Physicochemical Properties and Cellular Uptake of Astaxanthin-Loaded Emulsions
Abstract
1. Introduction
2. Results and Discussion
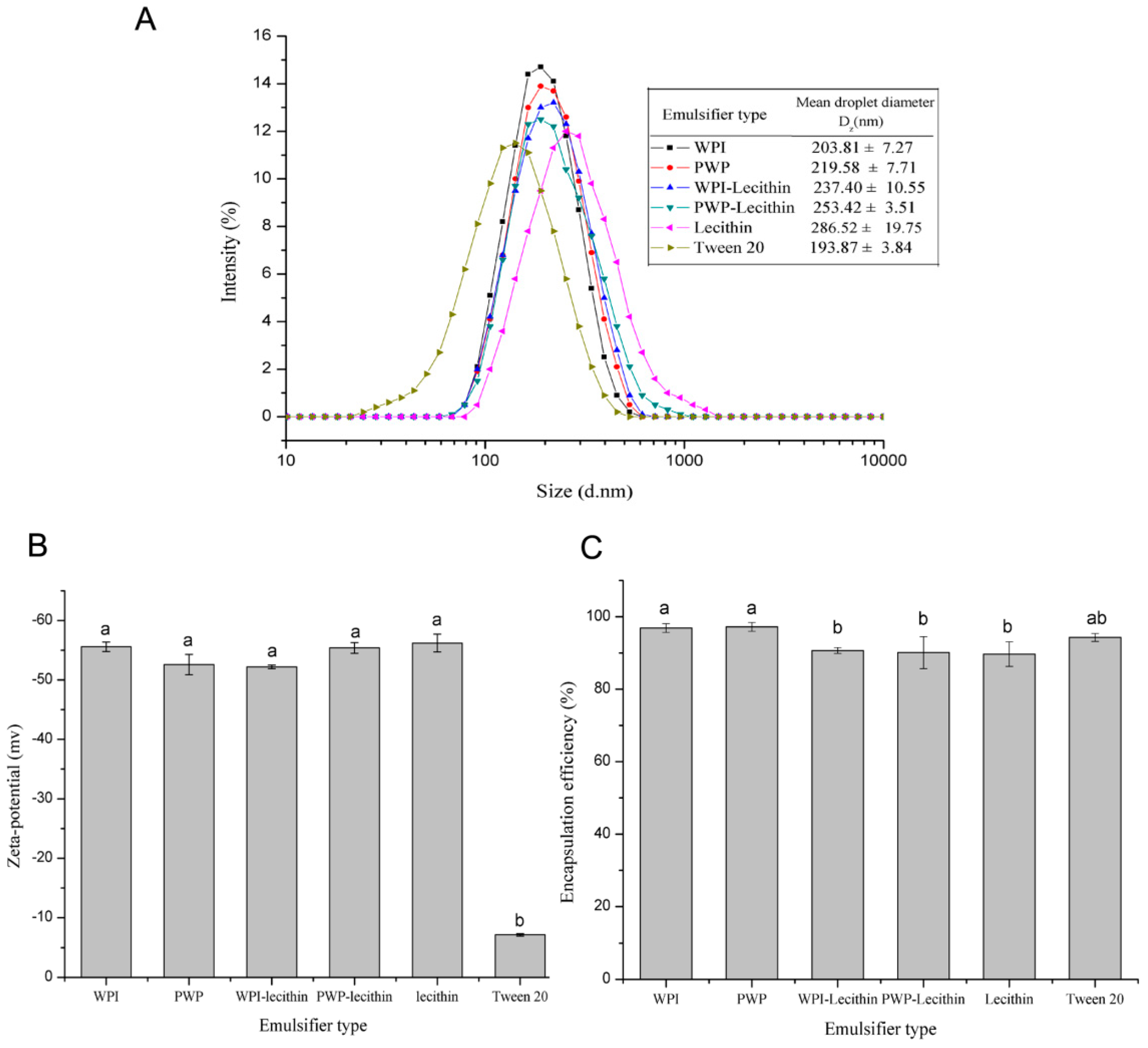
2.1. Characterization of Astaxanthin-Loaded Emulsions
2.2. Storage Stability of Astaxanthin-Loaded Emulsions
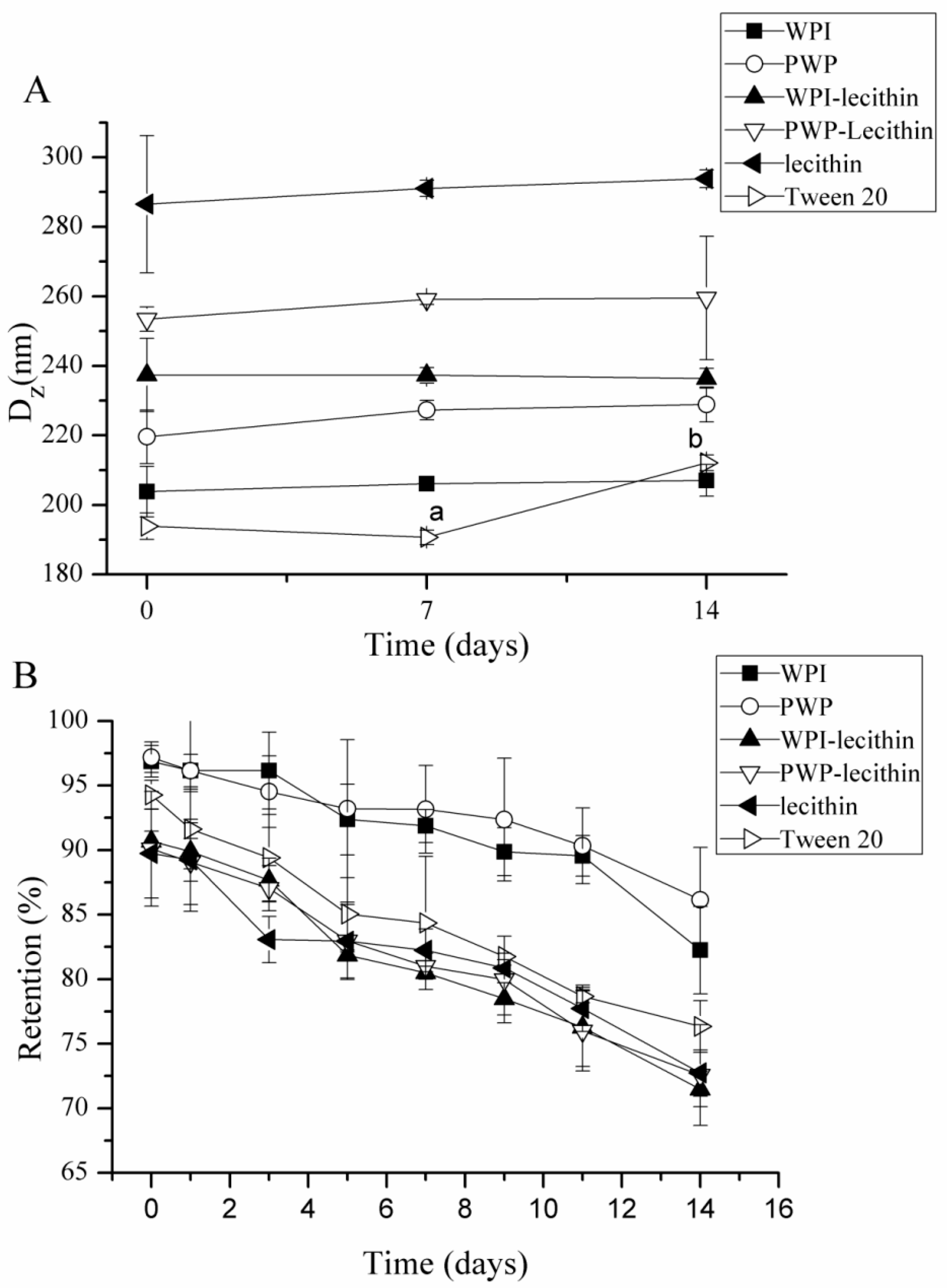
2.2.1. Effects of Storage Temperature on Physical and Chemical Stability of Astaxanthin-Loaded Emulsions
2.2.2. Effects of Emulsifier Type on Physical and Chemical Stability of Astaxanthin-Loaded Emulsions
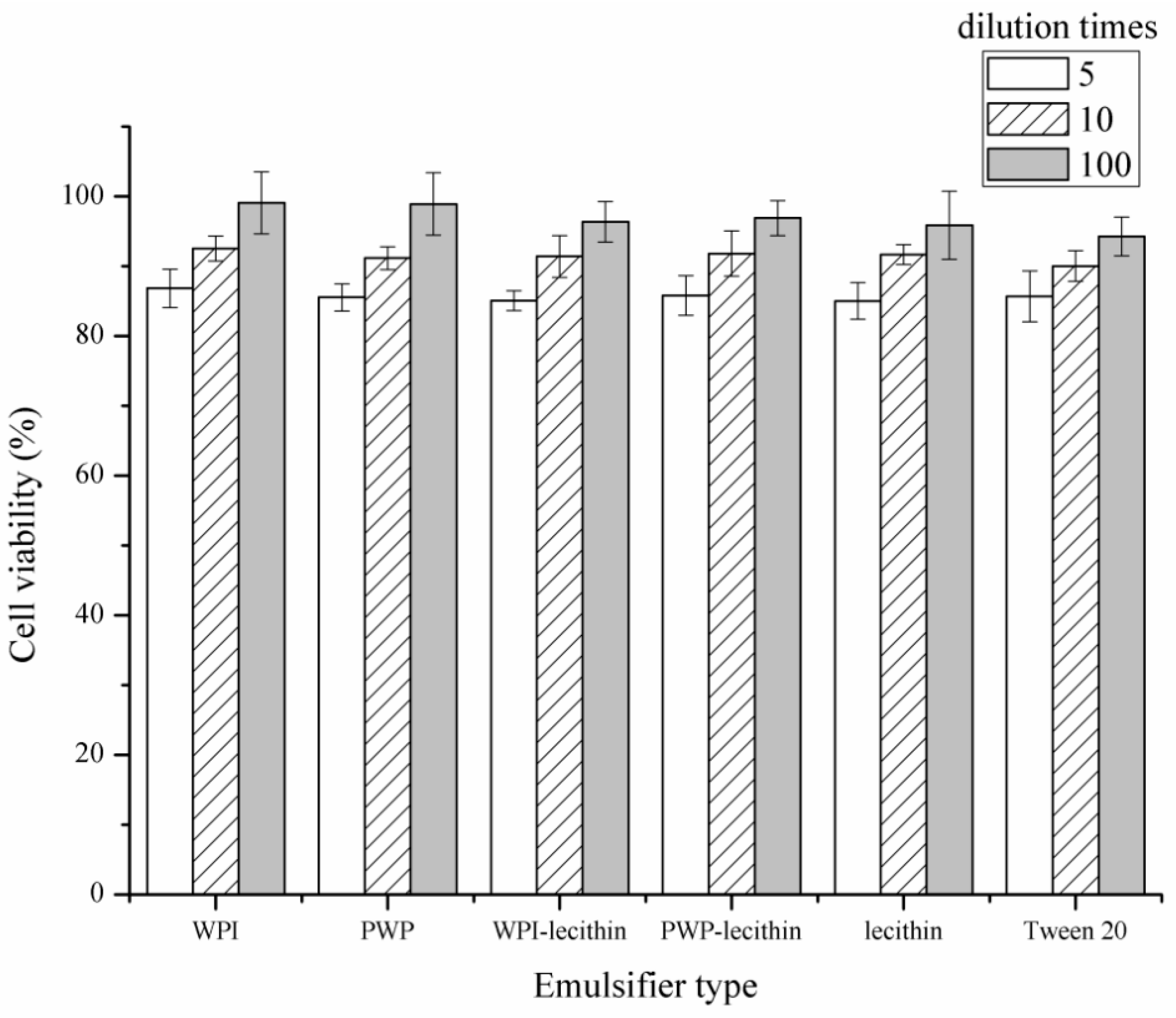
2.3. Cytotoxicity of Astaxanthin-Loaded Emulsions
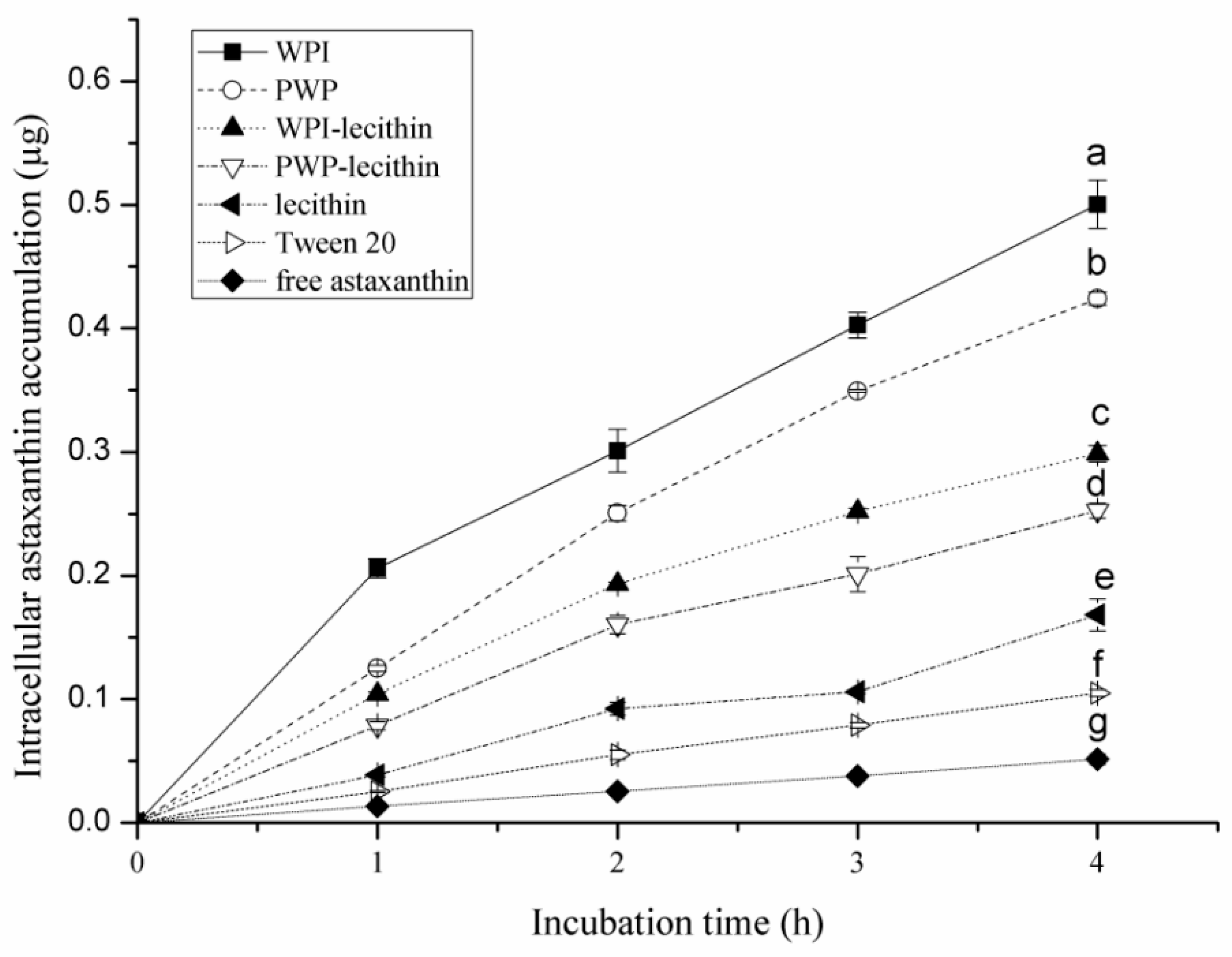
2.4. Cellular Uptake of Encapsulated Astaxanthin in Emulsion Delivery Systems
3. Material and Methods
3.1. Materials
3.2. Preparation of Astaxanthin-Loaded Emulsions
3.3. Determination of Particle Size and Zeta Potential
3.4. Extraction and Determination of Astaxanthin in Emulsions
3.5. High Performance Liquid Chromatography (HPLC)
3.6. Storage Stability of Astaxanthin Emulsions
3.7. Determination of Caco-2 Cell Viability
3.8. Caco-2 Cellular Uptake Assay
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ambati, R.R.; Moi, P.S.; Ravi, S. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Breithaupt, D.E. Modern application of xanthophylls in animal feeding—A review. Trends Food Sci. Technol. 2007, 18, 501–506. [Google Scholar] [CrossRef]
- Martin, G. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; Satomi, Y.; Jinno, K. Cancer prevention by phytochemicals. Oncology 2005, 69, 38–40. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer Int. J. 2000, 36, 59–65. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef]
- Parisi, V.; Tedeschi, M.; Gallinaro, G.; Varano, M.; Saviano, S.; Piermarocchi, S.; CARMIS Study Group. Carotenoids and antioxidants in age-related maculopathy italian study: Multifocal electroretinogram modifications after 1 year. Ophthalmology 2008, 115, 324–333. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. J. Clin. 2011, 16, 355–364. [Google Scholar]
- Félixvalenzuela, L. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar]
- Zhang, X.; Yin, W.; Qi, Y.; Li, X.; Zhang, W.; He, G. Microencapsulation of astaxanthin in alginate using modified emulsion technology: Preparation, characterization, and cytostatic activity. Can. J. Chem. Eng. 2017, 95, 412–419. [Google Scholar] [CrossRef]
- Higueraciapara, I.; Felixvalenzuela, L.; Goycoolea, F.M.; Arguellesmonal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Peng, C.H.; Chang, C.H.; Peng, R.Y.; Chyau, C.C. Improved membrane transport of astaxanthine by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Guerrero, J.M.; Briviba, K.; Rechkemmer, G.; Schuchmann, H.P.; Schubert, H. Cellular uptake of carotenoid-loaded oil-in-water emulsions in colon carcinoma cells in vitro. J. Agric. Food Chem. 2006, 54, 9366–9369. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Chemical stability of astaxanthin nanodispersions in orange juice and skimmed milk as model food systems. Food Chem. 2013, 139, 527–531. [Google Scholar] [CrossRef]
- Mcclements, D.J. Emulsion Design to improve the delivery of functional lipophilic components. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Nik, A.M.; Wright, A.J.; Corredig, M. Impact of interfacial composition on emulsion digestion and rate of lipid hydrolysis using different in vitro digestion models. Colloids Surf. B Biointerfaces 2011, 83, 321–330. [Google Scholar]
- Qiu, C.; Zhao, M.; Decker, E.A.; Mcclements, D.J. Influence of protein type on oxidation and digestibility of fish oil-in-water emulsions: Gliadin, caseinate, and whey protein. Food Chem. 2015, 175, 249–257. [Google Scholar] [CrossRef]
- Hur, S.J.; Decker, E.A.; Mcclements, D.J. Influence of initial emulsifier type on microstructural changes occurring in emulsified lipids during in vitro digestion. Food Chem. 2009, 114, 253–262. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, Y.; Fei, L.; Gao, Y. Investigation into the in vitro release properties of β-carotene in emulsions stabilized by different emulsifiers. LWT Food Sci. Technol. 2014, 59, 867–873. [Google Scholar] [CrossRef]
- Ho, A.K.K.H.Y.; Schroën, K.; Martín-González, M.F.; Berton-Carabin, C.C. Synergistic and antagonistic effects of plant and dairy protein blends on the physicochemical stability of lycopene-loaded emulsions. Food Hydrocoll. 2018, 81, 180–190. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef]
- Teo, A.; Lee, S.J.; Goh, K.K.; Wolber, F.M. Kinetic stability and cellular uptake of lutein in WPI-stabilised nanoemulsions and emulsions prepared by emulsification and solvent evaporation method. Food Chem. 2017, 221, 1269–1276. [Google Scholar] [CrossRef]
- Hu, M.; Mcclements, D.J.; Decker, E.A. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, C.; Lu, J.; Guo, M.R. Physicochemical properties of whey-protein-stabilized astaxanthin nanodispersion and its transport via a Caco-2 monolayer. J. Agric. Food Chem. 2018, 66, 1472–1478. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Cavallieri, Â.L.; Netto, F.M.; Cunha, R.L. Stability and in vitro digestibility of emulsions containing lecithin and whey proteins. Food Funct. 2013, 4, 1322–1331. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- Hsu, J.P.; Nacu, A. Behavior of soybean oil-in-water emulsion stabilized by nonionic surfactant. J. Colloid Interface Sci. 2003, 259, 374–381. [Google Scholar] [CrossRef]
- Beysseriat, M.; Decker, E.A.; Mcclements, D.J. Preliminary study of the influence of dietary fiber on the properties of oil-in-water emulsions passing through an in vitro human digestion model. Food Hydrocoll. 2006, 20, 800–809. [Google Scholar] [CrossRef]
- Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Chemical and physical stability of astaxanthin-enriched emulsion-based delivery systems. Food Biophys. 2016, 11, 302–310. [Google Scholar] [CrossRef]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An overview of ultrasound-assisted food-grade nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Kim, H.J.; And, E.A.D.; Mcclements, D.J. Role of postadsorption conformation changes of β-lactoglobulin on its ability to stabilize oil droplets against flocculation during heating at neutral pH. Langmuir 2002, 18, 7577–7583. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; Mcclements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Yang, X.; Tian, H.; Ho, C.T.; Huang, Q. Inhibition of citral degradation by oil-in-water nanoemulsions combined with antioxidants. J. Agric. Food Chem. 2011, 59, 6113–6119. [Google Scholar] [CrossRef]
- Rao, J.J.; Mcclements, D.J. Stabilization of phase inversion temperature nanoemulsions by surfactant displacement. J. Agric. Food Chem. 2010, 58, 7059–7066. [Google Scholar] [CrossRef]
- Mao, L.; Yang, J.; Xu, D.; Yuan, F.; Gao, Y. Effects of homogenization models and emulsifiers on the physicochemical properties of β-carotene nanoemulsions. J. Dispers. Sci. Technol. 2009, 31, 986–993. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- He, W.; Tan, Y.; Tian, Z.; Chen, L.; Hu, F.; Wu, W. Food protein-stabilized nanoemulsions as potential delivery systems for poorly water-soluble drugs: Preparation, in vitro characterization, and pharmacokinetics in rats. Int. J. Nanomed. 2011, 6, 521–533. [Google Scholar]
- Wei, L.; Kelly, A.L.; Maguire, P.; Zhang, H.; Stanton, C.; Song, M. Correlation of emulsion structure with cellular uptake behaviour of encapsulated bioactive nutrients: Influence of droplet size and interfacial structure. J. Agric. Food Chem. 2016, 64, 8659–8666. [Google Scholar]
- Frede, K.; Henze, A.; Khalil, M.; Baldermann, S.; Schweigert, F.J.; Rawel, H. Stability and cellular uptake of lutein-loaded emulsions. J. Funct. Foods 2014, 8, 118–127. [Google Scholar] [CrossRef]
- Park, S.A.; Ahn, J.B.; Choi, S.H.; Lee, J.S.; Lee, H.G. The effects of particle size on the physicochemical properties of optimized astaxanthin-rich Xanthophyllomyces dendrorhous-loaded microparticles. LWT Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Tan, T.B.; Chu, W.C.; Yussof, N.S.; Abas, F.; Mirhosseini, H.; Cheah, Y.K.; Nehdi, I.A.; Tan, C.P. Physicochemical, morphological and cellular uptake properties of lutein nanodispersions prepared by using surfactants with different stabilizing mechanisms. Food Funct. 2016, 7, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Bortlik, K.S.; Hager, C.; Lambelet, P.; Baur, M. A food-based formulation provides lycopene with the same bioavailability to humans as that from tomato paste. J. Nutr. 2002, 132, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Fang, T.; Gao, F.; Guo, M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017, 63, 668–676. [Google Scholar] [CrossRef]
- Slütter, B.; Plapied, L.; Fievez, V.; Sande, M.A.; Rieux, A.D.; Schneider, Y.J.; Riet, E.V.; Jiskoot, W.; Préat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Estimation of carotenoid bioavailability from fresh stir-fried vegetables using an in vitro digestion/Caco-2 cell culture model. J. Nutr. Biochem. 2000, 11, 574–580. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Fang, T.; Zheng, J.; Guo, M. Physicochemical Properties and Cellular Uptake of Astaxanthin-Loaded Emulsions. Molecules 2019, 24, 727. https://doi.org/10.3390/molecules24040727
Shen X, Fang T, Zheng J, Guo M. Physicochemical Properties and Cellular Uptake of Astaxanthin-Loaded Emulsions. Molecules. 2019; 24(4):727. https://doi.org/10.3390/molecules24040727
Chicago/Turabian StyleShen, Xue, Tianqi Fang, Jian Zheng, and Mingruo Guo. 2019. "Physicochemical Properties and Cellular Uptake of Astaxanthin-Loaded Emulsions" Molecules 24, no. 4: 727. https://doi.org/10.3390/molecules24040727
APA StyleShen, X., Fang, T., Zheng, J., & Guo, M. (2019). Physicochemical Properties and Cellular Uptake of Astaxanthin-Loaded Emulsions. Molecules, 24(4), 727. https://doi.org/10.3390/molecules24040727






