Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging
Abstract
:1. Introduction
2. Alkali Metal Ions
3. Alkaline Earth Metal Ions
4. Transition Metal Ions
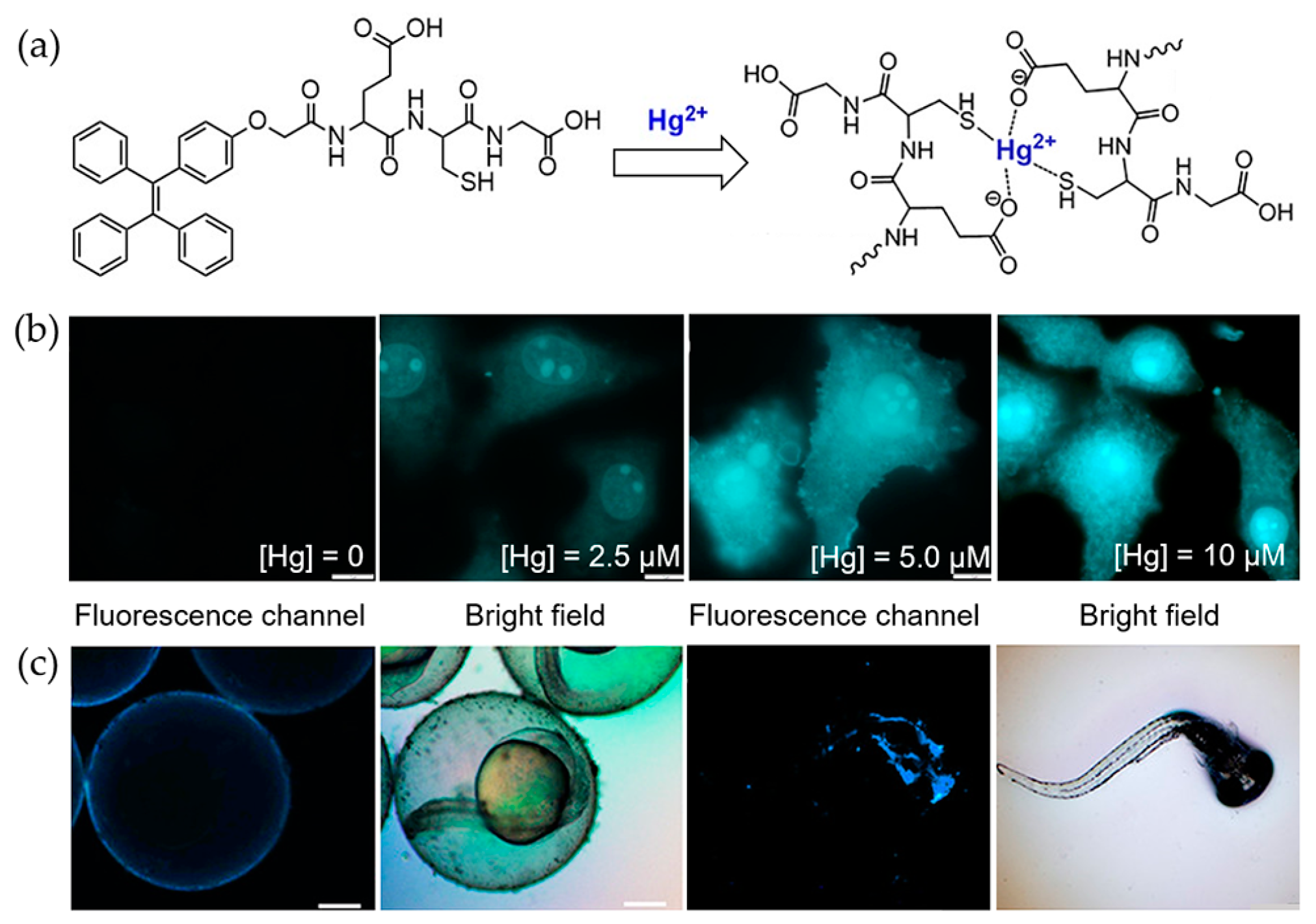
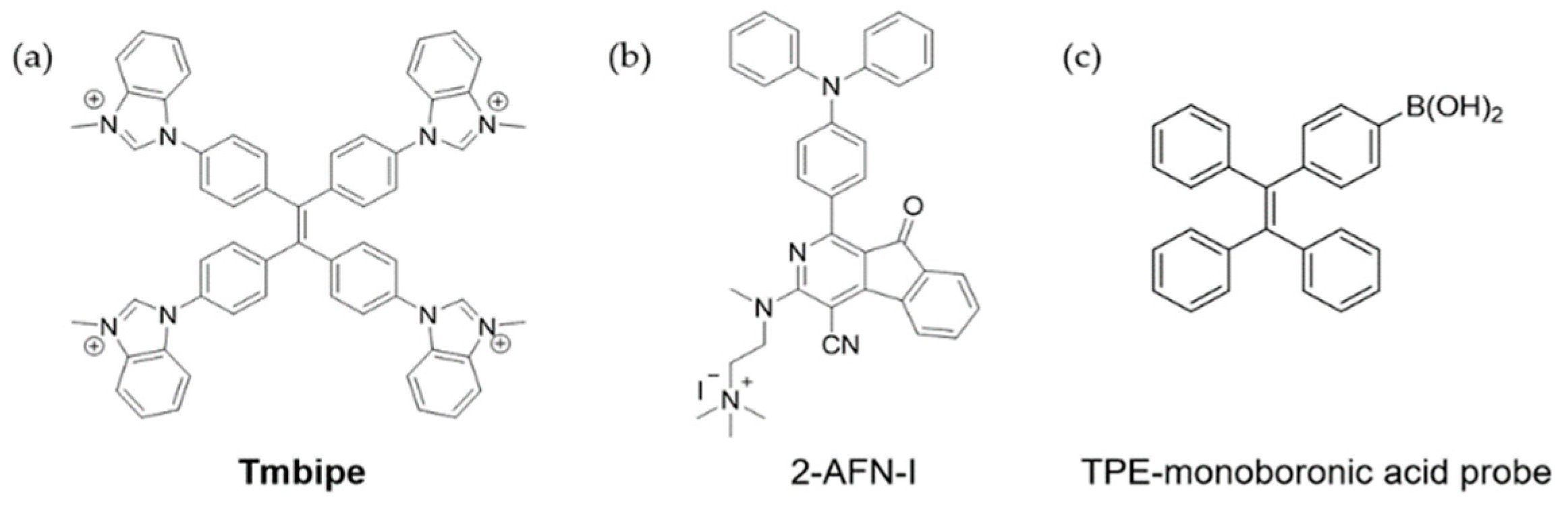
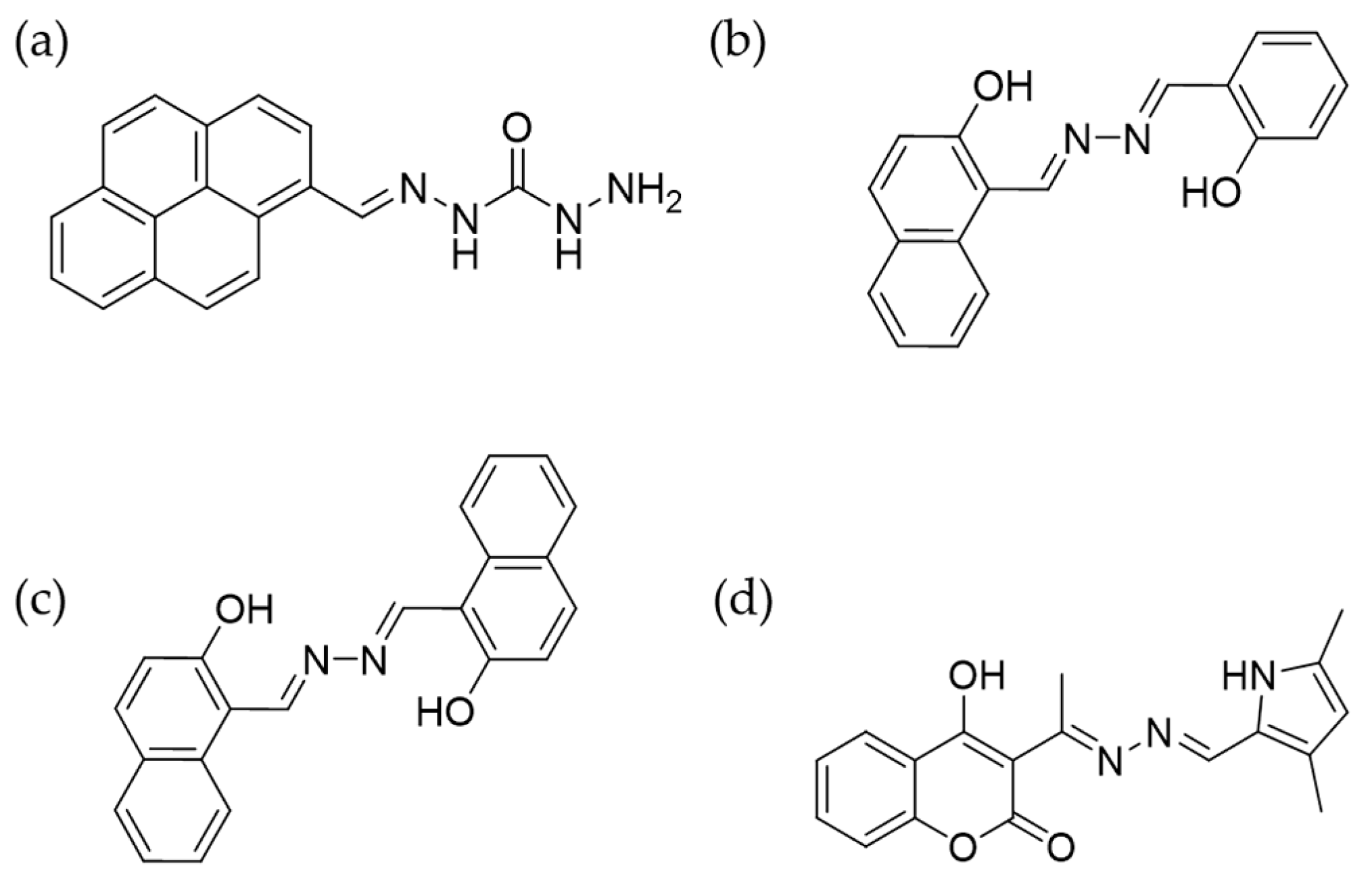
4.1. Mercury Ions
4.2. Cu2+
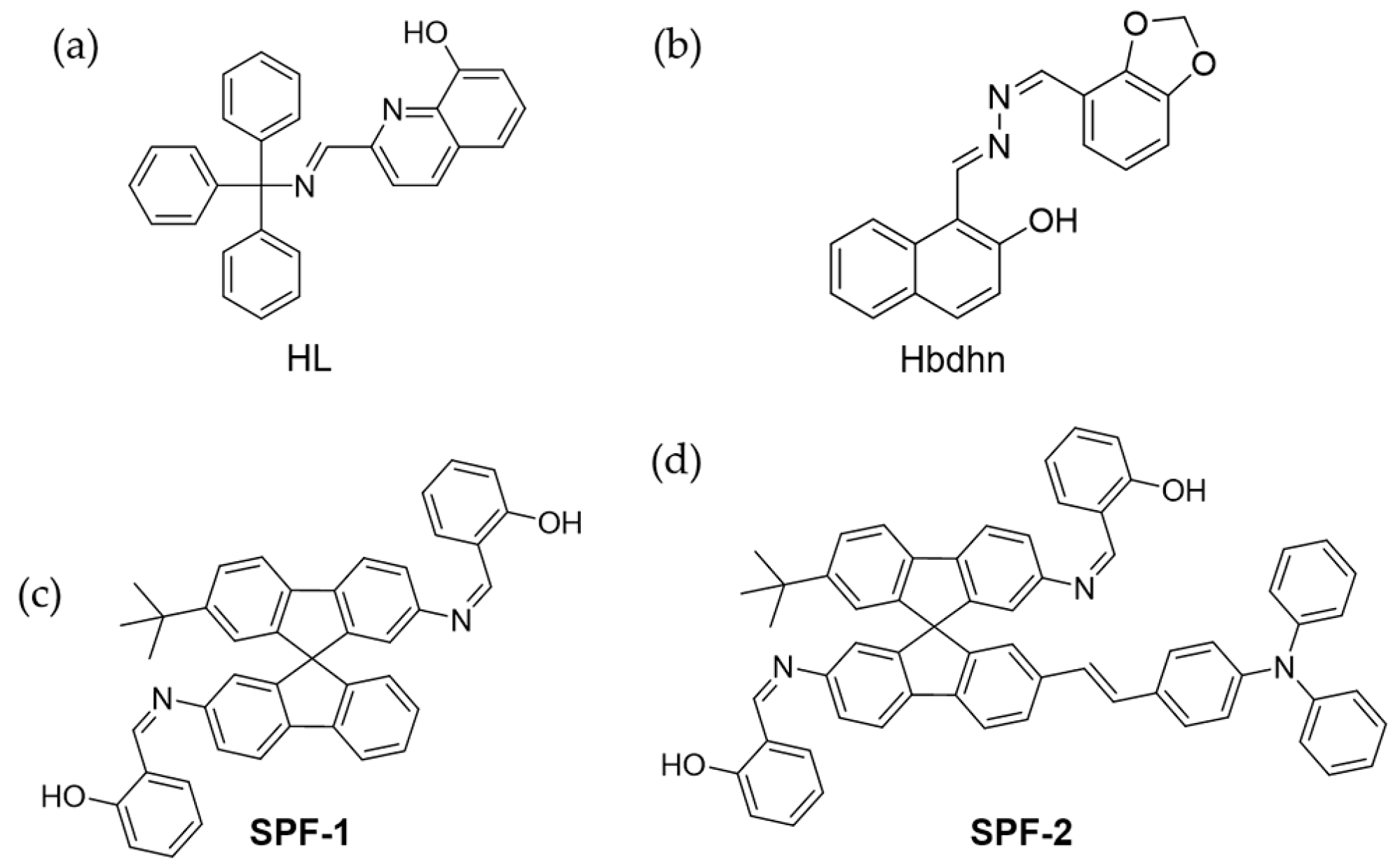
4.3. Zn2+
4.4. Fe3+
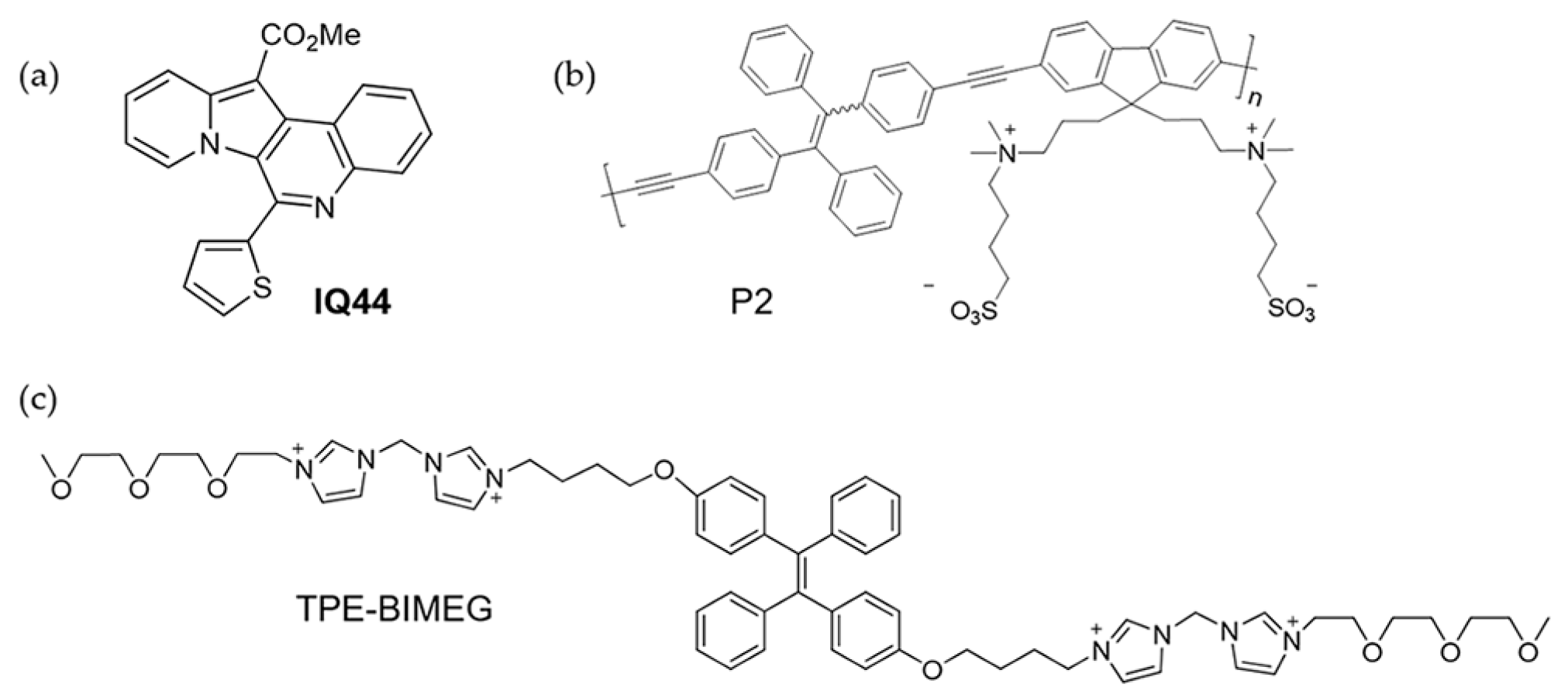
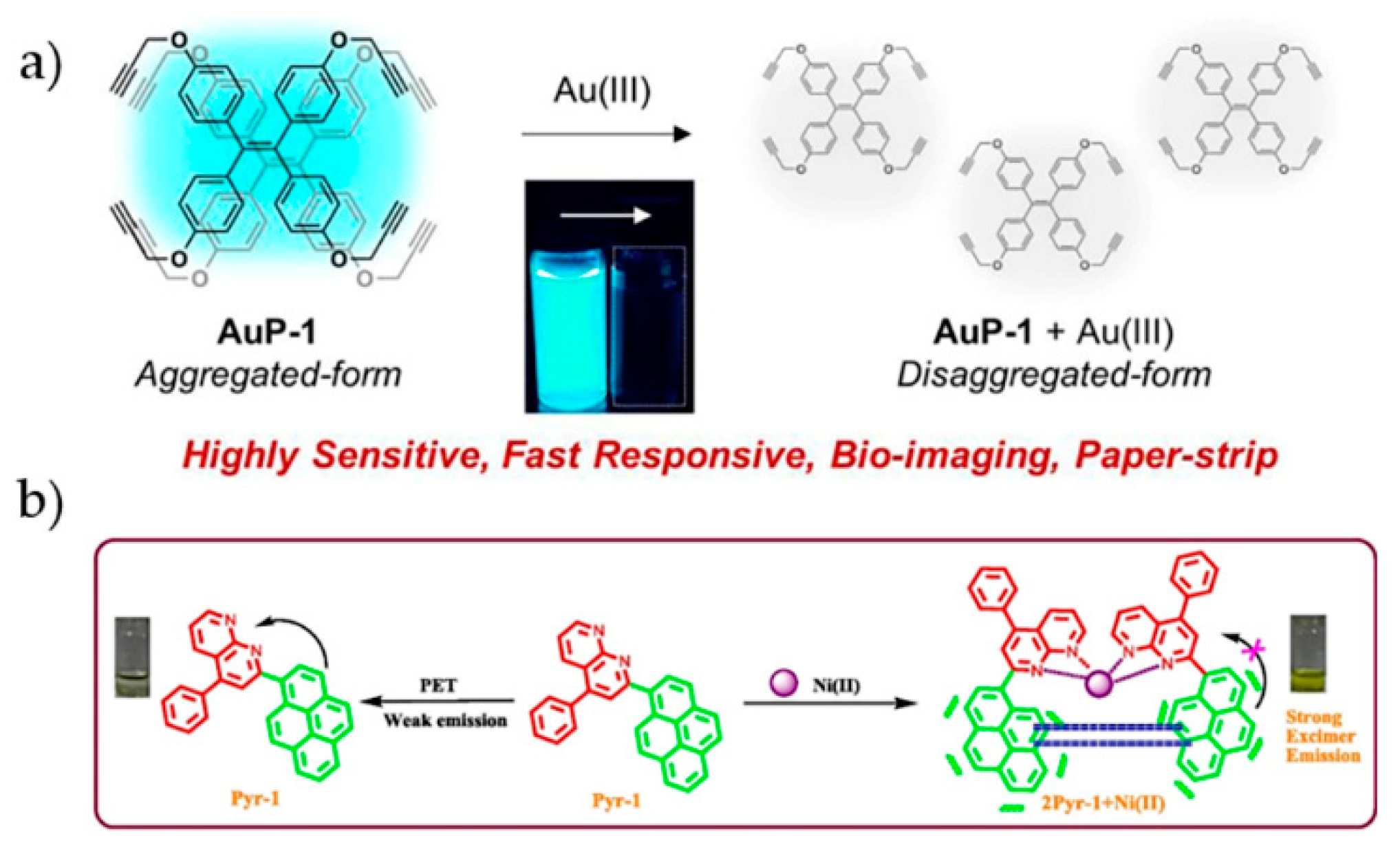
4.5. Other Transition Metal Ions
5. Other Metal Ions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.F.; Connors, B.; Paradiso, M. Neuroscience: Exploring the Brain, 3rd ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2015. [Google Scholar]
- Bischof, H.; Burgstaller, S.; Waldeck-Weiermair, M.; Rauter, T.; Schinagl, M.; Ramadani-Muja, J.; Graier, W.F.; Malli, R. Live-cell imaging of physiologically relevant metal ions using genetically encoded FRET-based probes. Cells 2019, 8, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; Worth Publishers: New York, NY, USA, 2012. [Google Scholar]
- Weaver, R.F. Molecular Biology, 5th ed.; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Rauk, A. The chemistry of Alzheimer’s disease. Chem. Soc. Rev. 2009, 38, 2698–2715. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.H.; Xu, Z.C. Fluorescence imaging of metal ions implicated in diseases. Chem. Soc. Rev. 2015, 44, 4487–4493. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Parker, D.R.; Clarke, J.M. Metals and micronutrients-food safety issues. Field Crop. Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.Q.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, H.M. Design principles of spectroscopic probes for biological applications. Chem. Sci. 2016, 7, 6309–6315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antina, E.V.; Bumagina, N.A.; V’yugin, A.I.; Solomonov, A.V. Fluorescent indicators of metal ions based on dipyrromethene platform. Dye. Pigment. 2017, 136, 368–381. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 12, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.E.; Roy, B.; Ahn, K.H. “Turn-on” fluorescent sensing with “reactive” probes. Chem. Commun. 2011, 47, 7583–7601. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.; Spence, M.T.Z. The Molecular Probes Handbook, 11th ed.; Invitrogen Corp.: Carlsbad, CA, USA, 2010. [Google Scholar]
- Gao, M.; Tang, B.Z. Fluorescent sensors based on aggregation-induced emission: Recent advances and perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 21, 1740–1741. [Google Scholar] [CrossRef]
- Niu, G.L.; Zhang, R.Y.; Gu, Y.; Wang, J.G.; Ma, C.; Kwok, R.T.K.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Wong, K.S.; et al. Highly photostable two-photo NIR AIEgens with tunable organelle specificity and deep tissue penetration. Biomaterials 2019, 208, 72–82. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Chen, J.W.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.P.; Lo, S.M.F.; Williams, I.D.; Zhu, D.B.; Tang, B.Z. Synthesis, light emission, nanoaggregation, and restricted intramolecular rotation of 1,1-substituted 2,3,4,5-tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Cai, Y.J.; Du, L.L.; Samedov, K.; Gu, X.G.; Qi, F.; Sung, H.H.Y.; Patrick, B.O.; Yan, Z.P.; Jiang, X.F.; Zhang, H.K.; et al. Deciphering the working mechanism of aggregation-induced emission of tetraphenylethylene derivatives by ultrafast spectroscopy. Chem. Sci. 2018, 9, 4662–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, Y.; Huang, F.J.; Xu, Q.; Zhang, M.S.; Lou, X.D.; Xia, F. Facile, fast-responsive, and photostable imaging of telomerase activity in living cells with a fluorescence turn-on manner. Anal. Chem. 2016, 886, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Hu, F.; Zhao, R.; Zhang, G.X.; Yang, H.; Zhang, D.Q. Tetraphenylethylene conjugated with a specific peptide as a fluorescence turn-on bioprobe for the highly specific detection and tracing of tumor markers in live cancer cells. Anal. Chem. 2014, 86, 7987–7995. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Huang, J.; Li, J.J.; Yan, J.W.; Qin, J.G.; Li, Z. A graphene oxide-based AIE biosensor with high selectivity toward bovine serum albumin. Chem. Commun. 2011, 47, 12385–12387. [Google Scholar] [CrossRef] [Green Version]
- Shustova, N.B.; Ong, T.C.; Cozzolino, A.F.; Michaelis, V.K.; Griffin, R.G.; Dinca, M. Phenyl ring dynamics in a Tetraphenylethylene-bridged metal-organic framework: Implications for the mechanism of aggregation-induced emission. J. Am. Chem. Soc. 2012, 13436, 15061–15070. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.B.; Liu, J.Z.; Geng, J.L.; Tang, B.Z.; Liu, B. Specific detection of integrin αvβ3 by light-up bioprobe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 13423, 9569–9572. [Google Scholar] [CrossRef]
- Gu, X.G.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIEgens for biological process monitoring and disease theranostics. Biomaterials 2017, 146, 115–135. [Google Scholar] [CrossRef]
- Hu, F.; Huang, Y.Y.; Zhang, G.X.; Zhao, R.; Yang, H.; Zhang, D.Q. Targeted bioimaging and photodynamic therapy of cancer cells with an activatable red fluorescent bioprobe. Anal. Chem. 2014, 86, 7987–7995. [Google Scholar] [CrossRef]
- Xu, C.H.; Zou, H.; Zhao, Z.; Zhang, P.F.; Kwok, R.T.K.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. A new strategy toward “simple” water-soluble AIE probes for hypoxia detection. Adv. Funct. Mater. 2019, 29, 1903278. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Sung, S.H.P.; Feng, G.X.; Zhang, C.J.; Kenry; Tang, B.Z.; Liu, B. Aggregation-induced emission probe for specific turn-on quantification of soluble transferrin receptor: An important disease marker for iron deficiency anemia and kidney diseases. Anal. Chem. 2018, 90, 1154–1160. [Google Scholar] [CrossRef]
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; He, L.; Wang, Y.; Xiong, M.; Hu, M.; Liang, H.; Huan, S.; Zhang, X.B.; Tan, W. Tetraphenylethene derivative modified DNA oligonucleotide for in situ potassium ion detection and imaging in living cells. Talanta 2017, 167, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Li, M.; Liao, N.X.; Bi, A.Y.; Jiang, Y.P.; Liu, S.; Gong, Z.C.; Zeng, W.B. Fluorescent probes for the detection of magnesium ions (Mg2+): From design to application. RSC Adv. 2018, 8, 12573–12587. [Google Scholar] [CrossRef] [Green Version]
- Carroll, M.F.; Schade, D.S. A practical approach to hypercalcemia. Am. Fam. Physician. 2003, 67, 1959–1966. [Google Scholar]
- Zaheer, A.; Murshed, M.; De Grand, A.M.; Morgan, T.G.; Karsenty, G.; Frangioni, J.V. Optical imaging of hydroxyapatite in the calcified vasculature of transgenic animals. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1132–1136. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.J.; Wang, L.Q.; Cao, F.X.; Tang, L.J. A simple fluorescence probe based on aggregation-induced emission (AIE) property for the detection of Mg2+ ions. J. Fluoresc. 2015, 26, 53–57. [Google Scholar] [CrossRef]
- Ishiwari, F.; Hasebe, H.; Matsumura, S.; Hajjaj, F.; Horii-Hayashi, N.; Nishi, M.; Someya, T.; Fukushima, T. Bioinspired design of a polymer gel sensor for the realization of extracellular Ca2+ imaging. Sci. Rep. 2016, 6, 24275. [Google Scholar] [CrossRef]
- Morishima, K.; Ishiwari, F.; Matsumura, S.; Fukushima, T.; Shibayama, M. Mesoscopic structural aspects of Ca2+-triggered polymer chain folding of a tetraphenylethene-appended poly(acrylic acid) in relation to its aggregation-induced emission behavior. Macromolecules 2017, 50, 5940–5945. [Google Scholar] [CrossRef]
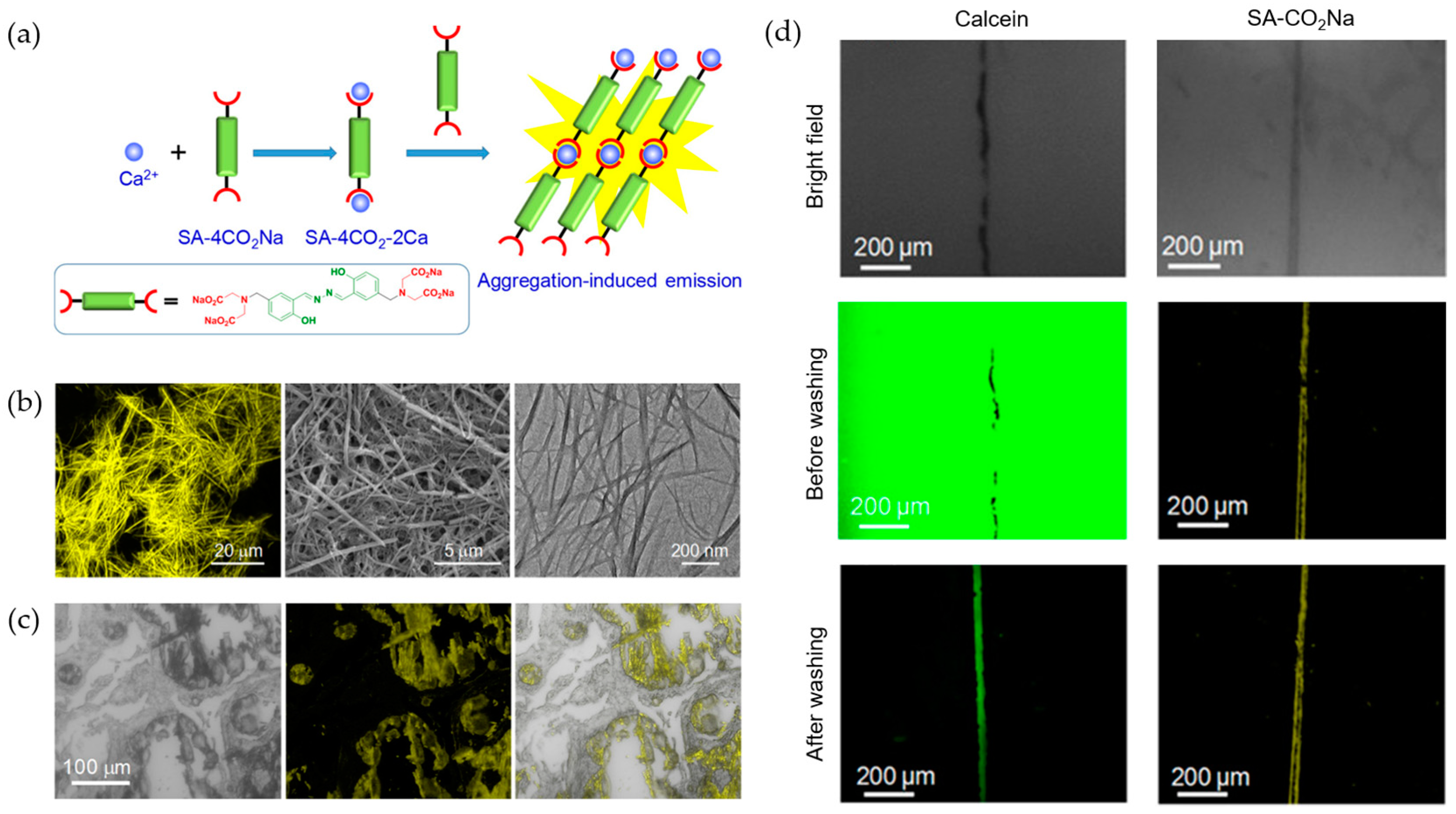
- Gao, M.; Li, Y.X.; Chen, X.H.; Li, S.W.; Ren, L.; Tang, B.Z. Aggregation-induced emission probe for light-up and in situ detection of calcium ions at high concentration. ACS Appl. Mater. Interfaces 2018, 10, 14410–14417. [Google Scholar] [CrossRef]
- Zhang, J.D.; Yan, Z.; Wang, S.; She, M.Y.; Zhang, Z.; Cai, W.Z.; Liu, P.; Li, J.L. Water soluble chemosensor for Ca2+, based on aggregation-induced emission characteristics and its fluorescence imaging in living cells. Dye. Pigment. 2017, 150, 112–120. [Google Scholar] [CrossRef]
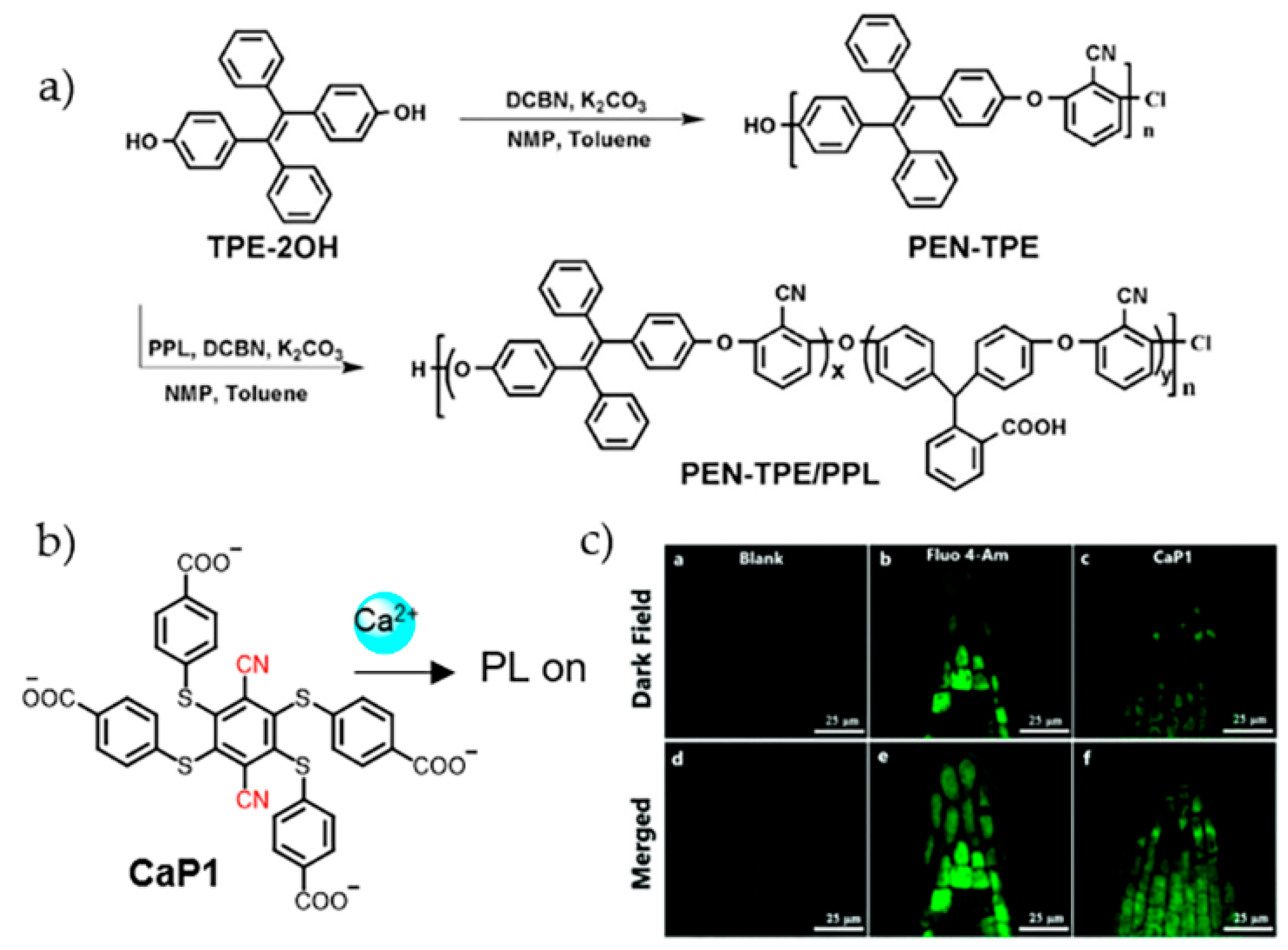
- Wang, P.; Jia, K.; Zhou, X.; Guan, X.; Wang, L.; Tian, Y.; Wu, C.; Liu, X. Ca2+ induced crosslinking of AIE-active polyarylene ether nitrile into fluorescent polymeric nanoparticles for cellular bioimaging. Macromol. Rapid Commun. 2017, 38, 1700360. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Zhou, Z.C.; Feng, H.; Zhang, C.Y.; Wang, Y.F.; Qian, Z.S.; Pan, J.W. An Aggregation-induced phosphorescence probe for calcium ion-specific detection and live-cell imaging in Arabidopsis Thaliana. Chem. Commun. 2019, 55, 4841–4844. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K. Flipping the light switch ‘ON’-the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2001, 57, 2161–2195. [Google Scholar] [CrossRef]
- Aron, A.T.; Ramos-Torres, K.M.; Cotruvo, J.A.; Chang, C.J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 2015, 48, 2434–2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, E.D.; Cohen, Y.; Winer, A.M. Environmental distribution and transformation of mercury compounds. Crit. Rev. Environ. Sci. Technol. 1996, 26, 1–43. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F. Health risk and significance of mercury in the environment. Environ. Sci. Pollut. Res. 2015, 22, 192–201. [Google Scholar] [CrossRef]
- Wang, K.; Li, J.J.; Ji, S.M.; Li, L.J.; Qiu, Z.P.; Pan, C.Q.; Zhang, J.Y.; Huo, Y.P. Fluorescence probes based on AIE luminogen: Application for sensing Hg2+ in aqueous media and cellular imaging. New J. Chem. 2018, 42, 13836–13846. [Google Scholar] [CrossRef]
- Zhang, G.B.; Ding, A.X.; Zhang, Y.; Yang, L.M.; Kong, L.; Zhang, X.J.; Tao, X.T.; Tian, Y.P.; Yang, J.X. Schiff base modified α-cyanostilbene derivative with aggregation-induced emission enhancement characteristics for Hg2+ detection. Sens. Actuator B-Chem. 2014, 202, 209–216. [Google Scholar] [CrossRef]
- Zhang, G.B.; Zhang, X.J.; Zhang, Y.; Wang, H.; Kong, L.; Tian, Y.P.; Tao, X.T.; Hong, B.; Yang, J.X. Design of turn-on fluorescent probe for effective detection of Hg2+ by combination of AIEE-active fluorophore and binding site. Sens. Actuator B-Chem. 2015, 221, 730–739. [Google Scholar] [CrossRef]
- Fang, W.Y.; Zhang, G.B.; Chen, J.; Kong, L.; Yang, L.M.; Bi, H.; Yang, J.X. An AIE active probe for specific sensing of Hg2+ based on linear conjugated bis-Schiff base. Sens. Actuator B-Chem. 2016, 229, 338–346. [Google Scholar] [CrossRef]
- Gui, S.L.; Huang, Y.Y.; Hu, F.; Jin, Y.L.; Zhang, G.X.; Zhang, D.Q.; Zhao, R. Bioinspired peptide for imaging Hg2+ distribution in living cells and Zebrafish based on coordination-mediated supramolecular assembling. Anal. Chem. 2018, 90, 9708–9715. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, D.X.; Zhu, L.N.; Lan, Y.L.; Cheng, M.; Zhang, L.M.; Chu, J.Q.; Li, X.Z.; Kong, D.M. Dinuclear HgII tetracarbene complex-triggered aggregation-induced emission for rapid and selective sensing of Hg2+ and organomercury species. Chem. Sci. 2019, 10, 4220–4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.T.; Li, S.W.; Ling, X.; Zhang, J.; Qin, A.J.; Zhuang, J.; Gao, M.; Tang, B.Z. Dual detection of bioaccumulated Hg2+ based on luminescent bacteria and aggregation-induced emission. Chem. Commun. 2019, 55, 7458–7461. [Google Scholar] [CrossRef] [PubMed]
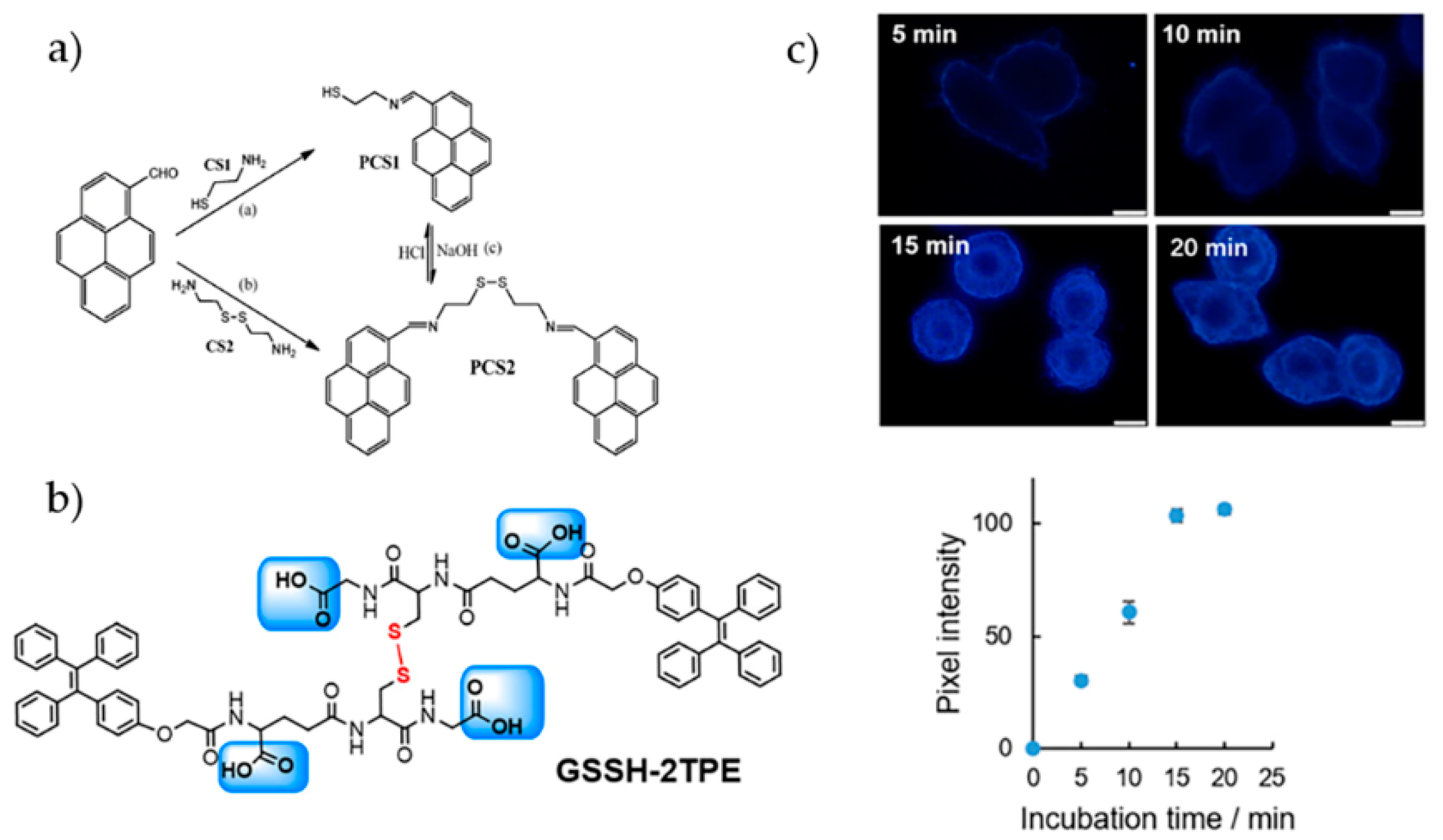
- Wu, Y.Q.; Wen, X.Y.; Fan, Z.F. An AIE active pyrene based fluorescent probe for selective sensing Hg2+ and imaging in live cells. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2019, 223, 117315. [Google Scholar] [CrossRef] [PubMed]
- Bahta, M.; Ahmed, N. Naphthalimide-amino acid conjugates chemosensors for Hg2+ detection: Based on chelation mediated emission enhancement in aqueous solution. J. Photochem. Photobiol. A-Chem. 2019, 378, 85–93. [Google Scholar] [CrossRef]
- Gabr, M.T.; Pigge, F.C. A turn-on AIE active fluorescent sensor for Hg2+ by combination of 1,1-bis(2-pyridyl)ethylene and thiophene/bithiophene fragments. Mater. Chem. Front. 2017, 1, 1654–1661. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, Q.Y.; Zheng, G.S.; Yang, L.; Zhang, J.; Wang, Y.F.; Zhang, H.T.; He, J.; Sun, H.Y.; Ho, D. An ultra-sensitive and ratiometric fluorescent probe based on the DTBET process for Hg2+ detection and imaging applications. Analyst 2019, 144, 1353–1360. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Molecular flexibility tuned emission in “V” shaped naphthalimides: Hg(II) detection and aggregation induced emission enhancement (AIEE). Chem. Commun. 2013, 49, 7292–7294. [Google Scholar] [CrossRef]
- Wang, A.Z.; Yang, Y.X.; Yu, F.F.; Xue, L.W.; Hu, B.W.; Fan, W.P.; Dong, Y.J. A highly selective and sensitive fluorescent probe for quantitative detection of Hg2+ based on aggregation-induced emission features. Talanta 2015, 132, 864–870. [Google Scholar] [CrossRef]
- Chatterjee, A.; Banerjee, M.; Khandare, D.G.; Gawas, R.U.; Mascarenhas, S.C.; Ganguly, A.; Gupta, R.; Joshi, H. Aggregation-induced emission-based chemodosimeter approach for selective sensing and imaging of Hg(II) and methylmercury species. Anal. Chem. 2017, 89, 12698–12704. [Google Scholar] [CrossRef]
- Gao, T.; Huang, X.Y.; Huang, S.; Dong, J.; Yuan, K.; Feng, X.P.; Liu, T.T.; Yu, K.Q.; Zeng, W.B. Sensitive water-soluble fluorescent probe based on umpolung and aggregation-induced emission strategies for selective detection of Hg2+ in living cells and Zebrafish. J. Agric. Food Chem. 2019, 67, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, T.; Bi, A.Y.; Cao, X.Z.; Feng, B.; Liu, M.; Du, T.; Feng, X.P.; Zeng, W.B. Revealing aggregation-induced emission effect of imidazolium derivatives and application for detection of Hg2+. Dye Pigment. 2020, 172, 107830. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.J.; Yan, M.M.; Tu, Q.; Chen, S.W.; Li, T.B.; Yuan, M.S.; Wang, J.Y. 2-Hydroxy benzothiazole modified rhodol: Aggregation-induced emission and dual-channel fluorescence sensing of Hg2+ and Ag+ ions. Sens. Actuator B-Chem. 2018, 255, 2086–2094. [Google Scholar] [CrossRef]
- Niu, C.X.; Liu, Q.L.; Shang, Z.H.; Zhao, L.; Ouyang, J. Dual-emission fluorescent sensor based on AIE organic nanoparticles and Au nanoclusters for the detection of mercury and melamine. Nanoscale 2015, 7, 8457–8465. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Squitti, R. Copper imbalance in Alzheimer’s disease: Convergence of the chemistry and the clinic. Coord. Chem. Rev. 2019, 397, 168–187. [Google Scholar] [CrossRef]
- Dujols, V.; Ford, F.; Czarnik, A.W. A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J. Am. Chem. Soc. 1997, 119, 7386–7387. [Google Scholar] [CrossRef]
- Xu, J.; Hou, Y.; Ma, Q.; Wu, X.; Feng, S.; Zhang, J.; Shen, Y. A highly selective fluorescent probe for Cu2+ based on rhodamine B derivative. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2014, 124, 416–422. [Google Scholar] [CrossRef]
- Wu, W.N.; Mao, P.D.; Wang, Y.; Mao, X.J.; Xu, Z.Q.; Xu, Z.H.; Zhao, X.L.; Fan, Y.C.; Hou, X.F. AEE active Schiff base-bearing pyrene unit and further Cu2+-induced self-assembly process. Sens. Actuator B-Chem. 2018, 258, 393–401. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.; Shellaiah, M.; Prakash, E.C.; Chang, H.C.; Raghunath, P.; Lin, M.C.; Lin, H.C.; Liu, B.; Zhou, H.L.; et al. Aggregation-induced emission activity and further Cu2+-induced self-assembly process of two Schiff compounds. Sens. Actuator B-Chem. 2017, 246, 554–562. [Google Scholar]
- Wang, Y.; Wu, H.; Wu, W.N.; Li, S.J.; Xu, Z.H.; Xu, Z.Q.; Fan, Y.C.; Zhao, X.L.; Liu, B.Z. An AIRE active Schiff base bearing coumarin and pyrrole unit: Cu2+ detection in either solution or aggregation states. Sens. Actuator B-Chem. 2018, 260, 106–115. [Google Scholar] [CrossRef]
- Ding, A.X.; Shi, Y.D.; Zhang, K.X.; Sun, W.; Tan, Z.L.; Lu, Z.L.; He, L. Self-assembled aggregation-induced emission micelle (AIE micelle) as interfacial fluorescence probe for sequential recognition of Cu2+ and ATP in water. Sens. Actuator B-Chem. 2018, 255, 440–447. [Google Scholar] [CrossRef]
- Maret, W. Crosstalk of the group IIa and IIb metals calcium and zinc in cellular signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 12325–12327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Rizzarelli, E. Zn2+ interaction with Amyloid-B: Affinity and speciation. Molecules 2019, 24, 2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Li, S.M.; Zheng, J.Q.; Kong, D.Y.; Zheng, X.J.; Fang, D.C.; Jin, L.P. Coordination-directed stacking and aggregation-induced emission enhancement of the Zn(II) Schiff base complex. Inorg. Chem. 2017, 56, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.Y.; Wang, Q.; Fan, Z.F. An active fluorescent probe based on aggregation-induced emission for intracellular bioimaging of Zn2+ and tracking of interactions with single-stranded DNA. Anal. Chim. Acta 2018, 1013, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Y.; Zhang, W.; Guo, H.D.; Liang, J.J.; Huang, D.Y.; Xiao, H.B. Two spirobifluorene-based fluorescent probes with aggregation-induced emission properties: Synthesis and application in the detection of Zn2+ and cell imaging. J. Mater. Chem. C 2019, 7, 2240–2249. [Google Scholar] [CrossRef]
- He, X.X.; Wang, X.M.; Zhang, L.; Fang, G.Z.; Liu, J.F.; Wang, S. Sensing and intracellular imaging of Zn2+ based on affinity peptide using an aggregation induced emission fluorescence “switch-on” probe. Sens. Actuator B-Chem. 2018, 271, 289–299. [Google Scholar] [CrossRef]
- Naskar, B.; Dhara, A.; Maiti, D.K.; Kukulka, M.; Mitoraj, M.P.; Srebro-Hooper, M.; Prodhan, C.; Chaudhuri, K.; Goswami, S. Aggregation-induced emission-based sensing platform for selective detection of Zn2+: Experimental and theoretical investigations. Chem. Phys. Chem. 2019, 20, 1630–1639. [Google Scholar] [CrossRef]
- Wang, J.X.; Lin, X.F.; Shu, T.; Su, L.; Liang, F.; Zhang, X.J. Self-assembly of metal nanoclusters for aggregation-induced emission. Int. J. Mol. Sci. 2019, 20, 1891. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.M.; Cao, F.P.; Lei, X.L.; Mang, L.H.; Cheng, S.J.; Song, J.T. Fluorescent copper nanoparticles: Recent advances in synthesis and applications for sensing metal ions. Nanoscale 2016, 8, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Hu, Y.F.; Zhang, L.L.; Huang, Y.; Zhao, S.L. Photoluminescence light-up detection of zinc ion and imaging in living cells based on the aggregation induced emission enhancement of glutathione-capped copper nanoclusters. Biosens. Bioelectron. 2017, 94, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.F.; Li, J.; Wang, E.K. Cu nanoclusters with aggregation induced emission enhancement. Small 2013, 9, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Z.H.; Wan, Z.H.; Yang, T.Z.; Wang, H.; Mei, X.F. One-pot development of water-soluble copper nanoclusters with red emission and aggregation induced fluorescence enhancement. RSC Adv. 2016, 6, 34090–34095. [Google Scholar] [CrossRef]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Gozzelin, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.; Baek, B.; Jang, K.; Lee, N.K.; Lee, J.H.; Lee, Y.; Kim, J.; Kang, S.W.; Park, J.; Kim, S.; et al. Novel turn-on fluorescent biosensors for selective detection of cellular Fe3+ in lysosomes: Thiophene as a selectivity-tuning handle for Fe3+ sensors. Dye. Pigment. 2019, 169, 51–59. [Google Scholar] [CrossRef]
- Yang, X.D.; Chen, X.L.; Lu, X.D.; Yan, C.G.; Xu, Y.K.; Hang, X.D.; Qu, J.Q.; Liu, R.Y. A highly selective and sensitive fluorescent chemosensor for detection of CN-, SO32- and Fe3+ based on aggregation-induced emission. J. Mater. Chem. C 2016, 4, 383–390. [Google Scholar] [CrossRef]
- Yang, D.L.; Li, F.; Luo, Z.M.; Bao, B.Q.; Hu, Y.L.; Weng, L.X.; Cheng, Y.X.; Wang, L.H. Conjugated polymer nanoparticles with aggregation induced emission characteristics for intracellular Fe3+ sensing. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1686–1693. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.Y.; Cui, Q.L.; Cao, Q.; Li, L.D. Self-assembly of fluorescent organic nanoparticles for iron(III) sensing and cellular imaging. ACS Appl. Mater. Interfaces 2016, 8, 7440–7448. [Google Scholar] [CrossRef]
- Sinha, N.; Stegemann, L.; Tan, T.T.Y.; Doltsinis, N.L.; Strassert, C.A.; Hahn, F.E. Turn-on fluorescence in Tetra-NHC ligands by rigidification through metal complexation: An alternative to aggregation-induced emission. Angew. Chem. Int. Ed. 2017, 56, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Won, M.; Kim, J.S.; Huh, Y.; Kim, D. A highly sensitive and fast responsive fluorescent probe for detection of Gold(III) ions based on the AIEgen disaggregation. Dye Pigment 2019, 160, 647–653. [Google Scholar] [CrossRef]
- Khan, R.I.; Ramu, A.; Pitchumani, K. Design and one-pot synthesis of a novel pyrene based fluorescent sensor for selective “turn on”, naked eye detection of Ni2+ ions, and live cell imaging. Sens. Actuator B-Chem. 2018, 266, 429–437. [Google Scholar] [CrossRef]
- Gui, S.L.; Huang, Y.Y.; Hu, F.; Jin, Y.I.; Zhang, G.X.; Yan, L.S.; Zhang, D.Q.; Zhao, R. Fluorescence turn-on chemosensor for highly selective and sensitive detection and bioimaging of Al3+ in living cells based on ion-induced aggregation. Anal. Chem. 2015, 87, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.F.; Bao, Z.Y.; Yu, C.Y.; Qiu, Q.Q.; Wei, M.R.; Xi, W.B.; Qian, Z.S.; Feng, H. A water-soluble molecular probe with aggregation-induced emission for discriminative detection of Al3+ and Pb2+ and imaging in seedling root of Arabidopsis. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2019, 223, 117335. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, R.; Shellaiah, M.; Prakash, E.C.; Chang, H.C.; Raghunath, P.; Lin, M.C.; Lin, H.C. A new pyrene-based aggregation induced ratiometric emission probe for selective detections of trivalent metal ions and its living cell application. Sens. Actuator B-Chem. 2015, 207, 338–345. [Google Scholar] [CrossRef]
- Simon, T.; Shellaiah, M.; Srinivasadesikan, V.; Lin, C.C.; Ko, F.H.; Sun, K.W.; Lin, M.C. A simple pyrene based AIEE active schiff base probe for selective naked eye and fluorescence off-on detection of trivalent cations with live cell application. Sens. Actuator B-Chem. 2016, 231, 18–29. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Srinivasadesikan, V.; Lin, C.M.; Sun, K.W.; Ko, F.H.; Lin, M.C.; Lin, H.C. Novel pyrene containing monomeric and dimeric supramolecular AIEE active nano-probes utilized in selective ‘’off-on’’ trivalent metal and highly acidic pH sensing with live cell applications. J. Mater. Chem. C 2016, 4, 2056–2071. [Google Scholar] [CrossRef]
- Gui, S.L.; Huang, Y.Y.; Zhu, Y.Y.; Jin, Y.L.; Zhao, R. Biomimetic sensing system for tracing Pb2+ distribution in living cells based on the metal-peptide supramolecular assembly. ACS Appl. Mater. Interfaces 2019, 11, 5804–5811. [Google Scholar] [CrossRef]
- Chen, X.T.; Peng, L.; Feng, M.L.; Xiang, Y.; Tong, A.J.; He, L.F.; Liu, B.; Tang, Y.P. An aggregation induced emission enhancement-based ratiometric fluorescent sensor for detecting trace uranyl ion (UO22+) and the application in living cells imaging. J. Lumines. 2017, 186, 301–306. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules 2019, 24, 4593. https://doi.org/10.3390/molecules24244593
Li Y, Zhong H, Huang Y, Zhao R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules. 2019; 24(24):4593. https://doi.org/10.3390/molecules24244593
Chicago/Turabian StyleLi, Yongming, Huifei Zhong, Yanyan Huang, and Rui Zhao. 2019. "Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging" Molecules 24, no. 24: 4593. https://doi.org/10.3390/molecules24244593