Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals
Abstract
1. Introduction
2. Results and Discussion
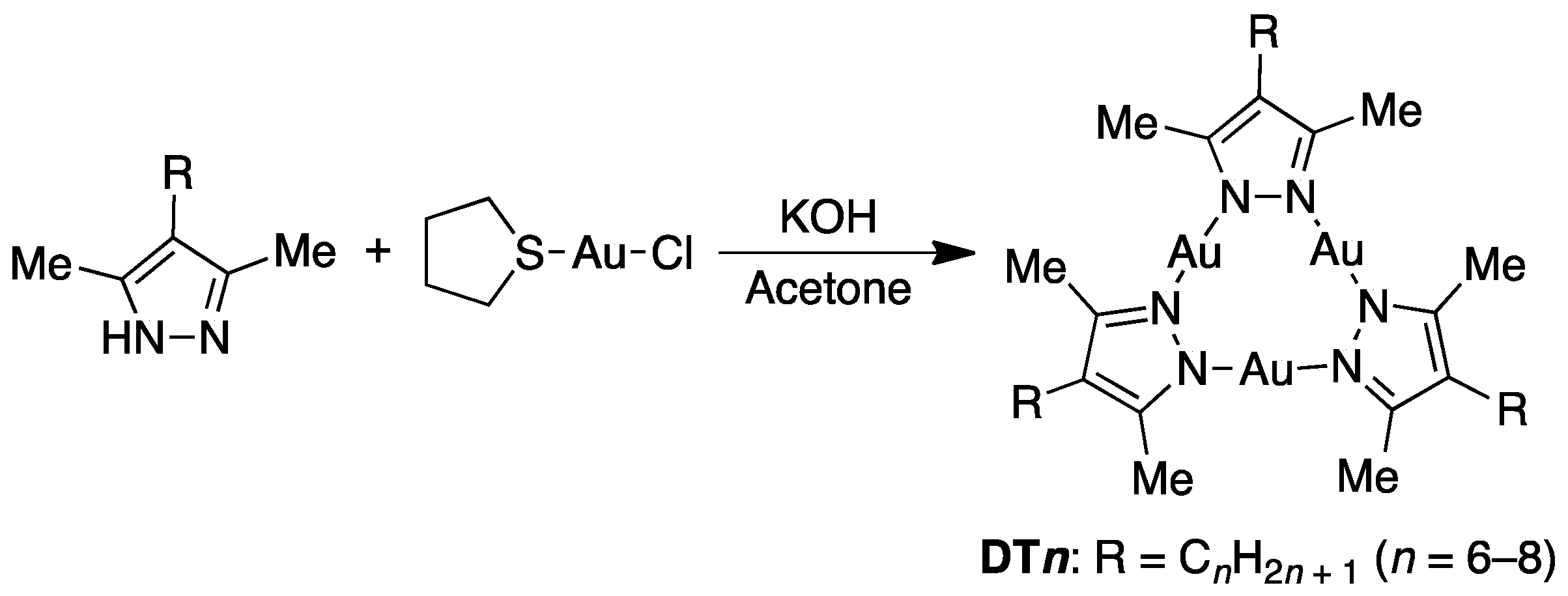
2.1. Synthesis and Characterization of Complexes
2.2. Photoluminescence Behavior of the Complexes
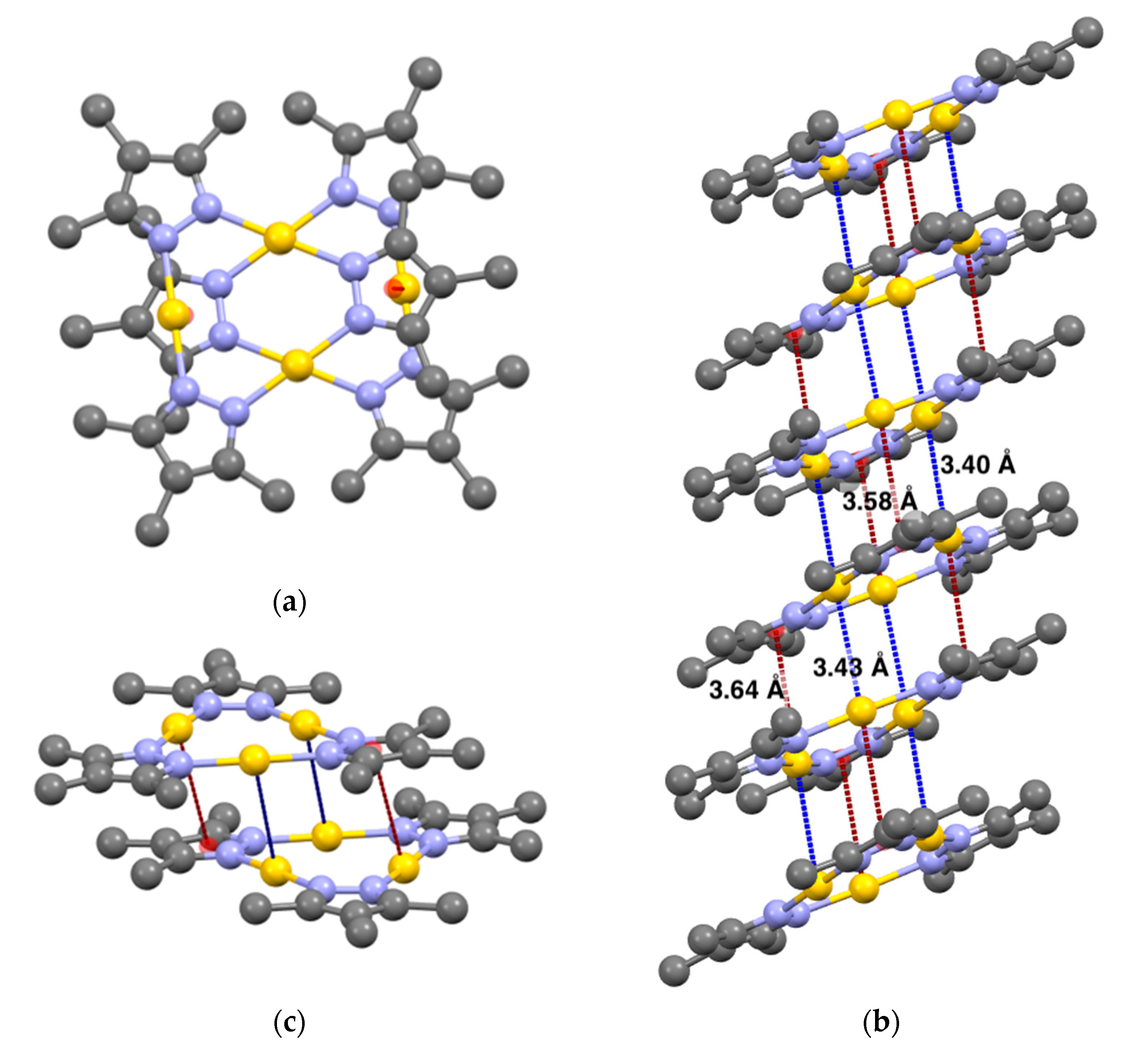
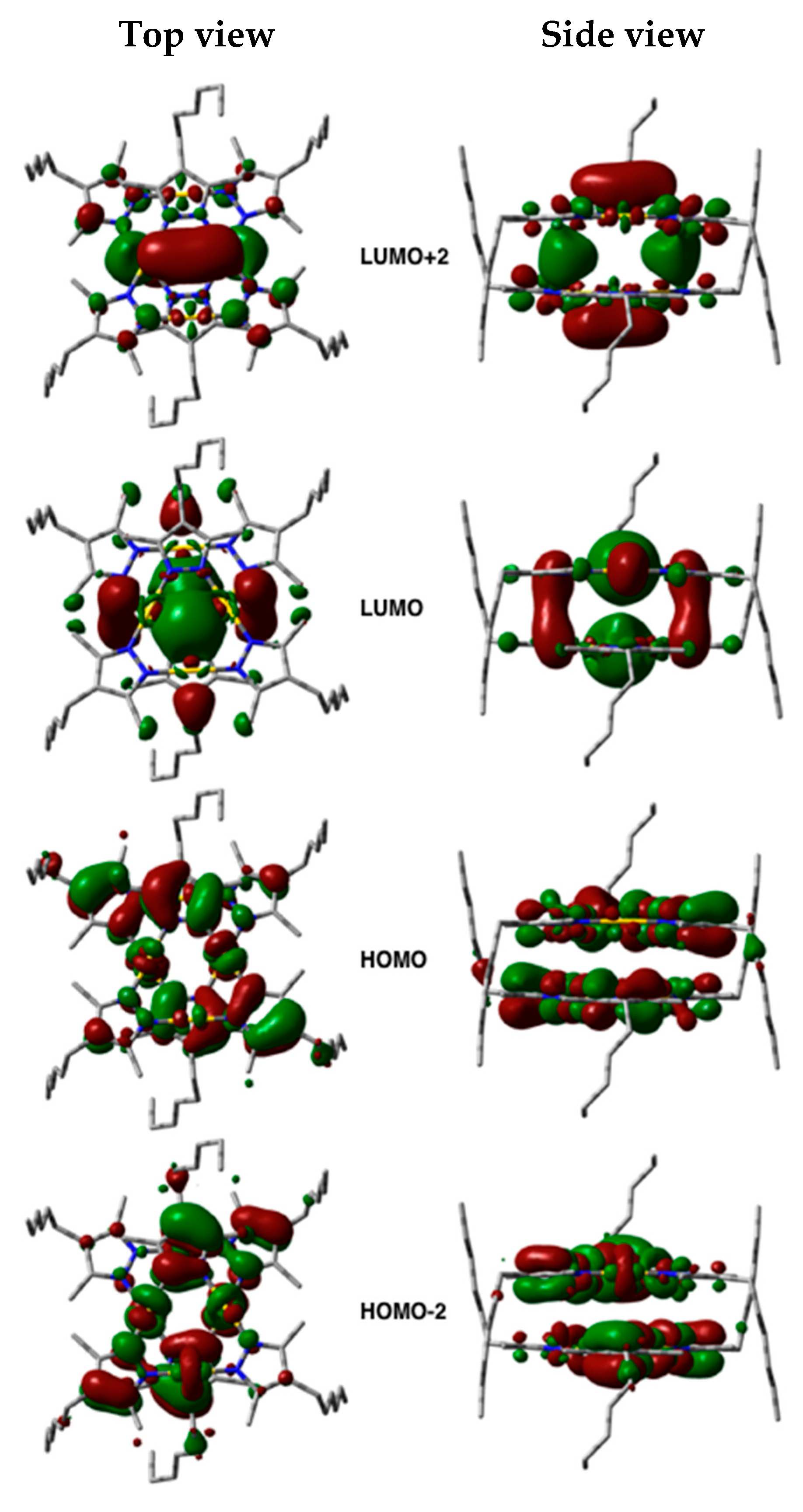
2.3. Relationship between the Room-Temperature Phosphorescence Properties and Aggregated Structure
3. Materials and Methods
3.1. Preparation of Materials
3.2. Single Crystal X-ray Structure
3.3. Photophysical Properties
3.4. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, Unite We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Tang, B.Z. Aggregation-Induced Emission: Fundamentals; John Wiley& Sons, Ltd.: West Sussex, UK, 2014. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Malkin, J. Photophysical and Photochemical Properties of Aromatic Compounds; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Ronda, C.R. Luminescence: From Theory to Applications; Ronda, C.R., Ed.; Wiley-VCH Verlag GmbH &Co. KGaA: Weinheim, Germany, 2008; pp. 1–34. [Google Scholar]
- Leung, C.W.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.; Tang, B.Z. A photostable AIE luminogen for specific mitochondrial imaging and tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.D.; Lam, J.W.Y.; Qin, W.; Li, J.; Xie, N.; Tang, B.Z. Molecular luminogens based on restriction of intramolecular motions through host-guest inclusion for cell imaging. Chem. Commun. 2014, 50, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Highly sensitive and selective fluorometric off-on K+ probe constructed via host-guest molecular recognition and aggregation-induced emission. J. Mater. Chem. 2012, 22, 8622–8628. [Google Scholar] [CrossRef]
- Huang, J.; Tang, R.; Zhang, T.; Li, Q.; Yu, G.; Xie, S.; Liu, Y.; Ye, S.; Qin, J.; Li, Z. A New Approch to prepare Efficient Blue AIE Emitters for Undoped OLEDs. Chemistry 2014, 20, 5317–5326. [Google Scholar] [CrossRef]
- Li, H.; Chi, Z.; Zhang, X.; Xu, B.; Liu, S.; Zhang, Y.; Xu, J. New Thermally Stable Aggregation-Induced Emission Enhancement Compounds for Non-Doped Red Light-Emitting Diodes. Chem. Commun. 2011, 47, 11273–11275. [Google Scholar] [CrossRef]
- Zhoa, W.; Cheung, T.S.; Jiang, N.; Huang, W.; Lam, J.W.Y.; Zhang, X.; He, Z.; Tang, B.Z. Boosting the Efficiency of Organic Persistent Room-Temperature Phosphorescence by Intramolecular Triplet-Triplet Energy Transfer. Nat. Commun. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Crespo, O. Modern Supramolecular Gold Chemistry; Laguna, A., Ed.; Wiley-VCH Verlag GmbH &Co. KGaA: Weinheim, Germany, 2009; pp. 65–129. [Google Scholar]
- Yam, V.W.W.; Au, V.K.; Leung, S.Y. Light-Emission Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Yang, C.; Messerschmidt, M.; Coppens, P.; Omary, M.A. Trinuclear Gold(I) Triazolates: A New Class of Wide-Band Phosphors and Sensors. Inorg. Chem. 2006, 45, 6592–6594. [Google Scholar] [CrossRef]
- Schmidbaur, H. The Aurophilicity Phenomenon: A Decade of Experimental Findings, Theoretical Concepts and Emerging Applications. Gold Bull. 2000, 33, 3–10. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Aurophilic Interactions as a Subject of Current Research: An Up-Date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Tubaro, C.; Biffis, A.; Basato, M.; Graiff, C.; Poater, A.; Cavallo, L.; Armaroli, N.; Accorsi, G. Blue-Emitting Dinuclear N-heterocyclic Dicarbene Gold(I) Complex Featuring a Nearly Unit Quantum Yield. Inorg. Chem. 2012, 51, 1178–1784. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Yamasita, T.; Aida, T. Phosphorescent Organogels via “Mettalophilic” Interactions for Reversible RGB-Color Switching. J. Am. Chem. Soc. 2005, 127, 179–183. [Google Scholar] [CrossRef] [PubMed]
- White-Morris, R.L.; Olmstead, M.M.; Attar, S.; Balch, A.L. Intermolecular Interactions in Polymorphs of Trinuclear Gold(I) Complexes: Insight into the Solvoluminescence of AuI3(MeN=COMe)3. Inorg. Chem. 2005, 44, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Balch, A.L.; Olmstead, M.M.; Vickery, J.C. Gold(I) Compounds without Significant Aurophilic Intermolecular Interactions: Synthesis, Structure, and Electronic Properties of Ph3PAuC(O)NHMe and Au3(PhCH2N=COMe)3: Comparative Monomeric and Trimeric Analogues of the Solvoluminescent Trimer, Au3(MeN=COMe)3. Inorg. Chem. 1999, 38, 3494–3499. [Google Scholar]
- Vickery, J.C.; Olmstead, M.M.; Fung, E.Y.; Balch, A.L. Solvent-Stimulated Luminescence from the Supramolecular Aggregation of a Trinuclear Gold (i) Complex that Displays Extensive Intermolecular AuċAu Interactions. Angew. Chem. Int. Ed. Engl. 1997, 36, 1179–1181. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Tsuge, K.; Sawamura, M. Reversible Mechanochromic Luminescence of [(C6F5Au]2(µ-1,4-diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044–10045. [Google Scholar] [CrossRef]
- Ito, H.; Muromoto, M.; Kurenuma, S.; Ishizaka, S.; Kitamura, N.; Sato, H.; Seki, T. Mechanical Stimulation and Solid Seeding Trigger Single-Crystal-to-Single-Crystal Molecular Domino Transformations. Nat. Commun. 2013, 4, 2009. [Google Scholar] [CrossRef]
- Seki, T.; Sakurada, K.; Muromoto, M.; Ito, H. Photoinduced Single-Crystal-to-Single-Crystal Phase Transition and Photosalient Effect of a Gold(I) Isocyanide Complex with Shortening of Intermolecular Aurophilic Bonds. Chem. Sci. 2015, 6, 1491–1497. [Google Scholar] [CrossRef]
- Seki, T.; Takamatsu, Y.; Ito, H. A Screening Approach for the Discover of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-to-Crystal Phase Transitions. J. Am. Chem. Soc. 2016, 138, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, A.; Nakamura, S.; Hisano, K.; Tsutsumi, O.; Srinivas, K.; Prabusankar, G. Controlling the Solid-State Luminescence of Gold(I) N-Heterocyclic Carbene Complexes through Change in the Structure of Molecular Aggregates. Sci. China Chem. 2018, 61, 957–965. [Google Scholar] [CrossRef]
- Yamada, S.; Rokusha, Y.; Kawano, R.; Fujisawa, K.; Tsutsumi, O. Mesogenic Gold Complexes Showing Aggregation-Induced Enhancement of Phosphorescence in Both Crystalline and Liquid-Crystalline Phases. Faraday Discuss. 2017, 196, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Younis, O.; Ando, A.; Rokusha, Y.; Yamada, S.; Tsutsumi, O. Photoluminescence from Au(I) Complexes Exhibiting Color Sensitivity to the Structure of the Molecular Aggregates. Chem. Lett. 2016, 45, 66–68. [Google Scholar] [CrossRef]
- Fujisawa, K.; Yamada, S.; Yanagi, Y.; Yoshioka, Y.; Kiyohara, A.; Tsutsumi, O. Tunning the Photoluminescence of Condensed-Phase Cyclic Trinuclear Au(I) Complexes through Control of Their Aggregated Structures by External Stimuli. Sci. Rep. 2015, 5, 7934. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Okuda, Y.; Izumi, Y.; Nagamatsu, A.; Rokusha, Y.; Sadaike, Y.; Tsutsumi, O. Reversible Thermal-Mode Control of Luminescence fromLiquid-Crystalline Gold(I) Complexes. J. Mater. Chem. C 2014, 2, 3549–3555. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kawakami, N.; Onishi, Y.; Izumi, Y.; Tamai, S.; Sugimoto, N.; Tsutsumi, O. Photoluminescent Properties of Liquid Crystalline Gold(I) Isocyanide Complexes with a Rod-Like Molecular Structure. J. Mater. Chem. C 2013, 1, 5359–5366. [Google Scholar] [CrossRef]
- Baeberá, J.; Elduque, A.; Giménez, R.; Ora, L.A.; Serrano, J.L. Pyrazolate “Golden” Rings: Trinuclear Complexes That Form Columnar Mesophases at Room Temperature. Angew. Chem. Int. Ed. Engl. 1996, 35, 2832–2835. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, S.H.; Park, K.M.; Kim, H.; Zin, W.C.; Choi, M.G.; Kim, K. Trinuclear Gold(I) Pyrazolate Complexes. Exhibiting Heaxagonal Columnar Mesophases with Only Three Side-Chains. Chem. Mater. 1998, 10, 1889–1893. [Google Scholar] [CrossRef]
- Cored, J.; Crespo, O.; Serrano, J.L.; Elduque, A.; Giménez, R. Decisive Influence of the Metal in Multifunctional Gold, Silver, and Copper Metallacycles: High Quantum Yield Phosphorescence, Color Switching, and Liquid Crystalline Behavior. Inorg. Chem. 2018, 57, 12632–12640. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Gold⋅⋅⋅π Aryl Interactions as Supramolecular Synthons. CrystEngComm 2009, 11, 1176–1186. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Aggregation-Induced Emission: Applications; Tang, B.Z., Qin, A., Eds.; John Wiley & Sons: London, UK, 2013; pp. 43–60. [Google Scholar]
- Yaun, W.Z.; Shen, X.Y.; Zhao, H.; Lam, J.W.Y.; Tang, L.; Lu, P.; Wang, C.; Liu, Y.; Wang, Z.; Zheng, Q.; et al. Crystallization-Induced Phosphorescence of Pure Organic Luminescence at Room Temperature. J. Phys. Chem. C 2010, 114, 6090–6099. [Google Scholar] [CrossRef]
- Sathish, V.; Ramdass, A.; Thanasekaran, P.; Lu, K.L.; Rajagopal, S. Aggregation-Induced Phosphorescence Enhancement (AIPE) Base on Transition Metal Complexes—An overview. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 25–44. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-2014, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and Refinement of Crystal Structure with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGx Suite for Small-Molecule Single-Crystal Crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, J.T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision, E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Qin, W.; Alifu, N.; Cai, Y.; Lam, J.W.Y.; He, X.; Su, H.; Zhang, P.; Qian, J.; Tang, B.Z. Synthesis of an Efficient Far-Red/Near-Infrared Luminogen with AIE Characteristic for in vivo Bioimaging Applications. Chem. Commun. 2019, 55, 5615–5618. [Google Scholar] [CrossRef]
- Kanosue, K.; Ando, S. Polymides with Heavy Halogens Exhibiting Room-Temperature Phosphorescence with Very Large Stokes Shifts. ACS Macro Lett. 2016, 5, 1301–1305. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Tian, X.; Gao, Y.; Zhao, X.; Grimsdale, A.C.; Lin, X.; Sun, H. An Organic Dye with Very Large Stockes-Shift and Broad Tunability of Fluorescence: Potentail Two-Photon Probe for Bioimaging and Ultra-Sensitive Solid-State Gas Sensor. Appl. Phys. Lett. 2016, 108, 011901. [Google Scholar] [CrossRef]
- Turrisi, R.; Sanguineti, A.; Sassi, M.; Savoie, B.; Takai, A.; Patriarca, G.E.; Salamone, M.M.; Ruffo, R.; Vaccaro, G.; Meinardi, F.; et al. Stokes Shift/Emission Efficiency Trade-Off in Donor-Acceptor Perylenemonoimides for Luminescenct Solar Concentrators. J. Mater. Chem. A 2015, 3, 8045–8054. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, Z.; Ai, B.; Lin, Y.; Zhou, W.; Zhang, J.; Yu, L.; Mi, Q.; Lian, S. Significant Improved Quantum Yield of CaAl12O19: Mn4+ Red Phosphor by Co-Doping Bi3+ and B3+ Ions and Dual Application for Plant Cultivations. J. Lumin. 2018, 201, 314–320. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
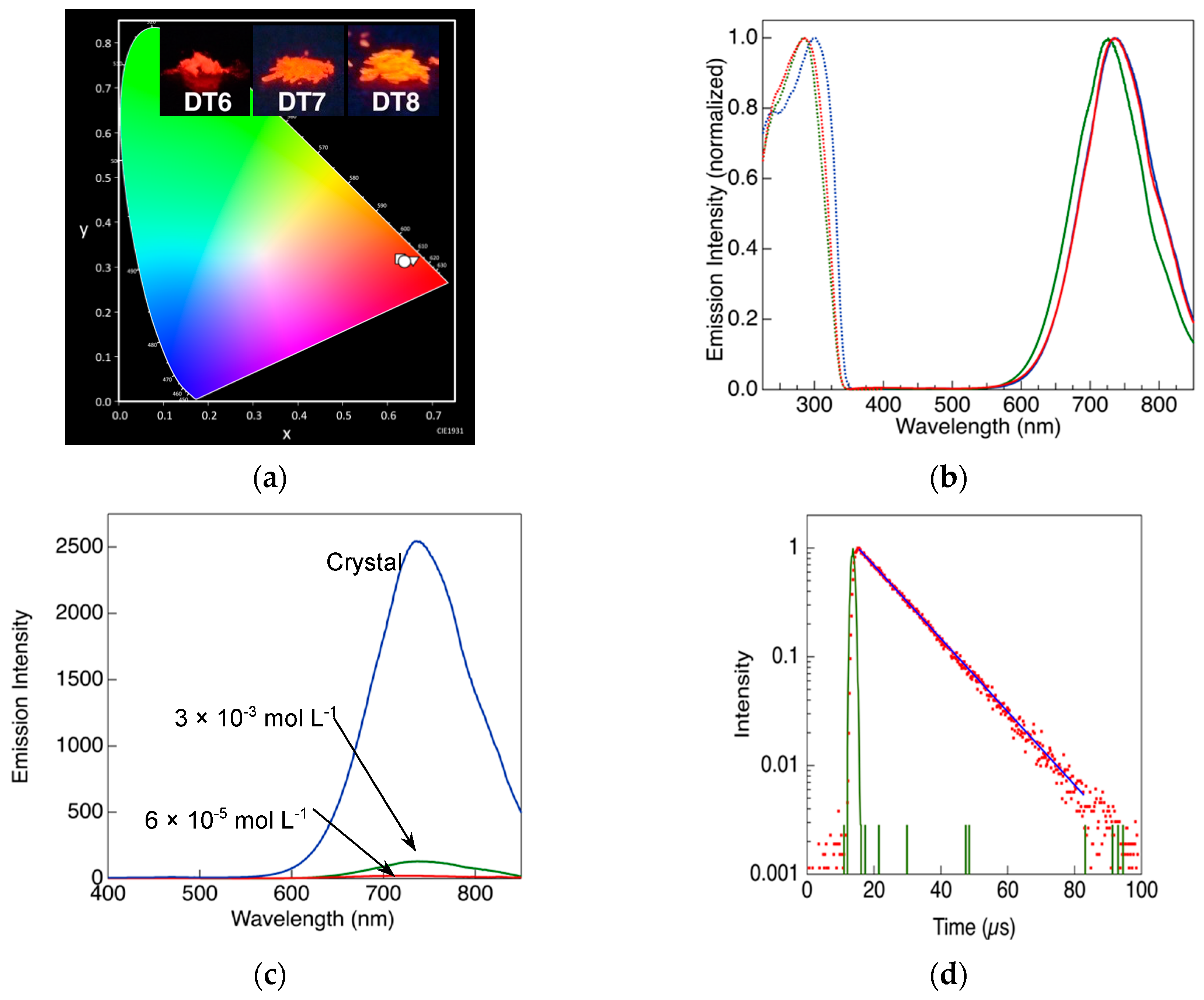
| Sample | λmaxlum[nm] a) | Φa,b) | τ [μs] | kr [s−1] c) | knr [s−1] c) |
|---|---|---|---|---|---|
| DT6 | 733 | 0.748 | 13 | 5.8 × 104 | 1.9 × 104 |
| DT7 | 726 | 0.609 | 13 | 4.7 × 104 | 3.0 × 104 |
| DT8 | 733 | 0.625 | 12 | 5.2 × 104 | 3.1 × 104 |
| Sample | Au–Au Distance [Å] | Au–π Distance [Å] | θ [deg] a) |
|---|---|---|---|
| DT6 | 3.40 | 3.58 | 15 |
| DT7 | 3.29 | 3.38 | 2.4 |
| DT8 | 3.25 | 3.39 | 7.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsutsumi, O.; Tamaru, M.; Nakasato, H.; Shimai, S.; Panthai, S.; Kuroda, Y.; Yamaguchi, K.; Fujisawa, K.; Hisano, K. Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals. Molecules 2019, 24, 4606. https://doi.org/10.3390/molecules24244606
Tsutsumi O, Tamaru M, Nakasato H, Shimai S, Panthai S, Kuroda Y, Yamaguchi K, Fujisawa K, Hisano K. Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals. Molecules. 2019; 24(24):4606. https://doi.org/10.3390/molecules24244606
Chicago/Turabian StyleTsutsumi, Osamu, Masakazu Tamaru, Hitoya Nakasato, Shingo Shimai, Supattra Panthai, Yuki Kuroda, Kenta Yamaguchi, Kaori Fujisawa, and Kyohei Hisano. 2019. "Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals" Molecules 24, no. 24: 4606. https://doi.org/10.3390/molecules24244606
APA StyleTsutsumi, O., Tamaru, M., Nakasato, H., Shimai, S., Panthai, S., Kuroda, Y., Yamaguchi, K., Fujisawa, K., & Hisano, K. (2019). Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals. Molecules, 24(24), 4606. https://doi.org/10.3390/molecules24244606







