Changes of Phytochemical Components (Urushiols, Polyphenols, Gallotannins) and Antioxidant Capacity during Fomitella fraxinea–Mediated Fermentation of Toxicodendron vernicifluum Bark
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes of Urushiols
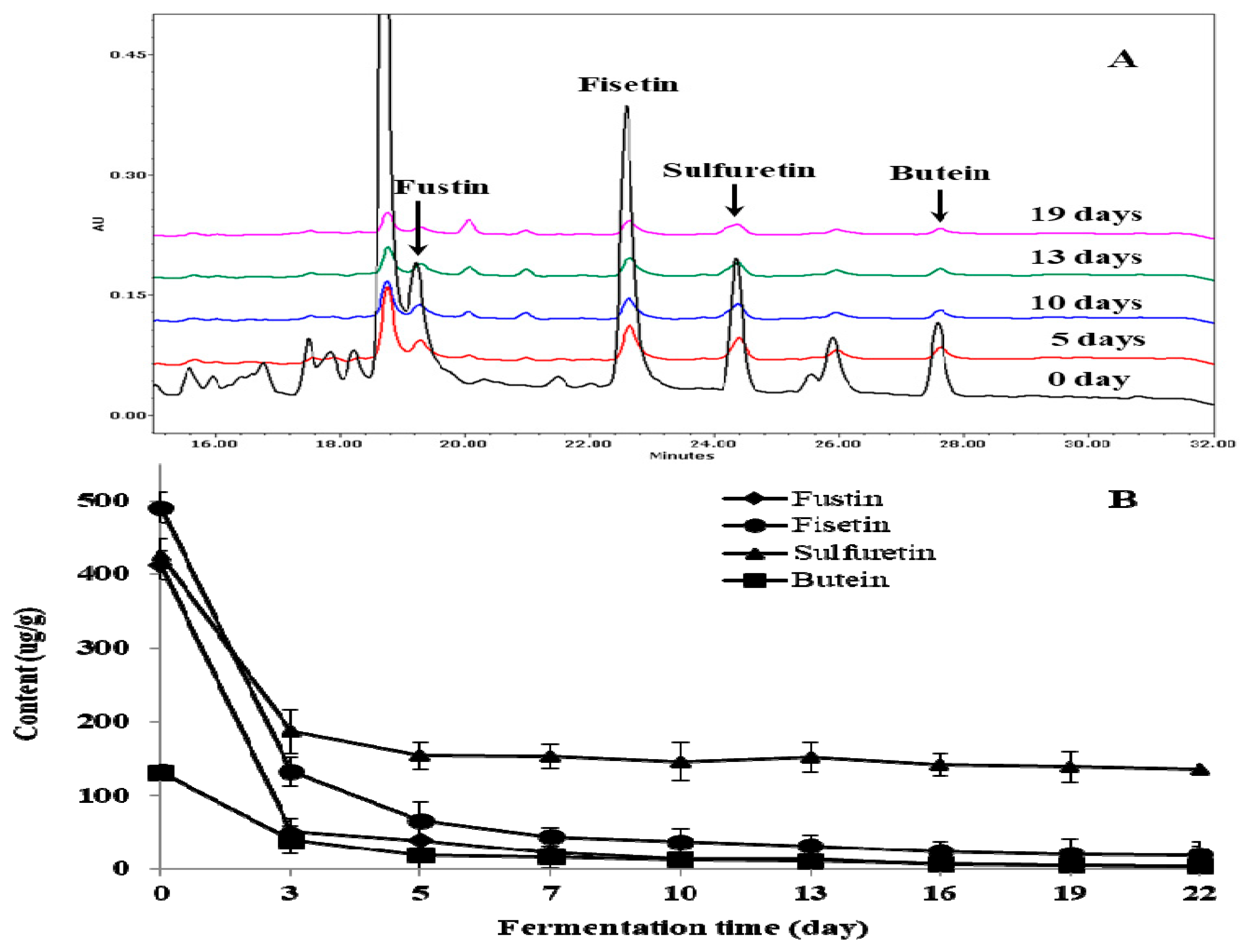
2.2. Change of Total Phenol, Total Flavonoid, and Individual Flavonoid
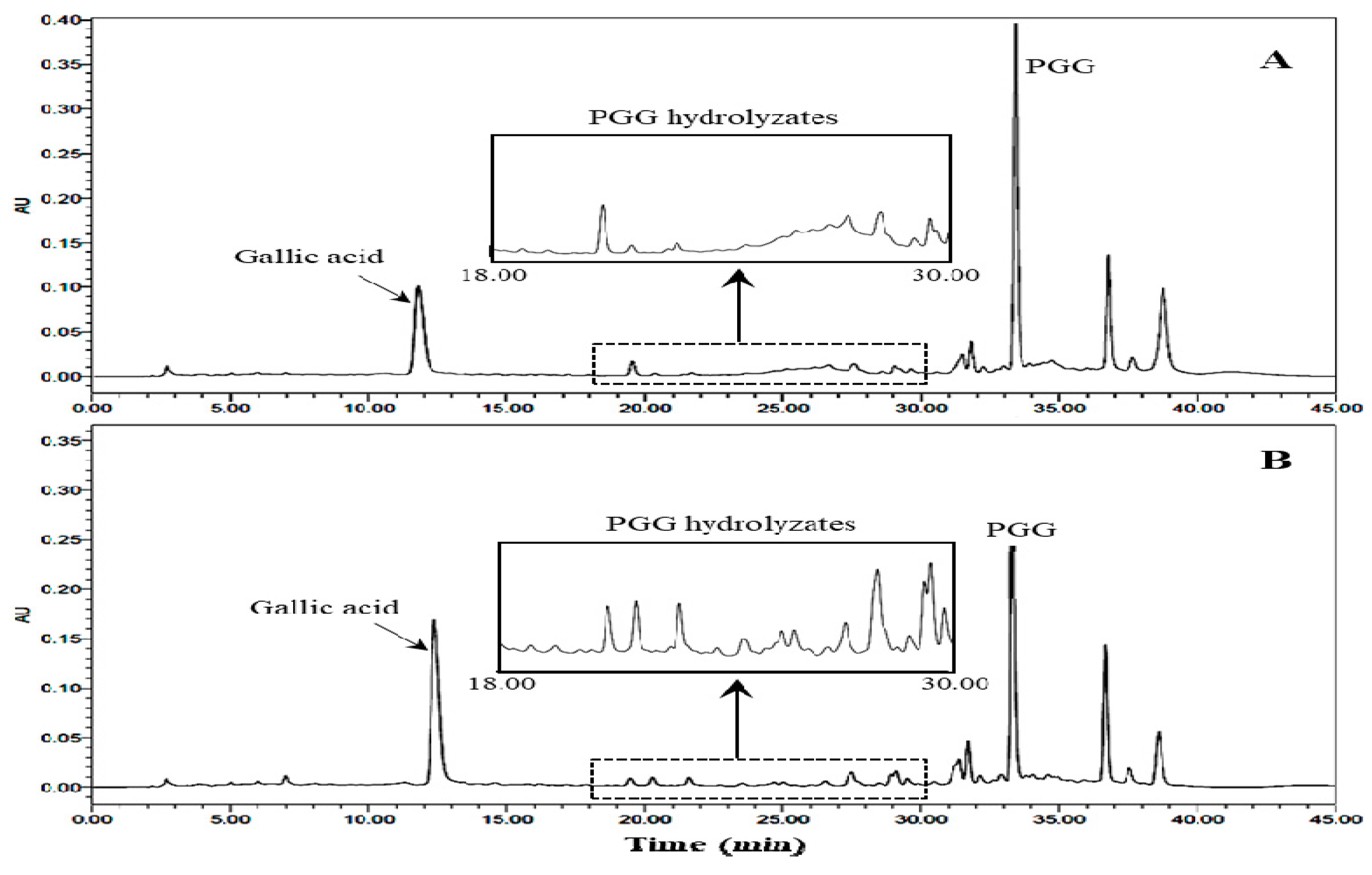
2.3. Changes of PGG
2.4. Characterization of PGG Hydrolysates by HPLC-MS
2.5. Changes of Antioxidant Activities
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Microorganism and Culture Conditions
3.4. Fermentation of TVSB by F. fraxinea
3.5. Extraction and HPLC Analysis of Urushiols.
3.5.1. Extraction
3.5.2. Thin-Layer Chromatography (TLC) Analysis
3.5.3. HPLC Analysis
3.6. Extraction for Polyphenols and Gallotannins
3.7. Total Phenol
3.8. Total Flavonoid
3.9. HPLC Analysis of Individual Flavonoids
3.10. Hydrolysis of PGG by Tannases
3.10.1. Preparation of PGG
3.10.2. Preparation of Crude Enzymes from Fermented TVSB
3.10.3. Tannase Assay
3.10.4. Enzymatic Hydrolysis of PGG
3.11. Analysis of PGG Hydrolysates in Fermented TVSB
3.11.1. HPLC
3.11.2. HPLC-MS
3.12. Antioxidant Activity
3.12.1. DPPH Free Radical Scavenging Activity
3.12.2. ABTS Free Radical Scavenging Activity
3.12.3. Ferric Reducing/Antioxidant Power (FRAP)
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiart, C. A note on Toxicodendron vernicifluum (Stokes) F.A. Barkley. Food Chem. Toxicol. 2014, 64, 410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, C.; Zheng, G.; Wei, S.; Hua, Z. Comparative studies of bark structure, lacquer yield and urushiol content of cultivated Toxicodendron vernicifluum varieties. N. Z. J. Bot. 2013, 51, 13–21. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, Y.C.; Ko, S.G. Integrating traditional medicine into modern inflammatory diseases care: Multitargeting by Rhus verniciflua Stokes. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.C.; Jung, H.S.; Kim, K.S.; Lee, S.K.; Yoon, S.W.; Park, J.H.; Kim, S.H.; Cheon, S.H.; Eo, W.K.; Lee, S.H. Rhus verniciflua Stokes against advanced cancer: A perspective from the Korean integrative cancer center. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Liu, C.S.; Nam, T.G.; Han, M.W.; Ahn, S.M.; Choi, H.S.; Kim, T.Y.; Chun, O.K.; Koo, I.K.; Kim, D.O. Protective effect of detoxified Rhus verniciflua Stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci. Biotechnol. Biochem. 2013, 77, 1682–1688. [Google Scholar] [CrossRef]
- Lim, K.T.; Hu, C.; Kitts, D.D. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. [Google Scholar] [CrossRef]
- Kim, M.O.; Yang, J.F.; Kwon, Y.S.; Kim, M.J. Antioxidant and anticancer effects of fermented Rhus verniciflua stem bark extracts in HCT-116 cells. ScienceAsia 2015, 41, 322–328. [Google Scholar] [CrossRef]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kim, S.Y.; Lee, Y.J.; Ko, B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.; Chang, K.H.; Hong, C.Y.; Na, C.S.; Dong, M.S.; Lee, D.; Lee, M.Y. Antiplatelet effects of Rhus verniciflua stokes heartwood and its active constituents–fisetin, butein, and sulfuretin–in rats. J. Med. Food. 2015, 18, 21–30. [Google Scholar]
- Lee, E.J.; Lee, G.H.; Sohn, S.H.; Bae, H.S. Extract of Rhus verniciflua Stokes enhances Th1 response and NK cell activity. Mol. Cell. Toxicol. 2016, 12, 399–407. [Google Scholar] [CrossRef]
- Byun, J.S.; Han, Y.H.; Hong, S.J.; Hwang, S.M.; Kwon, Y.S.; Lee, H.J.; Kim, S.S.; Kim, M.J.; Chun, W.J. Bark constituents from mushroom-detoxified Rhus verniciflua suppress kainic acid-induced neuronal cell death in mouse hippocampus. Korean J. Physiol. Pharmacol. 2010, 14, 279–283. [Google Scholar] [CrossRef]
- Cho, N.K.; Choi, J.H.; Yang, H.J.; Jeong, E.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.J.; Choi, S.U.; Pang, C.H.; Kim, S.Y.; Lee, K.R. Identification of cytotoxic and anti-inflammatory constituents from the bark of Toxicodendron vernicifluum (Stokes) F. A. Barkley. J. Ethnopharmacol. 2015, 162, 231–237. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, K.Y.; Kim, J.; Na, C.S.; Jung, N.C.; Chung, G.H.; Jang, Y.S. Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem. Toxicol. 2004, 42, 1383–1388. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, W.C.; Yoon, S.W. Impact of standardized Rhus verniciflua Stokes extract as complementary therapy on metastatic colorectal cancer: A Korean single-center experience. Integr. Cancer Ther. 2009, 8, 148–152. [Google Scholar] [CrossRef]
- Rayne, S.; Mazza, G. Biological activities of extracts from Sumac (Rhus spp.): A review. Plant Foods Hum. Nutr. 2007, 62, 165–175. [Google Scholar] [CrossRef]
- Gross, M.; Baer, H.; Fales, H.M. Urushiols of poisonous Anacardiaceae. Phytochem. 1975, 14, 2263–2266. [Google Scholar] [CrossRef]
- Ma, X.M.; Lu, R.; Miyakoshi, T. Recent advances in research on lacquer allergy. Allergol. Int. 2012, 61, 45–50. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, M.K.; Park, H.S.; Yun, S.E.; Mun, S.P.; Kim, J.S.; Sapkota, K.; Kim, S.; Kim, T.Y.; Kim, S.J. Biological detoxification of lacquer tree (Rhus verniciflua Stokes) stem bark by mushroom species. Food Sci. Biotechnol. 2007, 16, 935–942. [Google Scholar]
- Cheong, S.H.; Choi, Y.W.; Min, B.S.; Choi, H.Y. Polymerized urushiol of the commercially available Rhus product in Korea. Ann. Dermatol. 2010, 22, 16–20. [Google Scholar] [CrossRef]
- Sapkota, K.; Kim, S.; Kim, M.K.; Kim, S.J. A detoxified extract of Rhus verniciflua Stokes upregulated the expression of BDNF and GDNF in the rat brain and the human dopaminergic cell line SH-SY5Y. Biosci. Biotechnol. Biochem. 2010, 74, 1997–2004. [Google Scholar] [CrossRef]
- Shin, S.H.; Koo, K.H.; Bae, J.S.; Cha, S.B.; Kang, I.S.; Kang, M.S.; Kim, H.S.; Heo, H.S.; Park, M.S.; Gil, G.H.; et al. Single and 90-day repeated oral dose toxicity studies of fermented Rhus verniciflua stem bark extract in Sprague–Dawley rats. Food Chem. Toxicol. 2013, 55, 617–626. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla chinensis—Folklore to modern evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Jin, M.J.; Kim, I.S.; Park, J.S.; Dong, M.S.; Na, C.S.; Yoo, H.H. Pharmacokinetic profile of eight phenolic compounds and their conjugated metabolites after oral administration of Rhus verniciflua extracts in rats. J. Agric. Food Chem. 2015, 63, 5410–5416. [Google Scholar] [CrossRef]
- Jang, J.Y.; Shin, H.; Lim, J.W.; Ahn, J.H.; Jo, Y.H.; Lee, K.Y.; Hwang, B.Y.; Jung, S.J.; Kang, S.Y.; Lee, M.K. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of Rhus verniciflua. PLoS ONE 2018, 13, e0200257. [Google Scholar]
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Rodríguez-Vázquez, R.; Delgado-Boada, J.M. Fungal laccases. Fungal Biol. Rev. 2013, 7, 67–82. [Google Scholar] [CrossRef]
- Minussi, R.C.; Pastore, G.A.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Ghidouche, S.; Es-Safi, N.E.; Ducrot, P.H. Trtametes versicolor laccase mediated oxidation of flavonoids. Influence of the hydroxylation pattern of ring B of flavonols. Amer. J. Food Technol. 2007, 2, 630–640. [Google Scholar]
- Kumanotani, J. Enzyme catalyzed durable and authentic oriental lacquer: A natural microgel-printable coating by polysaccharide–glycoprotein–phenolic lipid complexes. Prog. Org. Coat. 1998, 34, 135–146. [Google Scholar] [CrossRef]
- Lu, R.; Miyakoshi, T. Studies on acetone powder and purified Rhus laccase immobilized on zirconium chloride for oxidation of phenols. Enzyme Res. 2012, 2012, 375309. [Google Scholar] [CrossRef]
- Motoda, S. Purification and characterization of polyphenol oxidase from Trametes sp. MS39401. J. Biosci. Bioeng. 1999, 87, 137–143. [Google Scholar] [CrossRef]
- Burke, R.M.; Cairney, J.W.G. Laccases and other polyphenol oxidases in ecto- and ericoid mycorrhizal fungi. Mycorrhiza 2002, 12, 105–116. [Google Scholar] [CrossRef]
- Martínková, L.; Kotik, M.; Marková, E.; Homolka, L. Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: A review. Chemosphere 2016, 149, 373–382. [Google Scholar] [CrossRef]
- Kubo, I.; Nihei, K.I.; Shimizu, K. Oxidation products of quercetin catalyzed by mushroom tyrosinase. Bioorg. Med. Chem. 2004, 12, 5343–5347. [Google Scholar] [CrossRef]
- Barry, K.M.; Davies, N.W.; Mohammed, C.L. Identification of hydrolysable tannins in the reaction zone of Eucalyptus nitens wood by high performance liquid chromatography–electrospray ionisation mass spectrometry. Phytochem. Anal. 2001, 12, 120–127. [Google Scholar] [CrossRef]
- Gόmez-Caravaca, A.M.; Lόpez-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernádez-Gutíerrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed, and seed husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Román, D.A.; Segura-Carretero, A. HPLC−DAD−ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Gordon, A.; Jungfer, E.; da Silva, B.A.; Maia, J.G.S.; Marx, F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the Amazon region. J. Agric. Food Chem. 2011, 59, 7688–7699. [Google Scholar] [CrossRef]
- Abdel-Nabey, M.A.; Sherief, A.A.; El-Tanash, A.B. Tannin biodegradation and some factors affecting tannase production by two Aspergillus sp. Biotechnol. 2011, 10, 149–158. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nakagawa, K.; Hayashi, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Yamaji, R.; Nakano, Y.; Inui, H. Hydrolysable tannins as antioxidants in the leaf extract of Eucalyptus globulus possessing tyrosinase and hyaluronidase inhibitory activities. Food Sci. Technol. Res. 2009, 15, 331–336. [Google Scholar] [CrossRef]
- Yao, J.; Guo, G.S.; Ren, G.H.; Liu, Y.H. Production, characterization and applications of tannase. J. Mol. Catal. B: Enzym. 2014, 101, 137–147. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Revathi, S.; Rameshkumar, N.; Krishnan, M.; Kayalvizhi, N. Microbial tannase: Current perspectives and biotechnological advances. Biocatal. Agric. Biotechnol. 2016, 6, 168–175. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Gorinstein, S.; Haruenkit, R.; Poovarodom, S.; Vearasilp, S.; Ruamsuke, P.; Namiesnik, J.; Leontowicz, M.; Leontowicz, H.; Suhaj, M.; ShengSome, G.P. Analytical assays for the determination of bioactivity of exotic fruits. Phytochem. Anal. 2010, 355–362. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 2812–2819. [Google Scholar] [CrossRef]
- Shama, P.; Singh, R.P. Evaluation of antioxidant activity in foods with special reference to TEAC method. Am. J. Food Technol. 2013, 8, 83–101. [Google Scholar]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef]
- Riebel, M.; Sabel, A.; Claus, H.; Fronk, P.; Xia, N.; Li, H.; König, H.; Decker, H. Influence of laccase and tyrosinase on the antioxidant capacity of selected phenolic compounds on human cell lines. Molecules 2015, 20, 17194–17207. [Google Scholar] [CrossRef]
- Sánchez-Mundo, M.L.; Escobedo-Crisantes, V.M.; Mendoza-Arvizu, S.; Jaramillo-Flores, M.E. Polymerization of phenolic compounds by polyphenol oxidase from bell pepper with increase in their antioxidant capacity. CYTA J. Food 2016, 14, 594–603. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. Based Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Chen, Y.; Hagerman, A.E. Characterization of soluble non-covalent complexes between bovine serum albumin and β-1,2,3,4,6-penta-O-galloyl-D-glucopyranose by MALDI-TOF MS. J. Agric. Food Chem. 2004, 52, 4008–4011. [Google Scholar] [CrossRef]
- Mondal, K.C.; Banerjee, D.; Jana, M.; Pati, B.R. Colorimetric assay method for determination of the tannase activity. Anal. Biochem. 2001, 295, 168–171. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosb, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Composit. Anal 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |



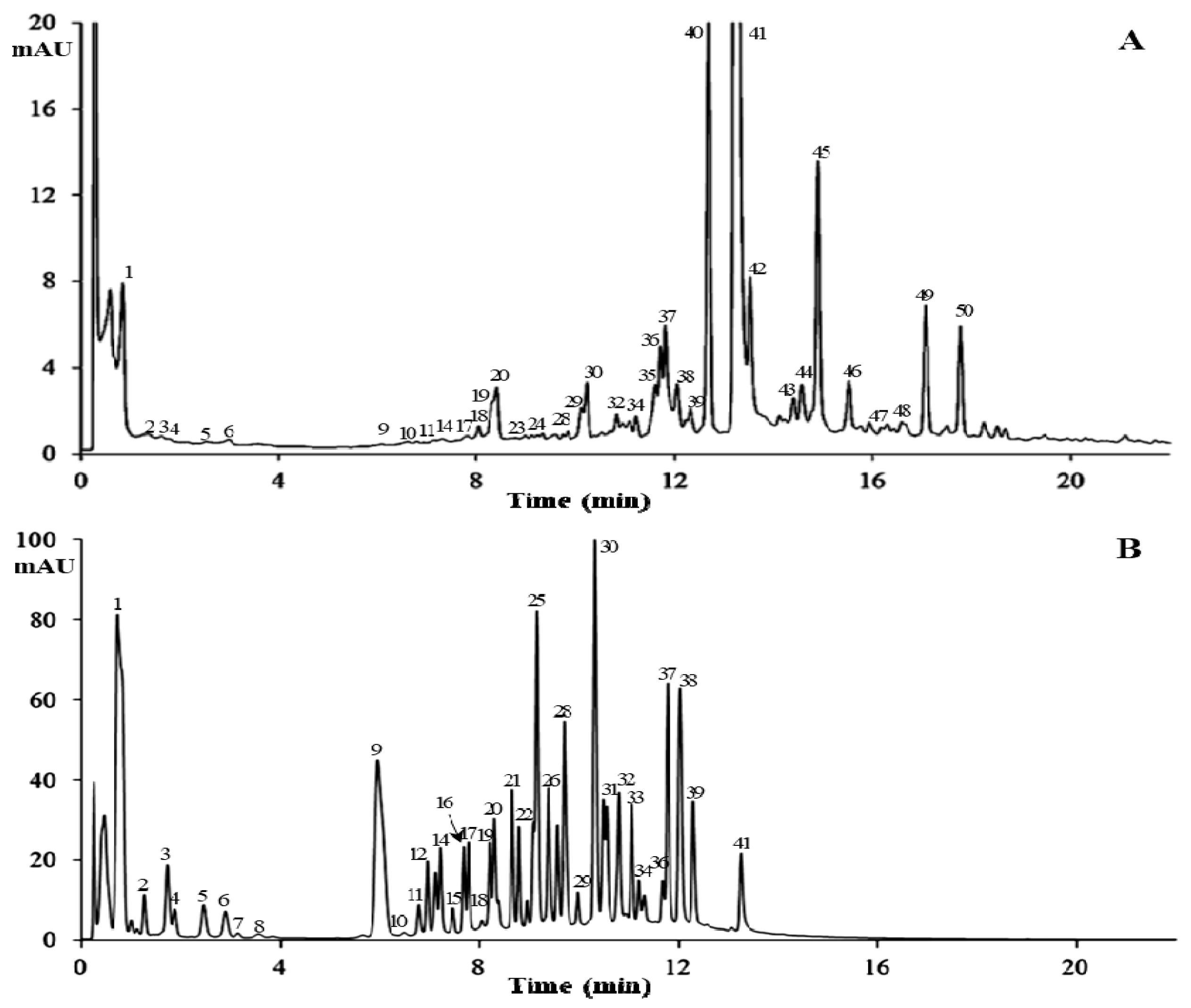
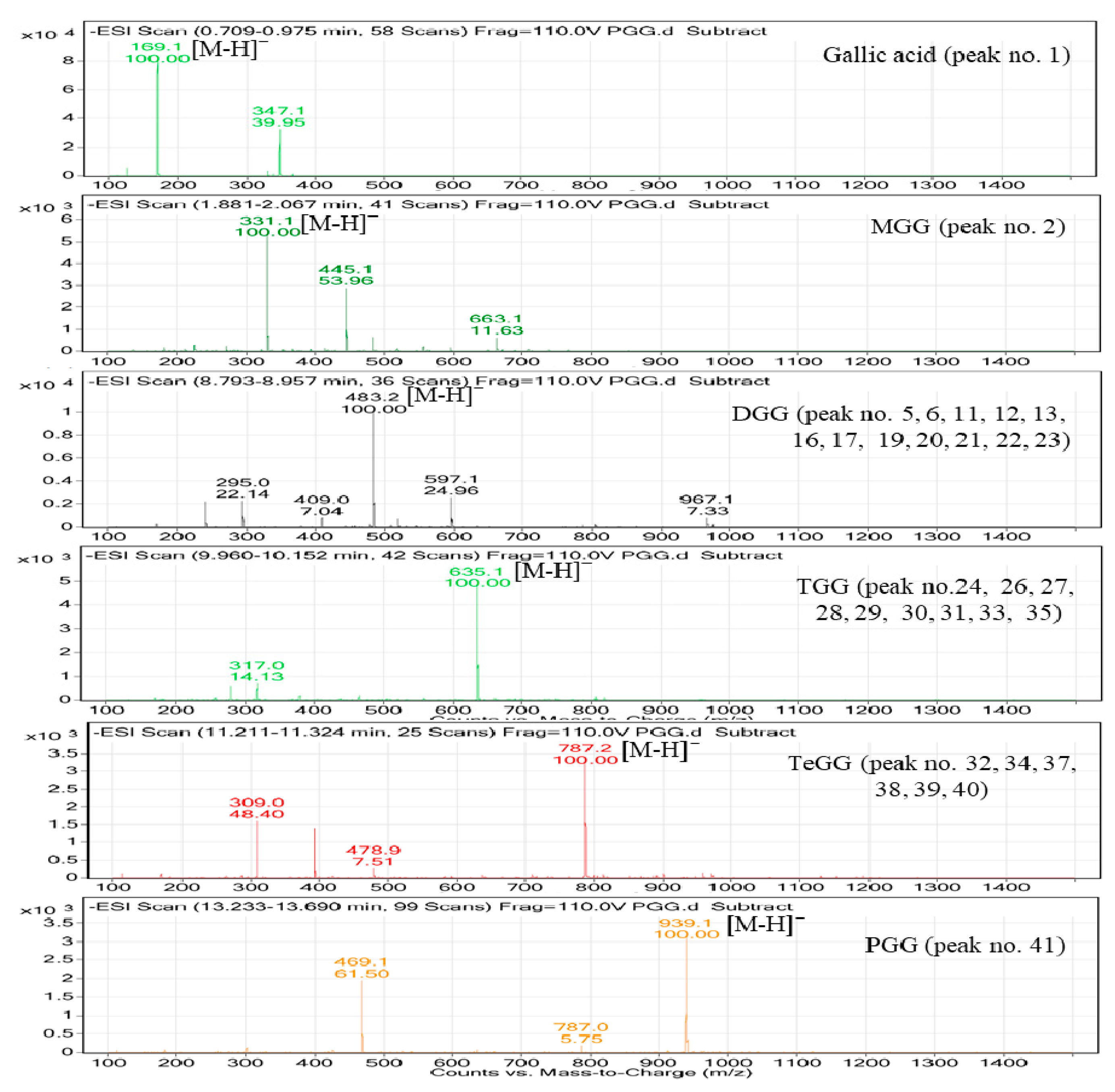

| Peak No. | tR (min) | UV (λmax, nm) | [M − H]− (m/z) | Other Fragment (m/z) | Identification | Detection | |
|---|---|---|---|---|---|---|---|
| FTVSB | PGGH | ||||||
| 1 | 0.714 | 272 | 169.0 | 331.1 | Gallic acid | O | X |
| 2 | 1.264 | 276 | 331.1 | 445.1, 271.0 | MGG | O | O |
| 3 | 1.699 | 276 | 483.1 | 597.0, 224.9 | DGG | O | O |
| 4 | 1.890 | 276 | 331.1 | 445.1, 270.9 | MGG (isomer) | O | O |
| 5 | 2.371 | 276 | 483.1 | 597.0 | DGG (isomer) | O | O |
| 6 | 2.781 | 273 | 483.1 | 181.0, 597.0 | DGG (isomer) | O | O |
| 7 | 3.141 | 255.1 | 123.0 | Unidentified | X | O | |
| 8 | 3.500 | 301.0 | 415.0, 197.8 | Unidentified | O | O | |
| 9 | 5.848 | 276 | 183.1 | 375.1 | Methyl gallate | O | O |
| 10 | 6.464 | 283.0 | 396.8 | Unidentified | O | O | |
| 11 | 6.744 | 276 | 483.1 | 241.0, 596.9 | DGG (isomer) | O | O |
| 12 | 6.950 | 275 | 483.1 | 597.1, 241.1 | DGG (isomer) | X | O |
| 13 | 7.118 | 273 | 483.1 | 597.0, 241.0 | DGG (isomer) | O | O |
| 14 | 7.234 | 276 | 225.1 | 483.1, 339.0 | Unidentified | O | O |
| 15 | 7.449 | 283.1 | 397.0, 575.0 | Unidentified | X | O | |
| 16 | 7.692 | 275 | 483.1 | 241.1, 597.0 | DGG (isomer) | X | O |
| 17 | 7.799 | 275 | 483.1 | 597.0, 241.1 | DGG (isomer) | O | O |
| 18 | 7.991 | 283.1 | 397.1 | Unidentified | O | O | |
| 19 | 8.215 | 277 | 483.1 | 241.0, 597.0 | DGG (isomer) | O | O |
| 20 | 8.313 | 277 | 483.1 | 597.0, 241.1 | DGG (isomer) | O | O |
| 21 | 8.658 | 216, 273 | 483.1 | 597.1, 241.0 | DGG (isomer) | X | O |
| 22 | 8.803 | 216, 273 | 483.2 | 597.0, 295.0 | DGG (isomer) | X | O |
| 23 | 8.962 | 275 | 483.2 | 597.1, 241.0 | DGG (isomer) | O | O |
| 24 | 9.097 | 276 | 635.1 | 749.1, 317.1 | TGG | O | O |
| 25 | 9.111 | 216, 279 | 431.2 | 499.1, 563.1 | Unidentified | X | O |
| 26 | 9.386 | 216, 276 | 635.1 | 749.1, 317.2 | TGG (isomer) | O | O |
| 27 | 9.578 | 217, 276 | 635.1 | 317.1 | TGG (isomer) | O | O |
| 28 | 9.708 | 216, 276 | 635.1 | 749.1, 317.1 | TGG (isomer) | O | O |
| 29 | 9.979 | 216, 276 | 635.1 | 317.0, 169.0 | TGG (isomer) | O | O |
| 30 | 10.315 | 217, 278 | 635.1 | 317.1, 169.0 | TGG (isomer) | O | O |
| 31 | 10.502 | 217, 278 | 635.1 | 317.2 | TGG (isomer) | O | O |
| 32 | 10.777 | 217, 278 | 787.1 | 635.1, 393.1 | TeGG | O | O |
| 33 | 11.057 | 217, 278 | 635.1 | 317.1, 749.1 | TGG (isomer) | O | O |
| 34 | 11.225 | 218, 277 | 787.1 | 393.2, 309.0 | TeGG (isomer) | O | O |
| 35 | 11.328 | 217, 278 | 635.1 | 317.2, 749.0 | TGG (isomer) | X | O |
| 36 | 11.688 | 217, 238 | 301.0 | 610.9 | Ellagic acid | O | O |
| 37 | 11.790 | 217, 278 | 787.1 | 393.2, 301.1 | TeGG (isomer) | O | O |
| 38 | 12.005 | 217, 279 | 787.1 | 393.2 | TeGG (isomer) | O | O |
| 39 | 12.280 | 217, 279 | 787.1 | 393.1 | TeGG (isomer) | O | O |
| 40 | 13.195 | 217, 279 | 787.1 | 393.1 | TeGG (isomer) | O | X |
| 41 | 13.256 | 217, 279 | 939.1 | 469.1 | PGG | O | O |
| 42 | 13.503 | 316, 360 | 285.1 | 113.0 | Fisetin | O | X |
| 43 | 14.344 | 255.1 | 432.9 | Unidentified | O | X | |
| 44 | 14.549 | 401.0 | 287.2, 723.4 | Unidentified | O | X | |
| 45 | 14.806 | 209.1 | 539.1 | Unidentified | O | X | |
| 46 | 15.441 | 265, 264 | 301.1 | 415.1, 603.0 | Quercetin | O | X |
| 47 | 16.430 | 423.1 | 271.1, 536.9 | Unidentified | O | X | |
| 48 | 16.995 | 261, 379 | 271.1 | 551.1, 385.1 | Butein | O | X |
| 49 | 21.014 | 417.1 | 531.0 | Unidentified | O | X | |
| 50 | 23.105 | 653.0 | 539.0, 518.7 | Unidentified | O | X | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Kim, M.-J.; Kim, D.-W.; Kim, G.-Y.; Kim, J.-K.; Gebru, Y.A.; Choi, H.-S.; Kim, Y.-H.; Kim, M.-K. Changes of Phytochemical Components (Urushiols, Polyphenols, Gallotannins) and Antioxidant Capacity during Fomitella fraxinea–Mediated Fermentation of Toxicodendron vernicifluum Bark. Molecules 2019, 24, 683. https://doi.org/10.3390/molecules24040683
Kim D-H, Kim M-J, Kim D-W, Kim G-Y, Kim J-K, Gebru YA, Choi H-S, Kim Y-H, Kim M-K. Changes of Phytochemical Components (Urushiols, Polyphenols, Gallotannins) and Antioxidant Capacity during Fomitella fraxinea–Mediated Fermentation of Toxicodendron vernicifluum Bark. Molecules. 2019; 24(4):683. https://doi.org/10.3390/molecules24040683
Chicago/Turabian StyleKim, Da-Ham, Min-Ji Kim, Dae-Woon Kim, Gi-Yoon Kim, Jong-Kuk Kim, Yoseph Asmelash Gebru, Han-Seok Choi, Young-Hoi Kim, and Myung-Kon Kim. 2019. "Changes of Phytochemical Components (Urushiols, Polyphenols, Gallotannins) and Antioxidant Capacity during Fomitella fraxinea–Mediated Fermentation of Toxicodendron vernicifluum Bark" Molecules 24, no. 4: 683. https://doi.org/10.3390/molecules24040683
APA StyleKim, D.-H., Kim, M.-J., Kim, D.-W., Kim, G.-Y., Kim, J.-K., Gebru, Y. A., Choi, H.-S., Kim, Y.-H., & Kim, M.-K. (2019). Changes of Phytochemical Components (Urushiols, Polyphenols, Gallotannins) and Antioxidant Capacity during Fomitella fraxinea–Mediated Fermentation of Toxicodendron vernicifluum Bark. Molecules, 24(4), 683. https://doi.org/10.3390/molecules24040683






