Catalytic Performance of Nitrogen-Doped Activated Carbon Supported Pd Catalyst for Hydrodechlorination of 2,4-Dichlorophenol or Chloropentafluoroethane
Abstract
1. Introduction
2. Results and Discussion
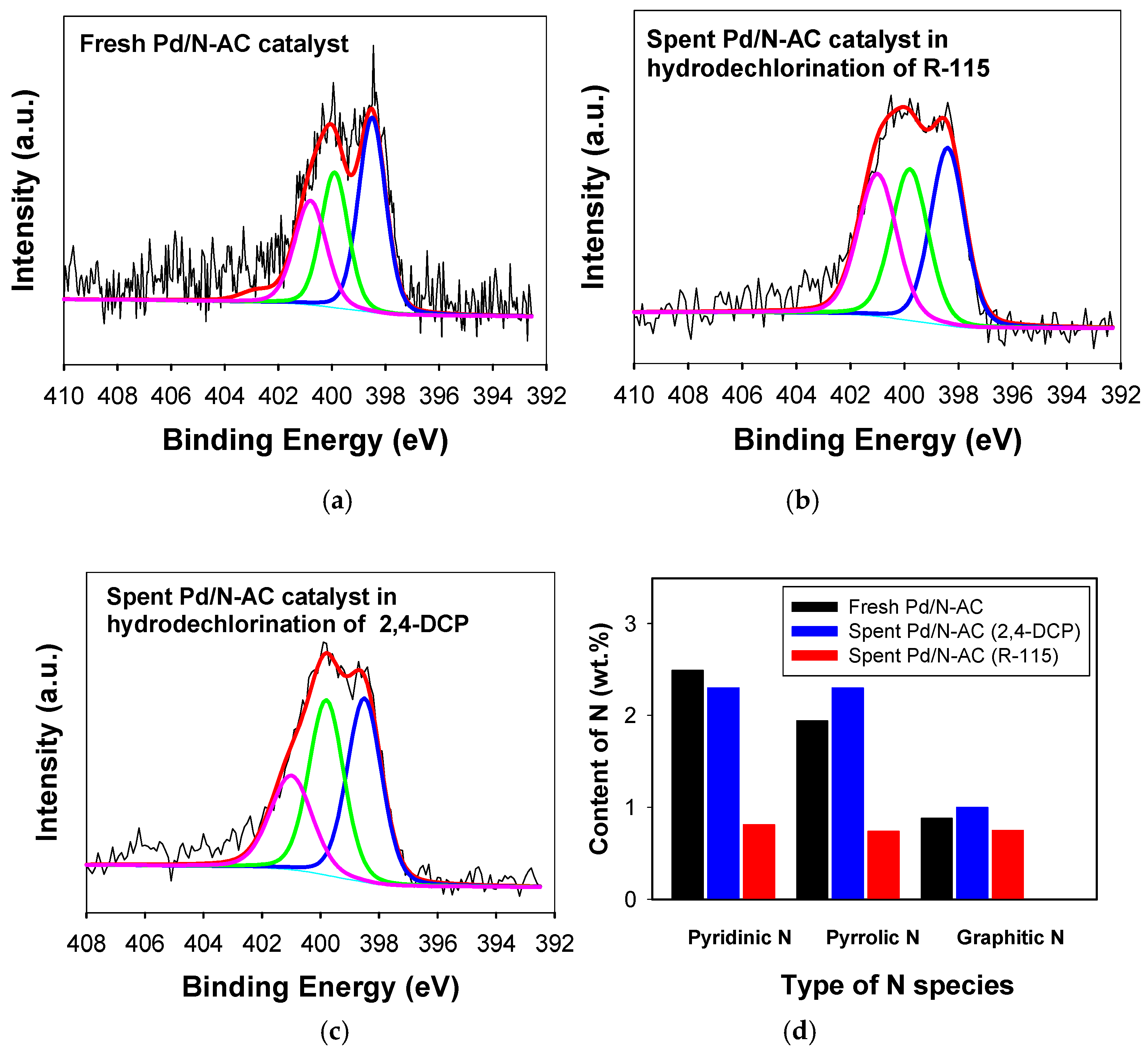
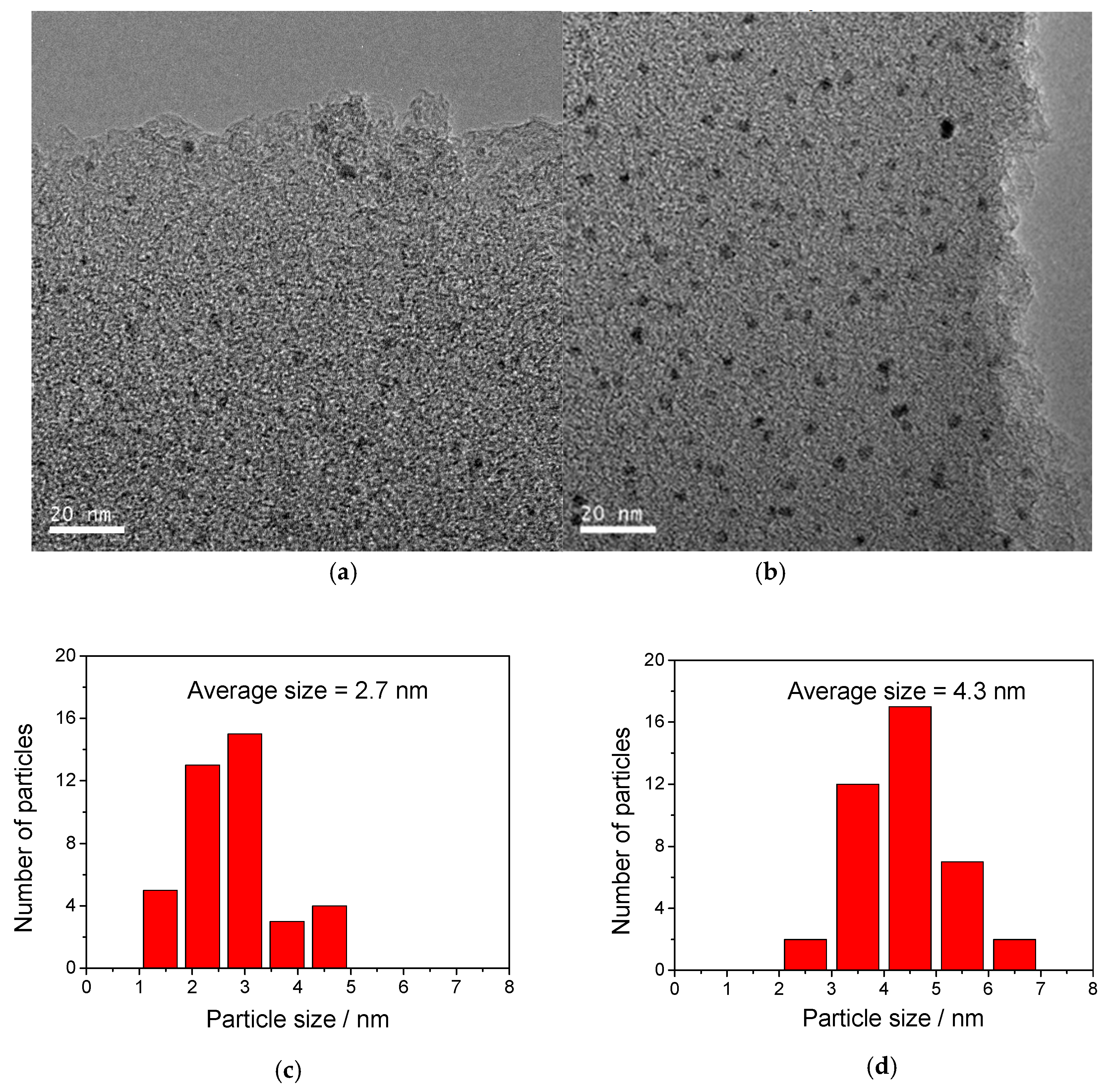
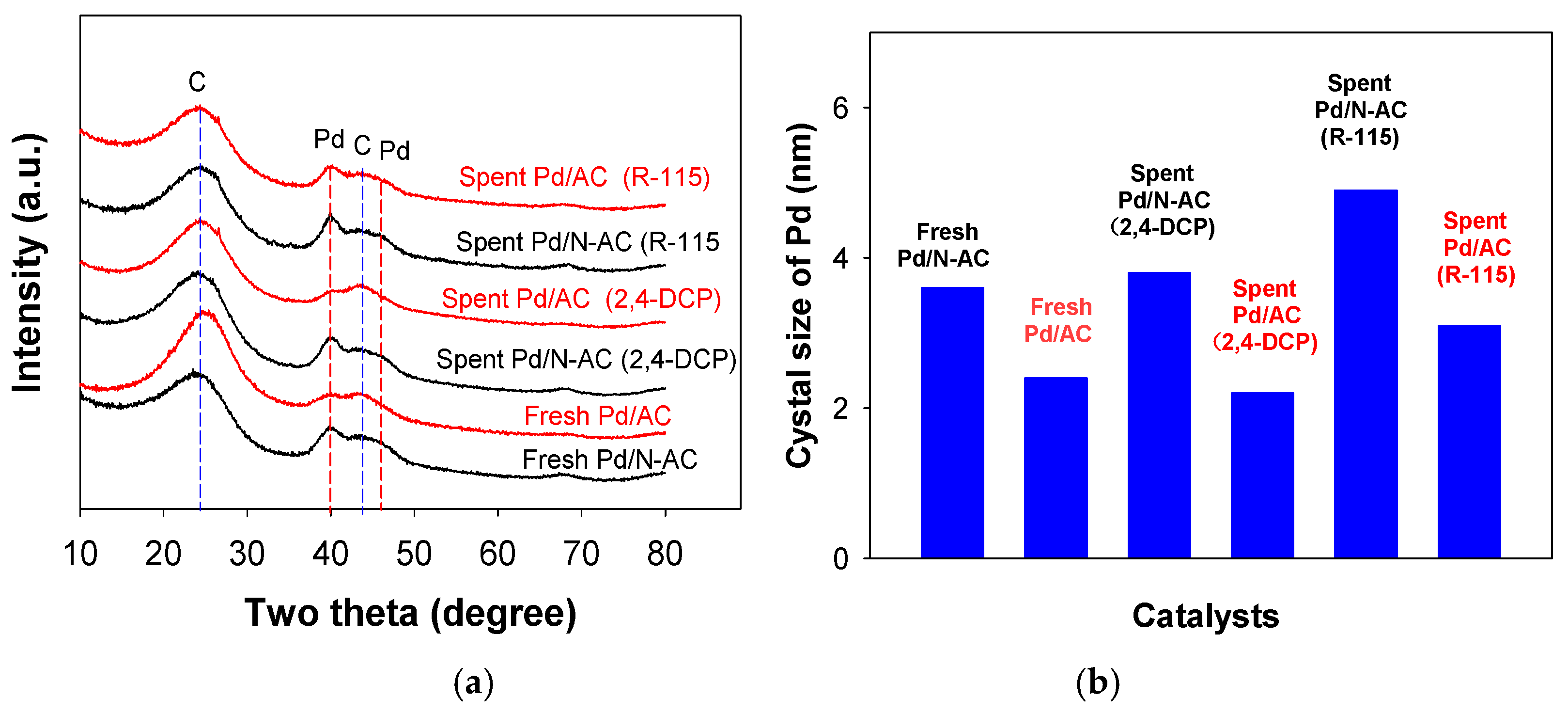
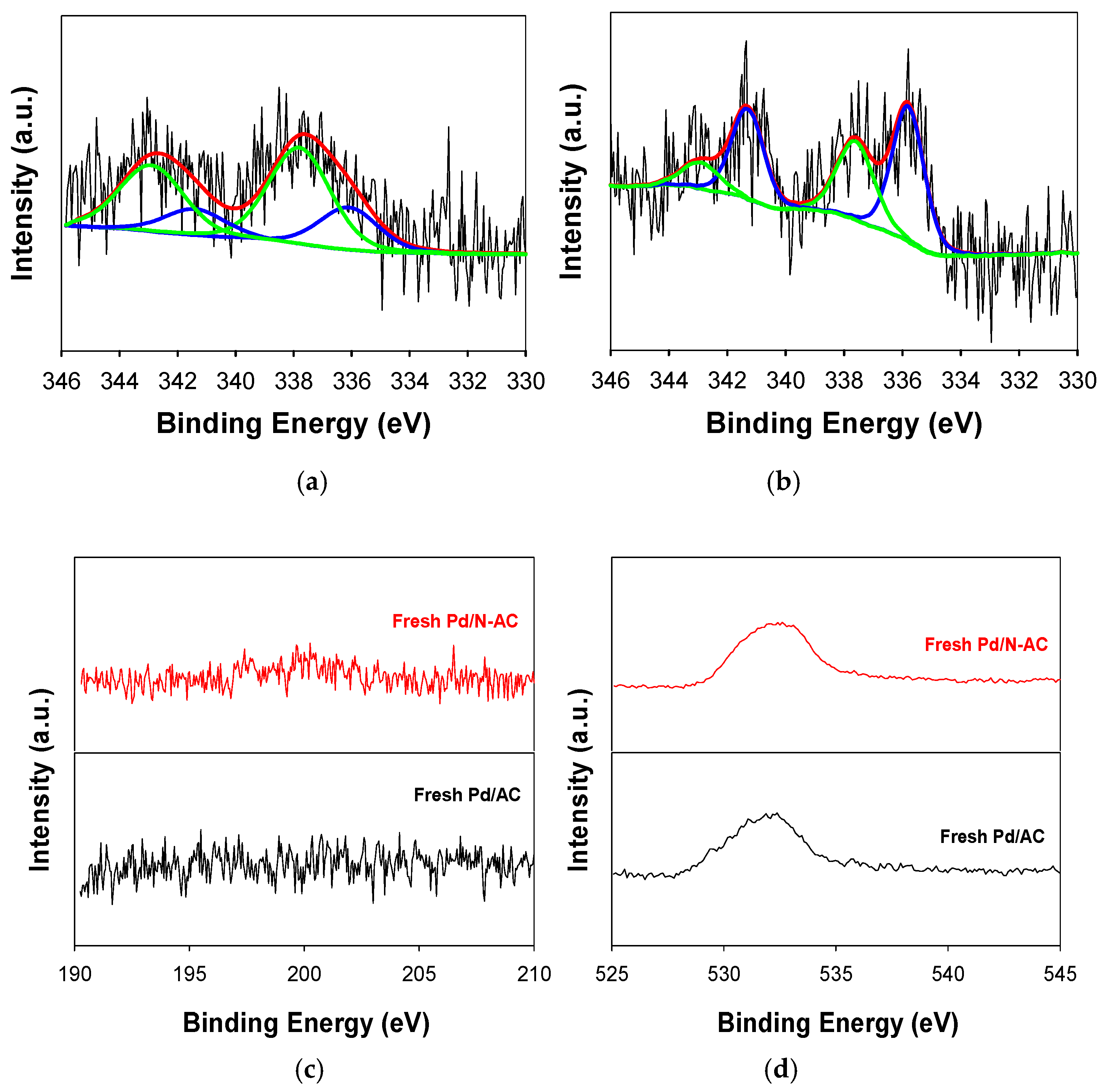
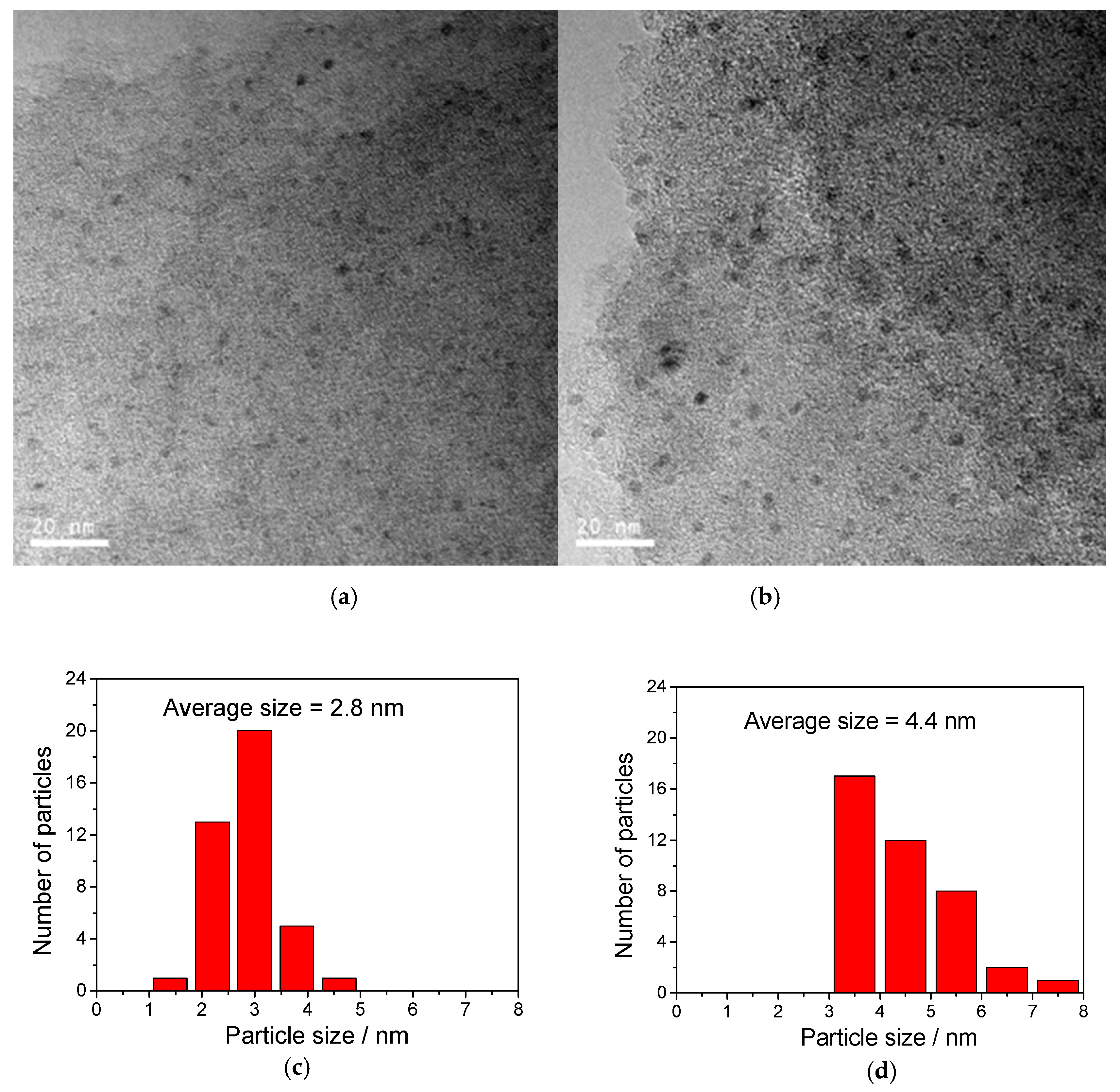
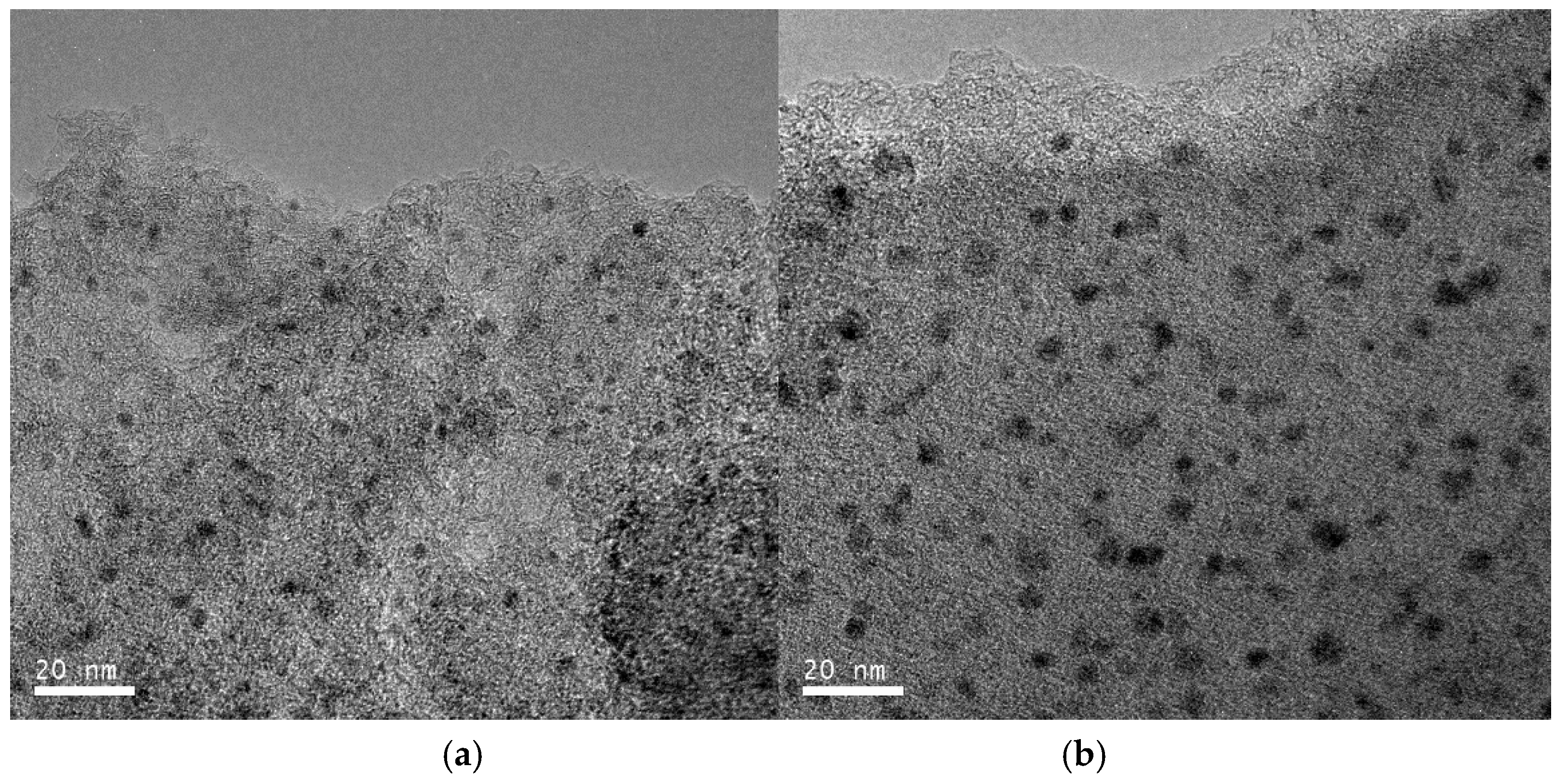
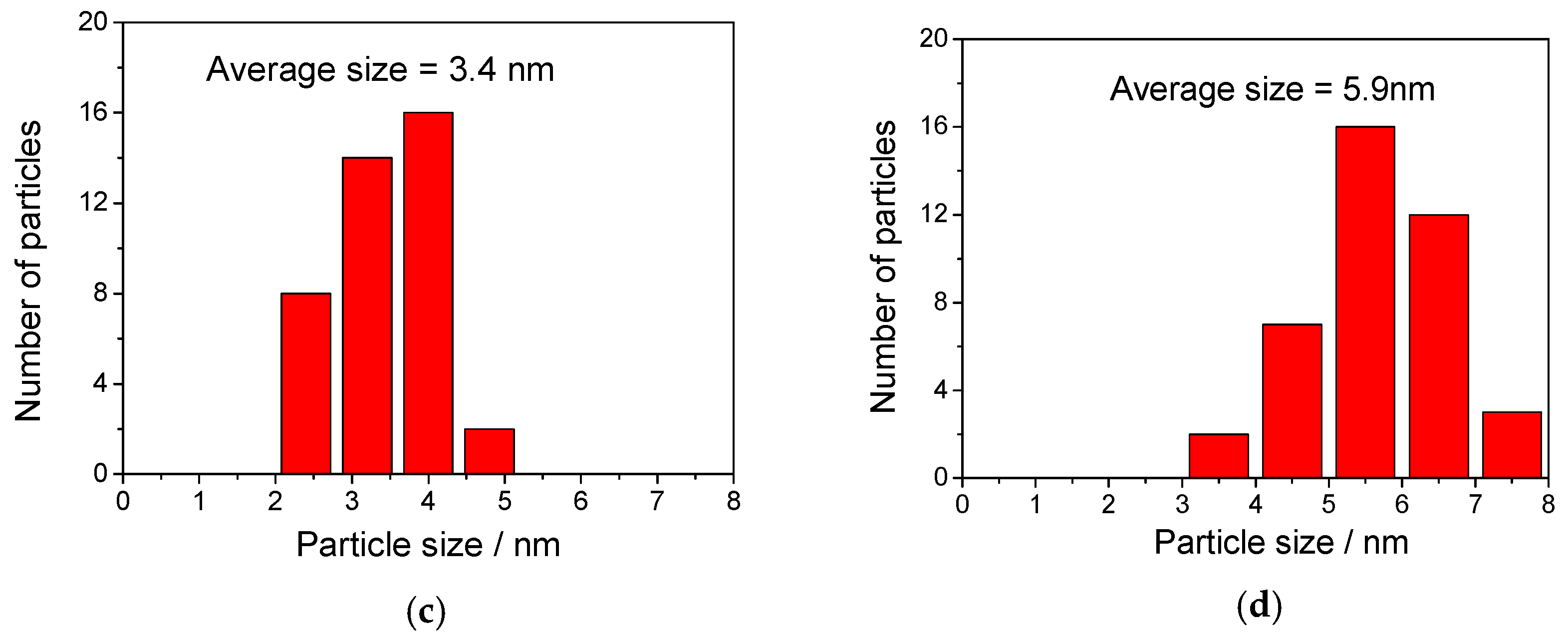
2.1. Catalyst Characterization
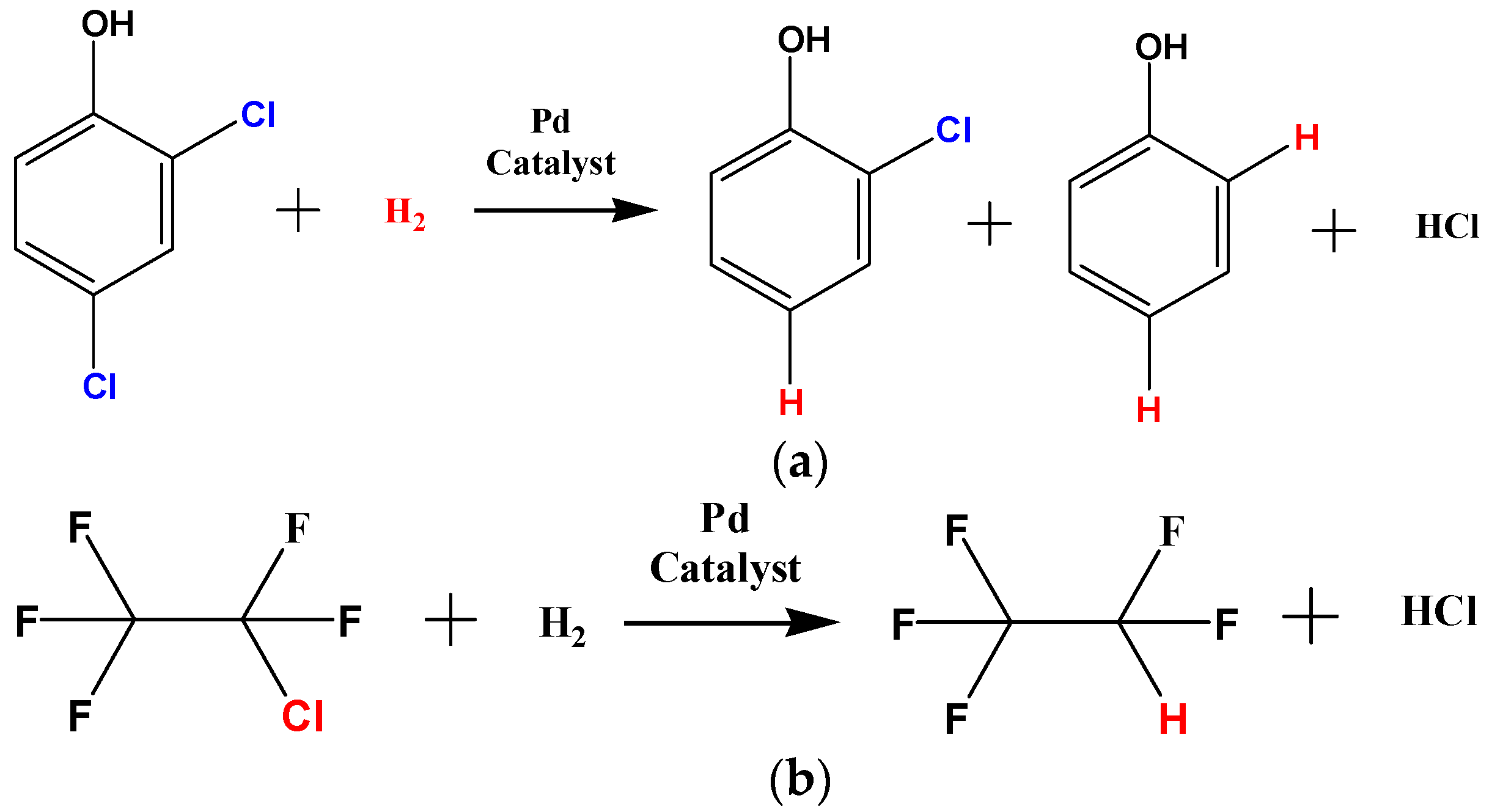
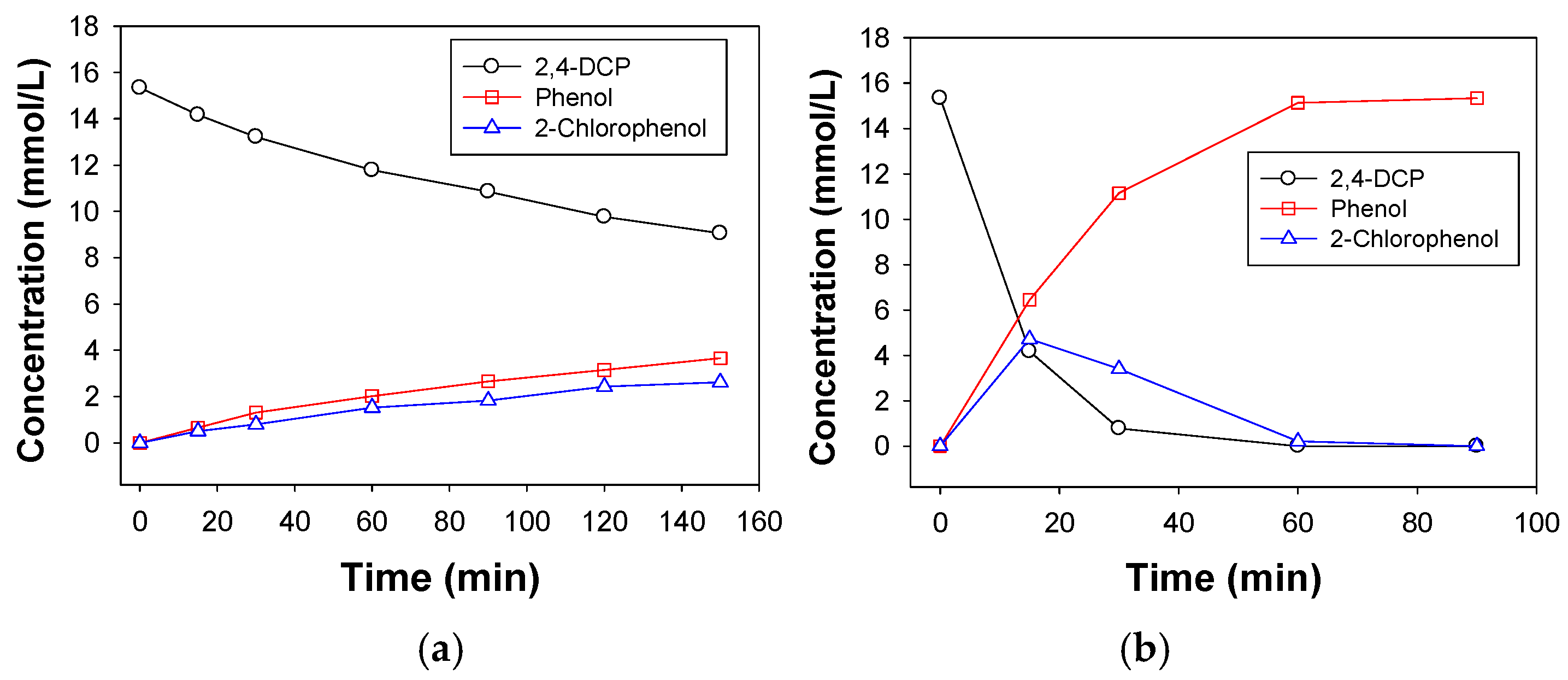
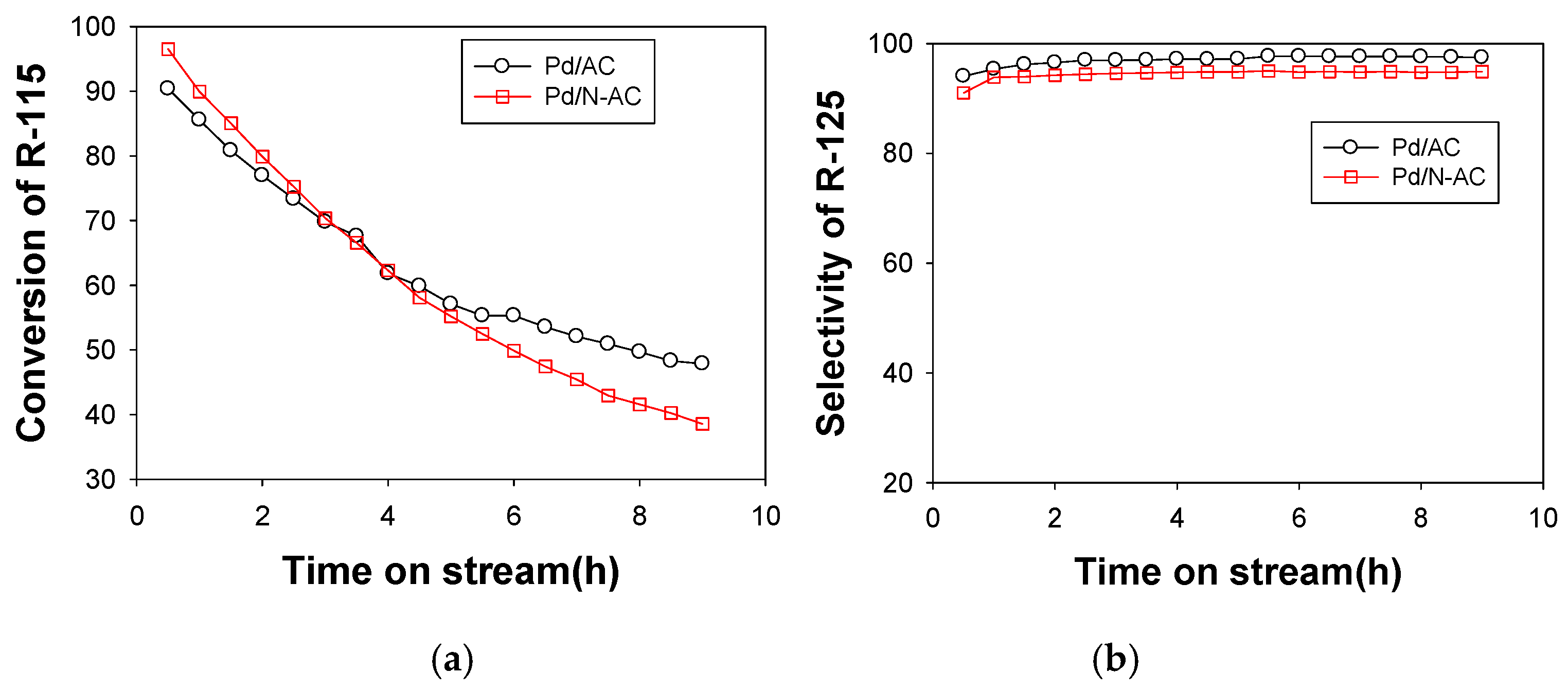
2.2. Catalytic Performance
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of N-AC
3.3. Preparation and Reduction of Supported Pd Catalysts
3.4. Characterization
3.5. Catalyst Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andreozzi, R.; Di, S.I.; Marotta, R.; Pinto, G.; Pollio, A.; Spasiano, D. Oxidation of 2,4-dichlorophenol and 3,4-dichlorophenol by means of Fe(III)-homogeneous photocatalysis and algal toxicity assessment of the treated solutions. Water Res. 2011, 45, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.M.; Yan, H.; Wang, S.L.; Chen, T.; Wang, G.Y. High selectivity to diphenyl carbonate synthesized via transesterification between dimethyl carbonate and phenol with C60-doped TiO2. Chem. Res. Chin. Univ. 2017, 33, 804–810. [Google Scholar] [CrossRef]
- Witońska, I.A.; Walock, M.J.; Binczarski, M.; Lesiak, M.; Stanishevsky, A.V.; Karski, S. Pd-Fe/SiO2 and Pd-Fe/Al2O3 catalysts for selective hydrodechlorination of 2, 4-dichlorophenol into phenol. J. Mol. Catal. A 2014, 393, 248–256. [Google Scholar] [CrossRef]
- Lovelock, J.E. Atmospheric Fluorine Compounds as Indicators of Air Movements. Nature 1971, 230, 379. [Google Scholar] [CrossRef]
- Molina, M.J.; Rowland, F.S. Rowland. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, K.; Wang, W.J.; Han, Y.X.; Xu, Z.Y.; Wan, H.Q.; Zheng, S.R.; Zhu, D.Q. Simultaneous removal of monochloroacetic acid and bromate by liquid phase catalytic hydrogenation over Pd/Ce1-xZrxO2. Appl. Catal. B 2015, 162, 85–92. [Google Scholar] [CrossRef]
- Menini, C.; Park, C.; Shin, E.J.; Tavoularis, G.; Keane, M.A. Catalytic hydrodehalogenation as a detoxification methodology. Catal. Today 2000, 62, 355–366. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, Z.Y.; Wan, H.Q.; Wan, Y.Q.; Chen, H.; Zhen, S.R.; Zhu, D.Q. Enhanced liquid phase catalytic hydrodechlorination of 2, 4-dichlorophenol over mesoporous carbon supported Pd catalysts. Catal. Commun. 2011, 12, 1405–1409. [Google Scholar] [CrossRef]
- Dong, Z.P.; Dong, C.X.; Liu, Y.S.; Le, X.D.; Jin, Z.C.; Ma, J.T. Hydrodechlorination and further hydrogenation of 4-chlorophenol to cyclohexanone in water over Pd nanoparticles modified N-doped mesoporous carbon microspheres. Chem. Eng. J. 2015, 270, 215–222. [Google Scholar] [CrossRef]
- Cui, X.L.; Zuo, W.; Tian, M.; Dong, Z.P.; Ma, J.T. Highly efficient and recyclable Ni MOF-derived N-doped magnetic mesoporous carbon-supported palladium catalysts for the hydrodechlorination of chlorophenols. J. Mol. Catal. A 2016, 423, 386–392. [Google Scholar] [CrossRef]
- Rioux, R.M.; Thompson, C.D.; Chen, N.; Ribeiro, F.H. Hydrodechlorination of chlorofluorocarbons CF3-CFCl2 and CF3-CCl3 over Pd/carbon and Pd black catalysts. Catal. Today 2000, 62, 269–278. [Google Scholar] [CrossRef]
- Mori, T.; Yasuoka, T.; Morikawa, Y. Hydrodechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane (CFC-113) over supported ruthenium and other noble metal catalysts. Catal. Today 2004, 88, 111–120. [Google Scholar] [CrossRef]
- Shekhar, S.C.; Murthy, J.K.; Rao, P.K.; Rao, K.S.R. Studies on the modifications of Pd/Al2O3 and Pd/C systems to design highly active catalysts for hydrodechlorination of CFC-12 to HFC-32. Appl. Catal. A 2004, 271, 95–101. [Google Scholar] [CrossRef]
- Zheng, X.; Qiang, X.; Zhang, Y.; Zhang, X.L.; Zhong, Y.J.; Zhou, W.D. Deactivation of Pd/C catalysts in the hydrodechlorination of the chlorofluorocarbons CFC-115 and CFC-12. Catal. Today 2011, 175, 615–618. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Gurrala, L.; Gogoi, P.; Chilukuri, S.V. Hydrogenation of cinnamaldehyde to hydrocinnamaldehyde over Pd nanoparticles deposited on nitrogen-doped mesoporous carbon. RSC Adv. 2016, 6, 44333–44340. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Gong, Y.; Zhang, P.F.; Li, H.R.; Wang, Y. Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade. J. Am. Chem. Soc. 2012, 134, 16987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Q.Y.; Han, Y.X.; Zheng, S.R. Enhanced catalytic hydrodechlorination of 2, 4-dichlorophenol over Pd catalysts supported on nitrogen-doped grapheme. RSC Adv. 2015, 5, 91363–91371. [Google Scholar] [CrossRef]
- Baeza, J.A.; Alonso-Morales, N.; Calvo, L.; Heras, F.; Rodriguez, J.J.; Gilarranz, M.A. Hydrodechlorination activity of catalysts based on nitrogen-doped carbons from low-density polyethylene. Carbon 2015, 87, 444–452. [Google Scholar] [CrossRef]
- Alonso-Morales, N.; Ruiz-Garcia, C.; Palomar, J.; Heras, F.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A. Hollow nitrogen- or boron-doped carbon submicrospheres with a porous shell: Preparation and application as supports for hydrodechlorination catalysts. Ind. Eng. Chem. Res. 2017, 56, 7665–7674. [Google Scholar] [CrossRef]
- Tang, H.D.; Xiang, M.; Xu, B.; Li, Y.; Han, W.F.; Liu, Z.J. One-step synthesis of N-doped mesoporous carbon as highly efficient support of Pd catalyst for hydrodechlorination of 2,4-dichlorophenol. Chem. Res. Chin. Univ. 2018, 34, 1004–1008. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Mao, S.J. Effects to Hydrogenation Derived from the Catalytic Surface Structure and Environment. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 10 March 2018. [Google Scholar]
- Xia, C.H.; Liu, Y.; Xu, J.; Yu, J.B.; Qin, W.; Liang, X.M. Catalytic hydrodechlorination reactivity of monochlorophenols in aqueous solutions over palladium/carbon catalyst. Catal. Commun. 2009, 10, 456–458. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Xu, B.; Xiang, M.; Chen, X.; Wang, Y.; Liu, Z. Catalytic Performance of Nitrogen-Doped Activated Carbon Supported Pd Catalyst for Hydrodechlorination of 2,4-Dichlorophenol or Chloropentafluoroethane. Molecules 2019, 24, 674. https://doi.org/10.3390/molecules24040674
Tang H, Xu B, Xiang M, Chen X, Wang Y, Liu Z. Catalytic Performance of Nitrogen-Doped Activated Carbon Supported Pd Catalyst for Hydrodechlorination of 2,4-Dichlorophenol or Chloropentafluoroethane. Molecules. 2019; 24(4):674. https://doi.org/10.3390/molecules24040674
Chicago/Turabian StyleTang, Haodong, Bin Xu, Meng Xiang, Xinxin Chen, Yao Wang, and Zongjian Liu. 2019. "Catalytic Performance of Nitrogen-Doped Activated Carbon Supported Pd Catalyst for Hydrodechlorination of 2,4-Dichlorophenol or Chloropentafluoroethane" Molecules 24, no. 4: 674. https://doi.org/10.3390/molecules24040674
APA StyleTang, H., Xu, B., Xiang, M., Chen, X., Wang, Y., & Liu, Z. (2019). Catalytic Performance of Nitrogen-Doped Activated Carbon Supported Pd Catalyst for Hydrodechlorination of 2,4-Dichlorophenol or Chloropentafluoroethane. Molecules, 24(4), 674. https://doi.org/10.3390/molecules24040674




