Novel Synthetic Approaches for Bisnaphthalimidopropyl (BNIP) Derivatives as Potential Anti-Parasitic Agents for the Treatment of Leishmaniasis
Abstract
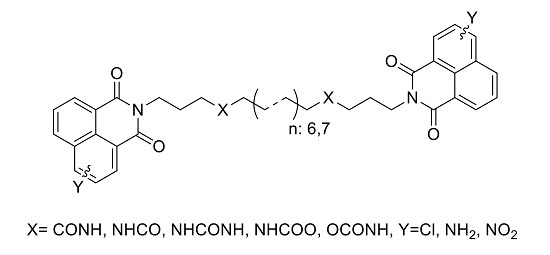
1. Introduction
2. Materials and Methods
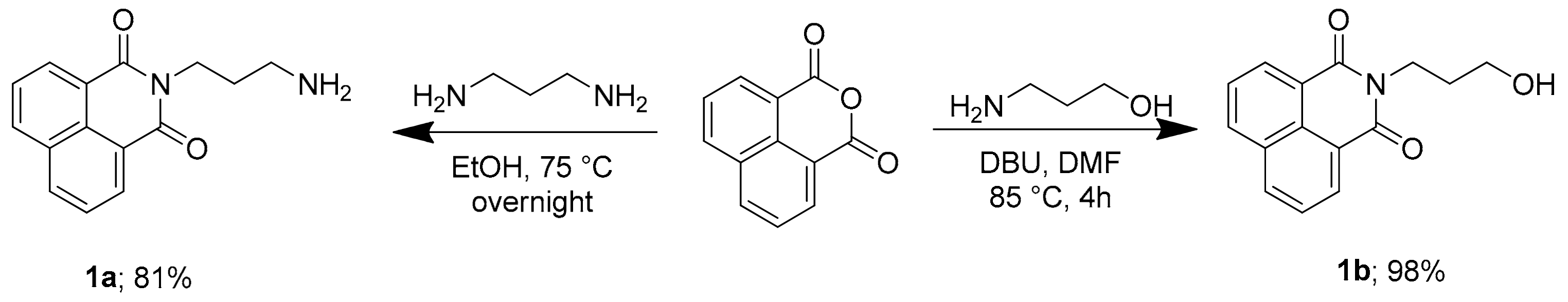
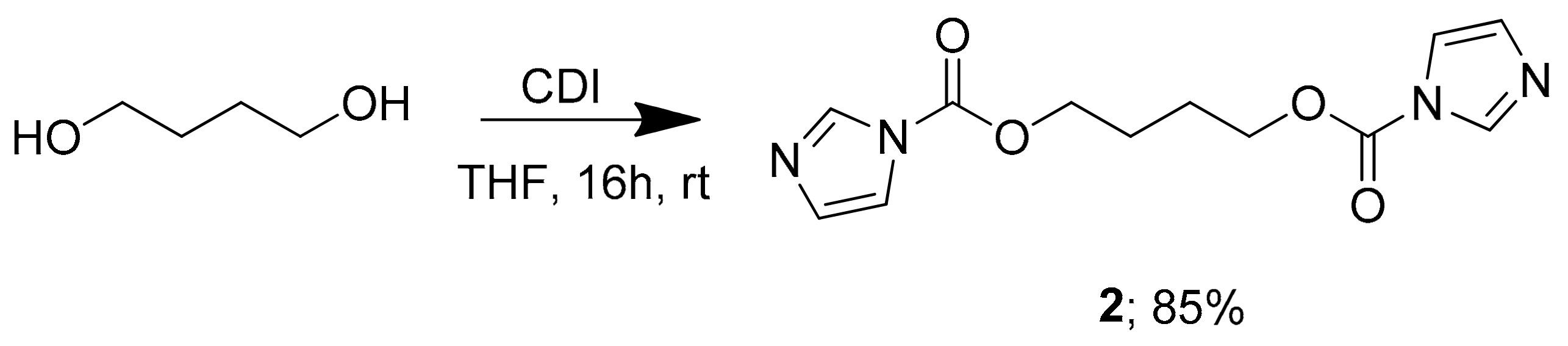
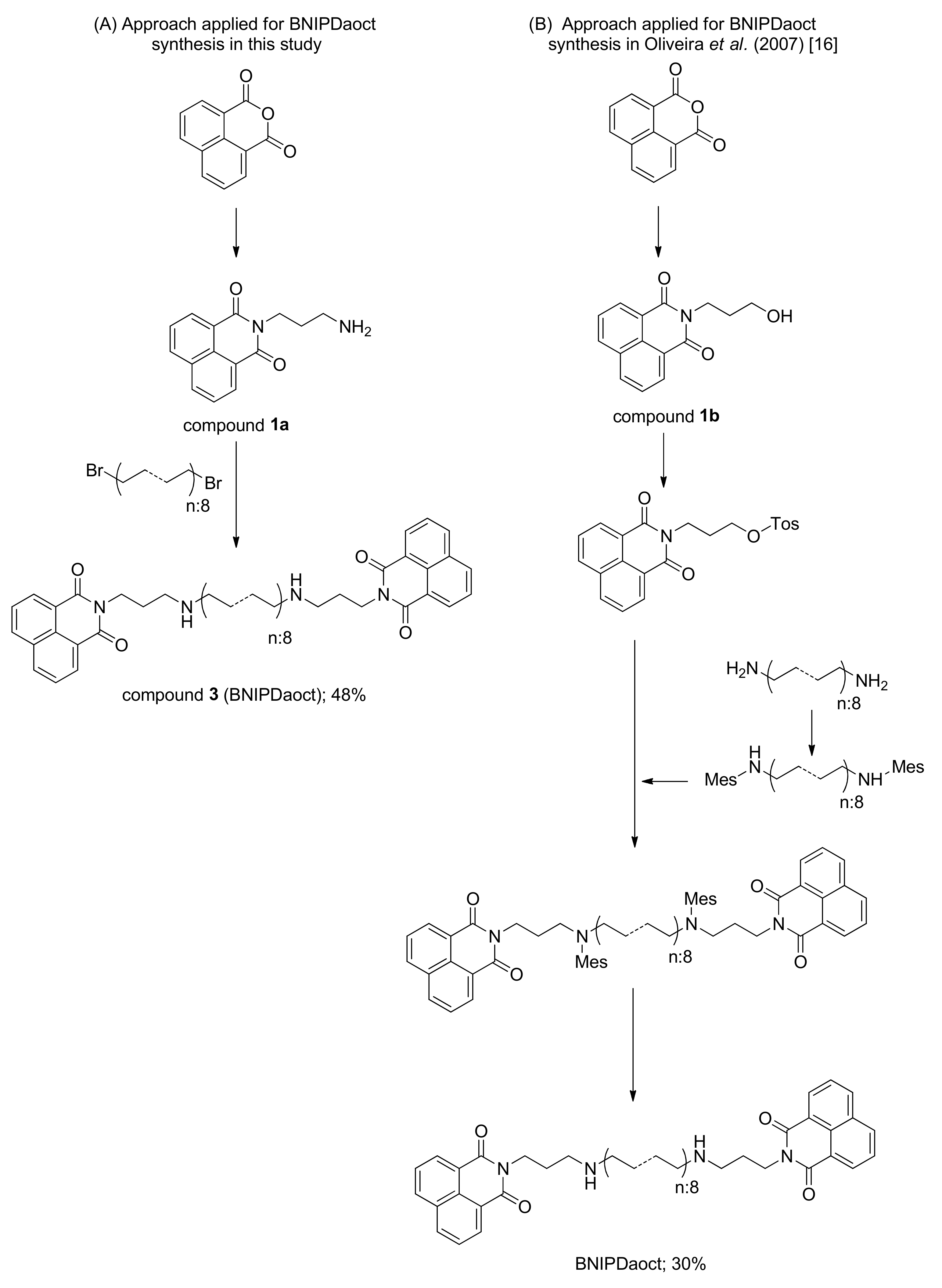
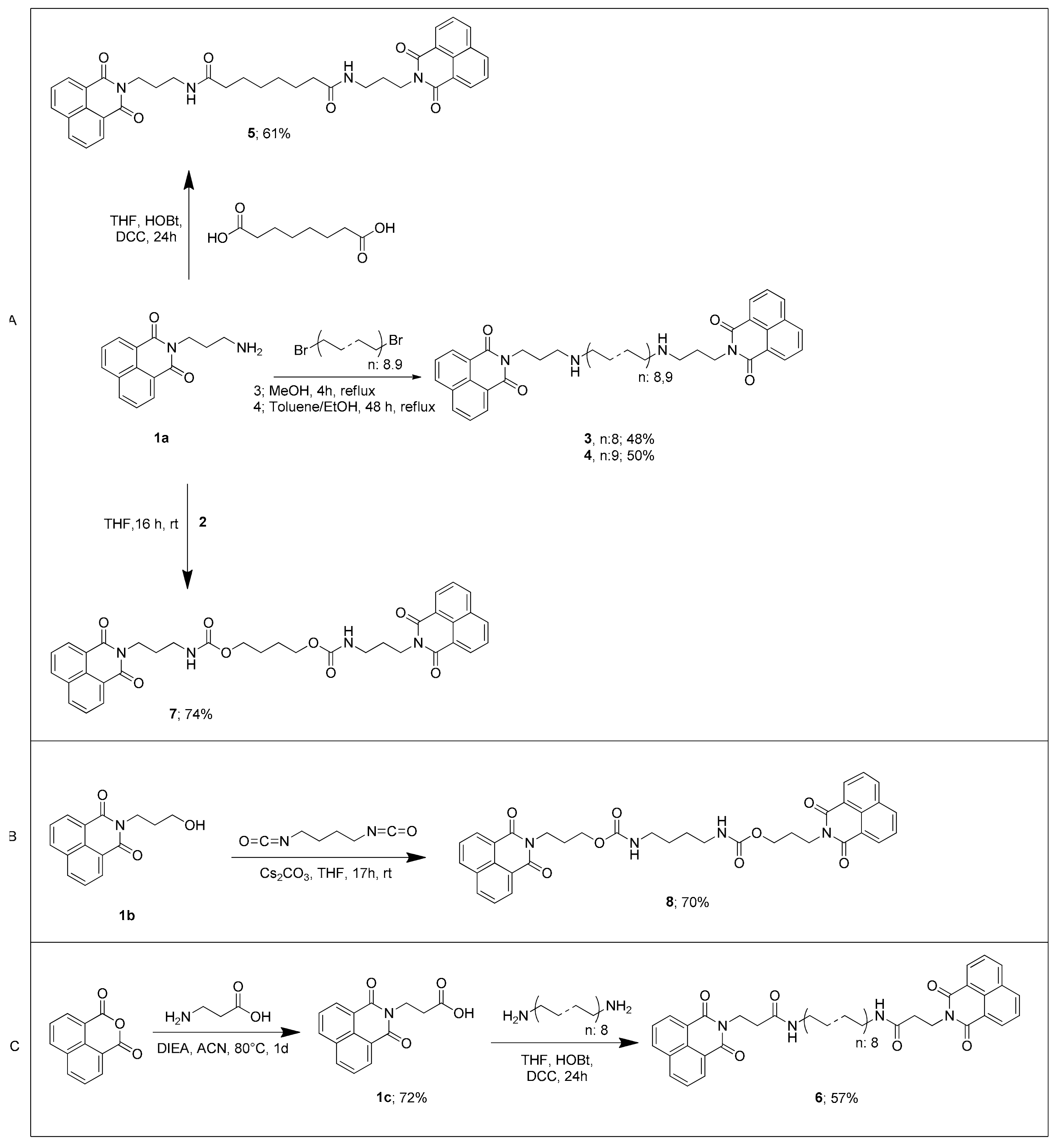
2.1. Chemistry
2.2. Experimental
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gradoni, L.; López-Vélez, R.; Mokni, M. Manual on Case Management and Surveillance of the Leishmaniases in the WHO European Region; World Health Organization: Copenhagen, Denmark, 2017. [Google Scholar]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.A.; Nguewa, P.A.; Castilla, J.; Alonso, C.; Pérez, J.M. Anticancer compounds as leishmanicidal drugs: Challenges in chemotherapy and future perspectives. Curr. Med. Chem. 2008, 15, 433–439. [Google Scholar] [PubMed]
- Tavares, J.; Ouaissi, A.; Lin, P.K.T.; Tomás, A.; Cordeiro-Da-Silva, A. Differential effects of polyamine derivative compounds against Leishmania infantum promastigotes and axenic amastigotes. Int. J. Parasitol. 2005, 35, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Muthuswami, R.; Madhubala, R. The mitochondrial SIR2 related protein 2 (SIR2RP2) impacts Leishmania donovani growth and infectivity. PLoS Negl. Trop. Dis. 2017, 11, e0005590. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Chen, D.; Yan, L.; Zheng, W. Sirtuin Inhibition: Strategies, Inhibitors, and Therapeutic Potential. Trends Pharmacol. Sci. 2017, 38, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Religa, A.A.; Waters, A.P. Sirtuins of parasitic protozoa: In search of function(s). Mol. Biochem. Parasitol. 2012, 185, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cui, C.H.; Bose, S.; Guo, D.; Shen, C.; Wong, W.P.; Halvorsen, K.; Farokhzad, O.C.; Teo, G.S.L.; Phillips, J.A.; et al. Bioinspired multivalent DNA network for capture and release of cells. Proc. Natl. Acad Sci. USA 2012, 109, 19626–19631. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Ouaissi, A.; Kong Thoo Lin, P.; Loureiro, I.; Kaur, S.; Roy, N.; Cordeiro-da-Silva, A. Bisnaphthalimidopropyl derivatives as inhibitors of Leishmania SIR2 related protein 1. ChemMedChem Chem. Enabling Drug Discov. 2010, 5, 140–147. [Google Scholar]
- Schuetz, A.; Min, J.; Antoshenko, T.; Wang, C.L.; Allali-Hassani, A.; Dong, A.; Loppnau, P.; Vedadi, M.; Bochkarev, A.; Sternglanz, R.; et al. Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure 2007, 15, 377–389. [Google Scholar] [CrossRef]
- Zheng, W. Sirtuins as emerging anti-parasitic targets. Eur. J. Med. Chem. 2013, 59, 132–140. [Google Scholar] [CrossRef]
- Sereno, D.; Vergnes, B.; Mathieu-Daude, F.; Cordeiro Da Silva, A.; Ouaissi, A. Looking for putative functions of the Leishmania cytosolic SIR2 deacetylase. Parasitol. Res. 2006, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fessel, M.R.; Lira, C.B.; Giorgio, S.; Ramos, C.H.I.; Cano, M.I.N. Sir2-Related Protein 1 from Leishmania amazonensis is a glycosylated NAD+-dependent deacetylase. Parasitology 2011, 138, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, H.; Yagita, K. Silent information regulator 2 proteins encoded by Cryptosporidium parasites. Parasitol. Res. 2010, 107, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, B.; Sereno, D.; Madjidian-Sereno, N.; Lemesre, J.-L.; Ouaissi, A. Cytoplasmic SIR2 homologue overexpression promotes survival of Leishmania parasites by preventing programmed cell death. Gene 2002, 296, 139–150. [Google Scholar] [CrossRef]
- Oliveira, J.; Ralton, L.; Tavares, J.; Codeiro-da-Silva, A.; Bestwick, C.S.; McPherson, A.; Lin, P.K.T. The synthesis and the in vitro cytotoxicity studies of bisnaphthalimidopropyl polyamine derivatives against colon cancer cells and parasite Leishmania infantum. Bioorg. Med. Chem. 2007, 15, 541–545. [Google Scholar] [CrossRef]
- Tavares, J.; Ouaissi, A.; Silva, A.M.; Kong Thoo Lin, P.; Roy, N.; Cordeiro-da-Silva, A. Anti-leishmanial activity of the bisnaphthalimidopropyl derivatives. Parasitol. Int. 2012, 61, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Ouaissi, A.; Santarém, N.; Sereno, D.; Vergnes, B.; Sampaio, P.; Cordeiro-Da-Silva, A. The Leishmania infantum cytosolic SIR2-related protein 1 (LiSIR2RP1) is an NAD+-dependent deacetylase and ADP-ribosyltransferase. Biochem. J. 2008, 415, 377–386. [Google Scholar] [CrossRef]
- Ronin, C.; Costa, D.M.; Tavares, J.; Faria, J.; Ciesielski, F.; Ciapetti, P.; Smith, T.K.; MacDougall, J.; Cordeiro-da-Silva, A.; Pemberton, I.K. The crystal structure of the Leishmania infantum Silent Information Regulator 2 related protein 1: Implications to protein function and drug design. PLoS ONE 2018, 13, e0193602. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Tavares, J.A.P.D.C.; Kong Thoo Lin, P. Bisnaphthalimidopropyl Derivative Compounds With Anti-Parasite and Anti-Cancer Activity. U.S. Patent No. 8,350,036, 8 June 2013. [Google Scholar]
- Kamal, A.; Bolla, N.R.; Srikanth, P.S.; Srivastava, A.K. Naphthalimide derivatives with therapeutic characteristics: A patent review. Expert Opin. Ther. Pat. 2013, 23, 299–317. [Google Scholar] [CrossRef]
- Kopsida, M.; Barron, G.A.; Bermano, G.; Kong Thoo Lin, P.; Goua, M. Novel bisnaphthalimidopropyl (BNIPs) derivatives as anticancer compounds targeting DNA in human breast cancer cells. Org. Biomol. Chem. 2016, 14, 9780–9789. [Google Scholar] [CrossRef]
- Barron, G.A.; Bermano, G.; Gordon, A.; Kong Thoo Lin, P. Synthesis, cytotoxicity and DNA-binding of novel bisnaphthalimidopropyl derivatives in breast cancer MDA-MB-231 cells. Eur. J. Med. Chem. 2010, 45, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Raquel, T.L.; Gemma, A.B.; Joanna, A.G.; Giovanna, B.; Simranjeet, K.; Nilanjan, R.; Helena Vasconcelos, M.P.K.L. Cytotoxicity and cell death mechanisms induced by a novel bisnaphthalimidopropyl derivative against the NCI-H460 non-small lung cancer cell line. Anticancer Agents Med. Chem. 2013, 3, 414–421. [Google Scholar]
- Bestwick, C.S.; Ralton, L.D.; Milne, L.; Kong Thoo Lin, P.; Duthie, S.J. The influence of bisnaphthalimidopropyl polyamines on DNA instability and repair in Caco-2 colon epithelial cells. Cell Biol Toxicol. 2011, 27, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Dance, A.M.; Ralton, L.; Fuller, Z.; Milne, L.; Duthie, S.; Bestwick, C.S.; Lin, P.K.T. Synthesis and biological activities of bisnaphthalimido polyamines derivatives: Cytotoxicity, DNA binding, DNA damage and drug localization in breast cancer MCF 7 cells. Biochem. Pharmacol. 2005, 69, 19–27. [Google Scholar] [CrossRef]
- Ralton, L.D.; Bestwick, C.S.; Milne, L.; Duthie, S.; Kong Thoo Lin, P. Bisnaphthalimidopropyl spermidine induces apoptosis within colon carcinoma cells. Chem. Biol. Interact. 2009, 177, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brana, M.F.; Cacho, M.; Gradillas, A.; Pascual-Teresa, B.; de Ramos, A. Intercalators as anticancer drugs. Curr. Pharm. Des. 2001, 7, 1745–1780. [Google Scholar] [CrossRef]
- Braña, M.F.; Ramos, A. Naphthalimides as Anticancer Agents: Synthesis and Biological Activity. Curr. Med. Chem. Agents 2001, 1, 237–255. [Google Scholar] [CrossRef]
- Filosa, R.; Peduto, A.; Di Micco, S.; de Caprariis, P.; Festa, M.; Petrella, A.; Capranico, G.; Bifulco, G. Molecular modelling studies, synthesis and biological activity of a series of novel bisnaphthalimides and their development as new DNA topoisomerase II inhibitors. Bioorg. Med. Chem. 2009, 17, 13–24. [Google Scholar] [CrossRef]
- Zhu, H.; Miao, Z.H.; Huang, M.; Feng, J.M.; Zhang, Z.X.; Lu, J.J.; Cai, Y.J.; Tong, L.J.; Xu, Y.F.; Qian, X.H.; et al. Naphthalimides Induce G2 Arrest Through the ATM-Activated Chk2-Executed Pathway in HCT116 Cells. Neoplasia 2009, 11, 1226–1234. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, X.; Zhang, H.; Xie, H.; Liu, J.; Xu, Y.; Zhu, W.; Wang, Y.; Wang, X.; Tan, S.; et al. A new class of naphthalimide-based antitumor agents that inhibit topoisomerase II and induce lysosomal membrane permeabilization and apoptosis. J. Med. Chem. 2010, 53, 2589–2600. [Google Scholar] [CrossRef]
- Bestwick, C.S.; Milne, L.; Dance, A.M.; Cochennec, G.; Cruickshank, G.; Allain, E.; Constable, L.; Duthie, S.J.; Lin, P.K.T. Caspase-independence and characterization of bisnaphthalimidopropyl spermidine induced cytotoxicity in HL60 cells. Toxicol. Vitr. 2018, 52, 342–350. [Google Scholar] [CrossRef]
- Kong Thoo Lin, P.; Dance, A.M.; Bestwick, C.; Milne, L. The biological activities of new polyamine derivatives as potential therapeutic agents. Biochem. Soc. Trans. 2003, 31, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Noro, J.; Maciel, J.; Duarte, D.; Olival, A.D.; Baptista, C.; Silva, A.C.D.; Alves, M.J.; Kong Thoo Lin, P. Evaluation of New Naphthalimides as Potential Anticancer Agents Against Breast Cancer MCF-7, Pancreatic Cancer BxPC-3 and Colon Cancer HCT-15 Cell Lines. 2015. Available online: www.researchgate.net/publication/284216907 (accessed on 15 August 2019).
- Brana, M.F.; Castellano, J.M.; Moran, M.; de Vega Pérez, M.J.; Romerdahl, C.R.; Qian, X.D.; Bousquet, P.; Emling, F.; Schlick, E.; Keilhauer, G. Bis-naphthalimides: A new class of antitumor agents. Anticancer Drug Des. 1993, 8, 257–268. [Google Scholar] [PubMed]
- Kong Thoo Lin, P.; Pavlov, V.A. The synthesis and in vitro cytotoxic studies of novel bis-naphthalimidopropyl polyamine derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1609–1612. [Google Scholar]
- Braña, M.F.; Cacho, M.; García, M.A.; de Pascual-Teresa, B.; Ramos, A.; Acero, N.; Llinares, F.; Muñoz-Mingarro, D.; Abradelo, C.; Rey-Stolle, M.F.; et al. Synthesis, antitumor activity, molecular modeling, and DNA binding properties of a new series of imidazonaphthalimides. J. Med. Chem. 2002, 45, 5813–5816. [Google Scholar] [CrossRef]
- Reger, D.L.; Debreczeni, A.; Horger, J.J.; Smith, M.D. Structures of bifunctional molecules containing two very different supramolecular synthons: Carboxylic acid and strong π⋯π stacking 1,8-naphthalimide ring. Cryst. Growth Des. 2011, 11, 4068–4079. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, X.; Wang, W.; Zhao, M.; Zheng, M.; Chang, H.W.; Wu, J.; Peng, S. A class of novel N-(3S-1, 2, 3, 4-tetrahydroisoquinoline-3-carbonyl)-L-amino acid derivatives: Their synthesis, anti-thrombotic activity evaluation, and 3D QSAR analysis. Eur. J. Med. Chem. 2009, 44, 4904–4919. [Google Scholar] [CrossRef]
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound 3 (BNIPDaoct) | 163.7 | 131.3 | 134.4 | 130.7 | 127.7 | 122.1 | 127.4 | 46.7 | 28.2 | 44.8 | 37.1 | 25.8 | 25.4 | 24.5 | - | ppm |
| Compound 4 (BNIPDanon) | 163.5 | 131.2 | 134.3 | 130.7 | 127.2 | 122.3 | 127.3 | 48.6 | 28.7 | 46.5 | 37.9 | 27.1 | 26.5 | 23.2 | 22.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keskin, E.; Ucisik, M.H.; Sucu, B.O.; Guzel, M. Novel Synthetic Approaches for Bisnaphthalimidopropyl (BNIP) Derivatives as Potential Anti-Parasitic Agents for the Treatment of Leishmaniasis. Molecules 2019, 24, 4607. https://doi.org/10.3390/molecules24244607
Keskin E, Ucisik MH, Sucu BO, Guzel M. Novel Synthetic Approaches for Bisnaphthalimidopropyl (BNIP) Derivatives as Potential Anti-Parasitic Agents for the Treatment of Leishmaniasis. Molecules. 2019; 24(24):4607. https://doi.org/10.3390/molecules24244607
Chicago/Turabian StyleKeskin, Elif, Mehmet Hikmet Ucisik, Bilgesu Onur Sucu, and Mustafa Guzel. 2019. "Novel Synthetic Approaches for Bisnaphthalimidopropyl (BNIP) Derivatives as Potential Anti-Parasitic Agents for the Treatment of Leishmaniasis" Molecules 24, no. 24: 4607. https://doi.org/10.3390/molecules24244607
APA StyleKeskin, E., Ucisik, M. H., Sucu, B. O., & Guzel, M. (2019). Novel Synthetic Approaches for Bisnaphthalimidopropyl (BNIP) Derivatives as Potential Anti-Parasitic Agents for the Treatment of Leishmaniasis. Molecules, 24(24), 4607. https://doi.org/10.3390/molecules24244607