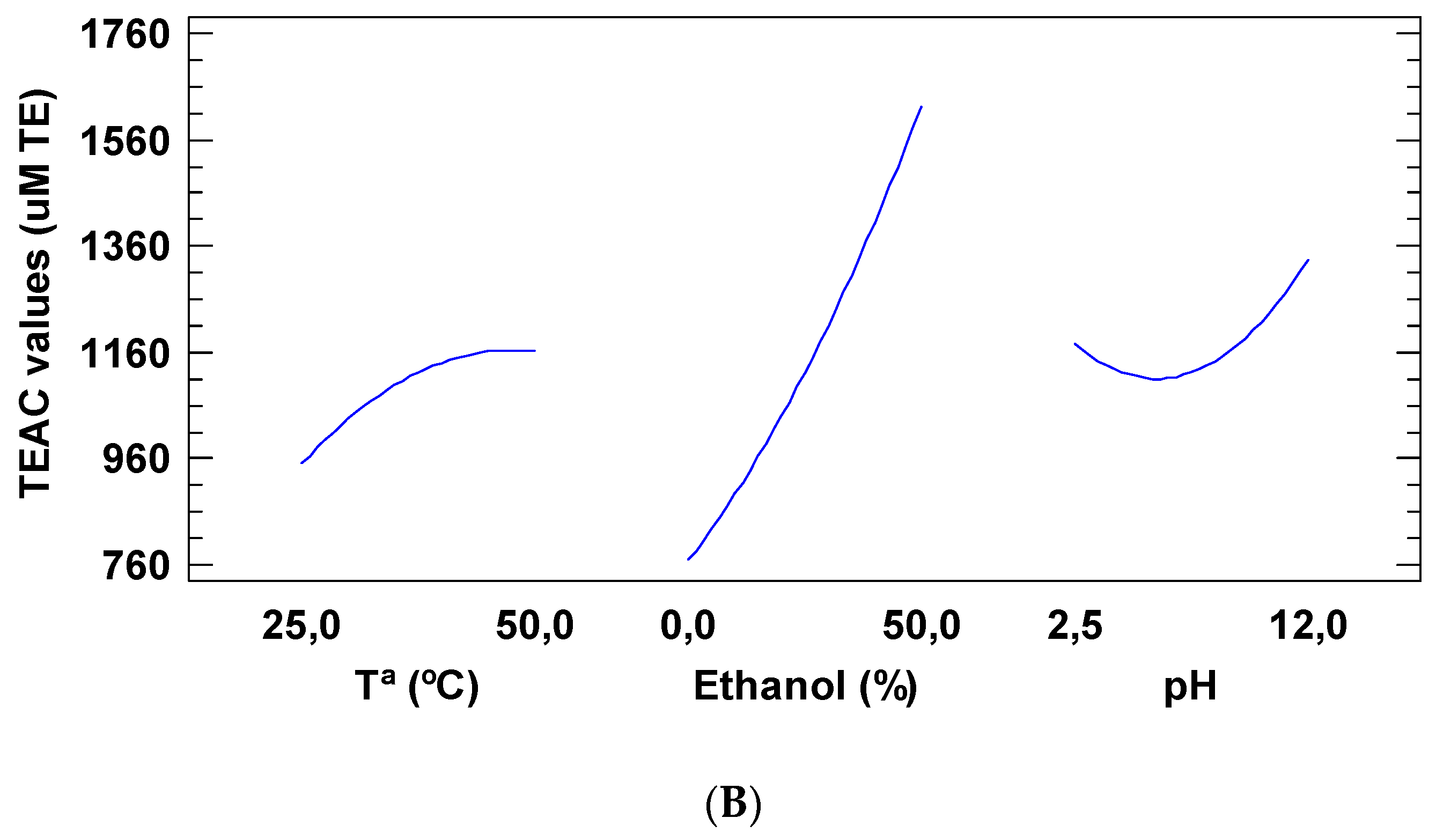

| Run# | T (°C) | Ethanol (%) | pH | TPC (mg GAE/100 g of Dry Matter) | TEAC Values (µM TE/g of Dry Matter) |
|---|---|---|---|---|---|
| 1 | 25 | 0 | 7.25 | 186.52 ± 4.58 | 705.31 ± 18.45 |
| 2 | 25 | 25 | 2.5 | 197.74 ± 1.38 | 996.68 ± 5.26 |
| 3 | 25 | 25 | 12 | 196.74 ± 2.09 | 1325.68 ± 109.73 |
| 4 | 25 | 50 | 7.25 | 200.06 ± 2.34 | 1185.43 ± 284.17 |
| 5 | 35 | 0 | 2.5 | 203.95 ± 2.64 | 617.80 ± 24.15 |
| 6 | 35 | 0 | 12 | 207.20 ± 13.06 | 862.10 ± 50.04 |
| 7 | 35 | 25 | 7.25 | 211.79 ± 3.75 | 1110.00 ± 216.41 |
| 8 | 35 | 25 | 7.25 | 206.92 ± 8.28 | 1094.15 ± 171.80 |
| 9 | 35 | 25 | 7.25 | 203.64 ± 2.39 | 1091.04 ± 50.88 |
| 10 | 35 | 50 | 2.5 | 222.58 ± 2.16 | 1948.07 ± 434.18 |
| 11 | 35 | 50 | 12 | 215.72 ± 1.51 | 1785.94 ± 84.12 |
| 12 | 50 | 0 | 7.25 | 186.78 ± 0.94 | 983.24 ± 101.28 |
| 13 | 50 | 25 | 2.5 | 209.17 ± 3.32 | 1101.18 ± 79.84 |
| 14 | 50 | 25 | 12 | 210.38 ± 4.31 | 1328.06 ± 76.90 |
| 15 | 50 | 50 | 7.25 | 220.48 ± 2.47 | 1644.27 ± 28.53 |
| TPC | TEAC | |||
|---|---|---|---|---|
| Source | p-Value | Sig. | p-Value | Sig. |
| A: Tª (ºC) | 0.0073 | ** | 0.2328 | n.s. |
| B: Ethanol (%) | 0.0007 | *** | 0.0029 | *** |
| C: pH | 0.7961 | n.s. | 0.3635 | n.s. |
| AA | 0.0036 | *** | 0.6071 | n.s. |
| AB | 0.0318 | * | 0.8815 | n.s. |
| AC | 0.7439 | n.s. | 0.9067 | n.s. |
| BB | 0.9735 | n.s. | 0.5495 | n.s. |
| BC | 0.2318 | n.s. | 0.3976 | n.s. |
| CC | 0.0497 | * | 0.3016 | n.s. |
| Ta (°C) | Ethanol (%) | pH | 1-AP | 7-HS | Cyanidin | Ethyl Vanillin | 4-Vinylphenol | Sinensetin | SGG | Cinnamic Acid | DPC | PC | FG+FG | Ferulaldehyde | 4-HB+BA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 0 | 7.25 | ND | ND | ND | 10.40 | 85.00 | ND | ND | 3.30 | 10.40 | ND | 4.40 | ND | ND |
| 25 | 25 | 2.5 | ND | ND | ND | 10.90 | 209.2 | ND | ND | 4.00 | 31.50 | ND | ND | 1.80 | 23.00 |
| 25 | 25 | 12 | ND | ND | ND | 10.60 | 258.8 | ND | ND | ND | 34.90 | ND | ND | 3.10 | 21.40 |
| 25 | 50 | 7.25 | ND | ND | ND | 11.00 | 264.3 | ND | ND | ND | 38.30 | ND | ND | 3.70 | 24.50 |
| 35 | 0 | 2.5 | 1.70 | ND | ND | 14.90 | 126.2 | ND | 35.50 | 6.10 | 13.50 | ND | 8.80 | 0.50 | ND |
| 35 | 0 | 12 | ND | 3.70 | ND | 21.50 | 20.10 | ND | 25.80 | ND | ND | 0.20 | 7.40 | ND | 0.60 |
| 35 | 25 | 7.25 | 4.30 | 3.00 | 2.30 | 10.60 | 229.9 | 50.10 | 85.00 | ND | ND | 28.00 | 8.70 | 3.00 | 21.30 |
| 35 | 25 | 7.25 | 4.40 | 4.10 | 2.40 | 11.60 | 248.6 | 50.00 | 86.00 | ND | ND | 33.50 | 8.00 | 3.90 | 22.80 |
| 35 | 25 | 7.25 | 4.30 | 4.50 | 2.00 | 12.20 | 234.7 | 50.00 | 81.90 | ND | ND | 31.90 | 8.50 | 3.80 | 22.60 |
| 35 | 50 | 2.5 | 3.90 | ND | 4.10 | 13.20 | 303.3 | 77.80 | 86.20 | ND | ND | 41.80 | 8.10 | 6.60 | 23.20 |
| 35 | 50 | 12 | 4.00 | ND | 5.50 | 11.10 | 297.4 | 76.10 | 95.40 | ND | ND | 38.90 | ND | 6.10 | 22.10 |
| 50 | 0 | 7.25 | 1.40 | 4.20 | ND | 26.00 | 22.30 | ND | 23.00 | 11.00 | ND | ND | 4.90 | ND | 11.40 |
| 50 | 25 | 2.5 | ND | ND | ND | 12.20 | 242.5 | 57.30 | 92.50 | ND | ND | 32.90 | ND | 5.30 | 25.00 |
| 50 | 25 | 12 | 4.60 | 4.40 | 3.50 | 11.90 | 302.6 | 60.40 | 92.80 | ND | ND | 42.10 | 9.30 | 5.20 | 28.80 |
| 50 | 50 | 7.25 | ND | ND | 5.30 | 18.70 | 352.7 | ND | ND | ND | ND | 48.00 | ND | 3.60 | 34.20 |
| Source | Lignans | Phenolic Acids | 4-Vinylphenol | Hydroxycinnamic Acids | ||||
|---|---|---|---|---|---|---|---|---|
| p-Value | Sig. | p-Value | Sig. | p-Value | Sig. | p-Value | Sig. | |
| A: Ta (°C) | 0.0251 | * | 0.0003 | *** | 0.3599 | n.s. | 0.3558 | n.s. |
| B: Ethanol (%) | 0.5893 | n.s. | 0.0000 | *** | 0.0002 | *** | 0.0039 | *** |
| C: pH | 0.0256 | * | 0.5304 | n.s. | 0.9698 | n.s. | 0.6837 | n.s. |
| AA | 0.0050 | ** | 0.0165 | * | 0.9056 | n.s. | 0.7121 | n.s. |
| AB | 0.1028 | n.s. | 0.5178 | n.s. | 0.0843 | n.s. | 0.4661 | n.s. |
| AC | 0.0344 | * | 0.1846 | n.s. | 0.6726 | n.s. | 0.1701 | n.s. |
| BB | 0.0153 | * | 0.0000 | *** | 0.0219 | * | 0.0804 | n.s. |
| BC | 0.3209 | n.s. | 0.5820 | n.s. | 0.2235 | n.s. | 0.5392 | n.s. |
| CC | 0.0473 | * | 0.2462 | n.s. | 0.5964 | n.s. | 0,9739 | n.s. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. https://doi.org/10.3390/molecules24040797
Roselló-Soto E, Martí-Quijal FJ, Cilla A, Munekata PES, Lorenzo JM, Remize F, Barba FJ. Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules. 2019; 24(4):797. https://doi.org/10.3390/molecules24040797
Chicago/Turabian StyleRoselló-Soto, Elena, Francisco J. Martí-Quijal, Antonio Cilla, Paulo E. S. Munekata, Jose M. Lorenzo, Fabienne Remize, and Francisco J. Barba. 2019. "Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization" Molecules 24, no. 4: 797. https://doi.org/10.3390/molecules24040797
APA StyleRoselló-Soto, E., Martí-Quijal, F. J., Cilla, A., Munekata, P. E. S., Lorenzo, J. M., Remize, F., & Barba, F. J. (2019). Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules, 24(4), 797. https://doi.org/10.3390/molecules24040797










