Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Coronarin D
2.2. In Vitro Antiproliferative Activity Assay
2.3. Cell Cycle Assay
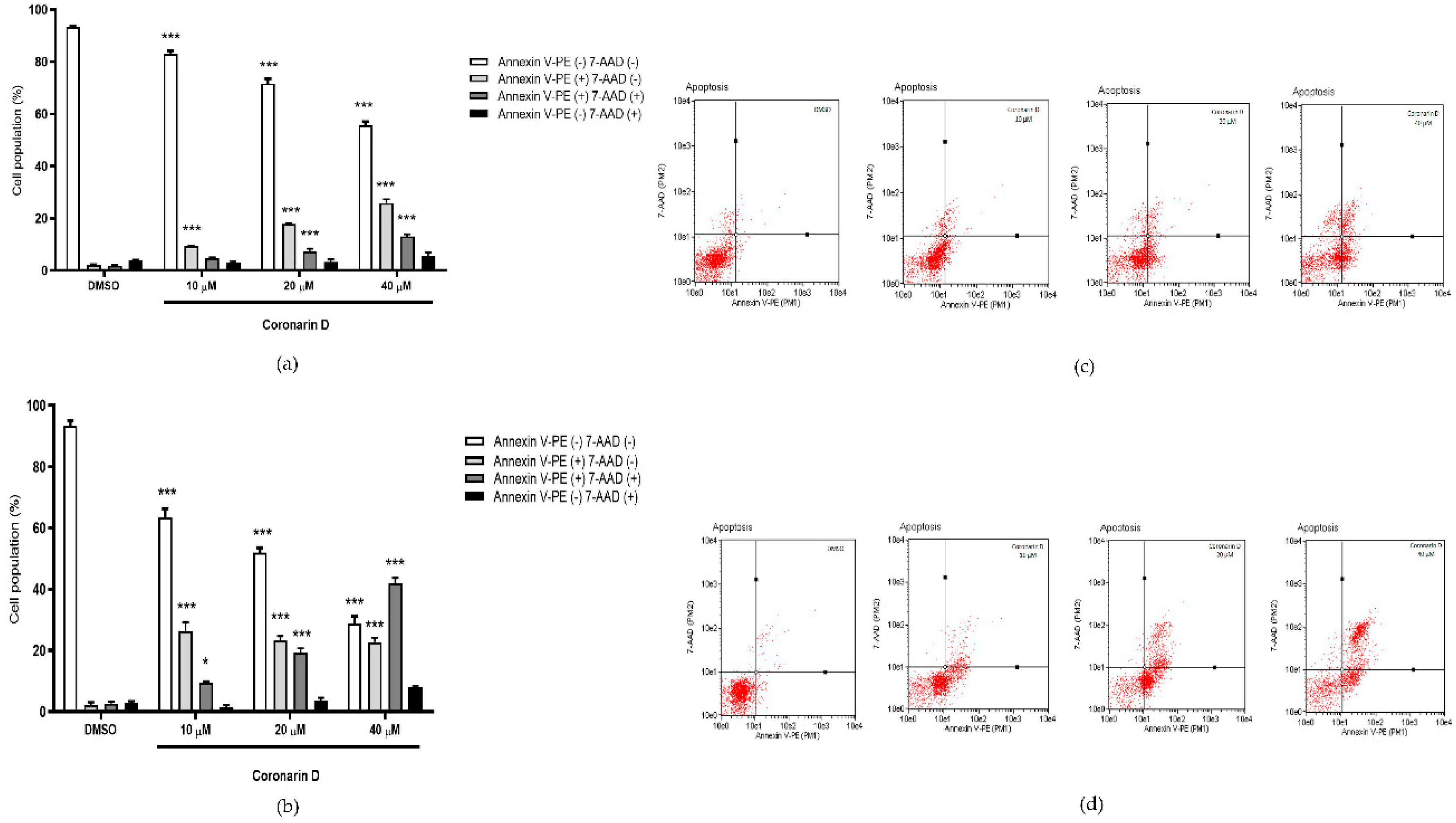
2.4. Phosphatidylserine (PS) Externalization Assay
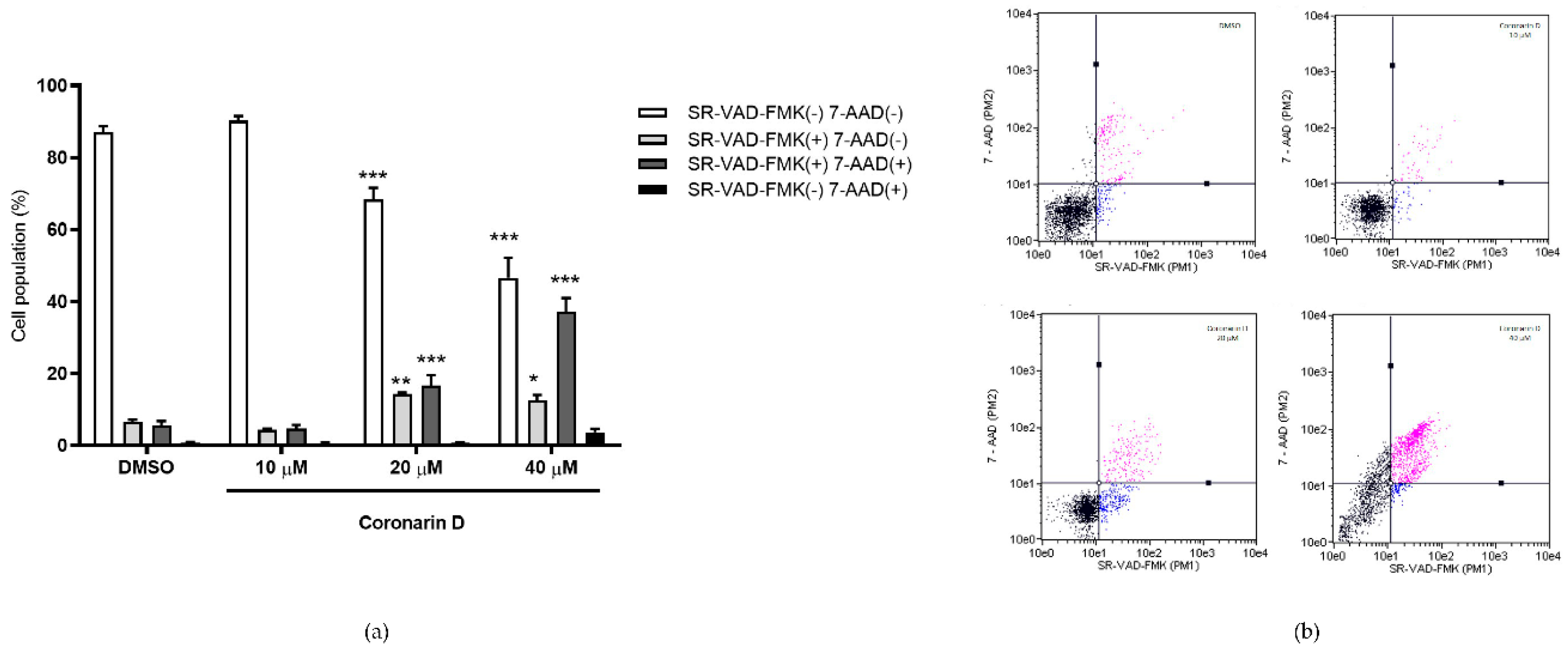
2.5. Detection of Activated Caspases
2.6. Mitochondrial Membrane Potential Assay
2.7. Measurement of Hydrogen Peroxide (H2O2) Generation
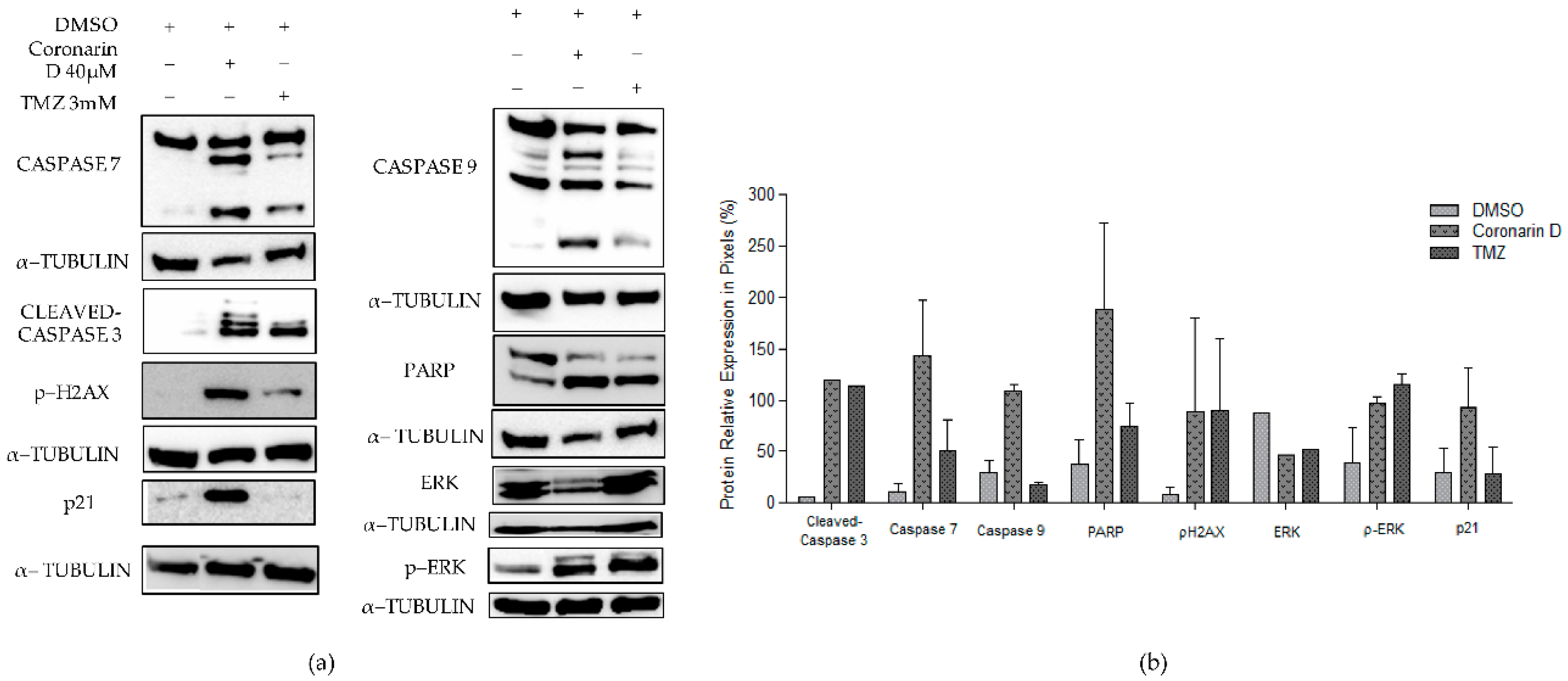
2.8. Western Blotting Assay
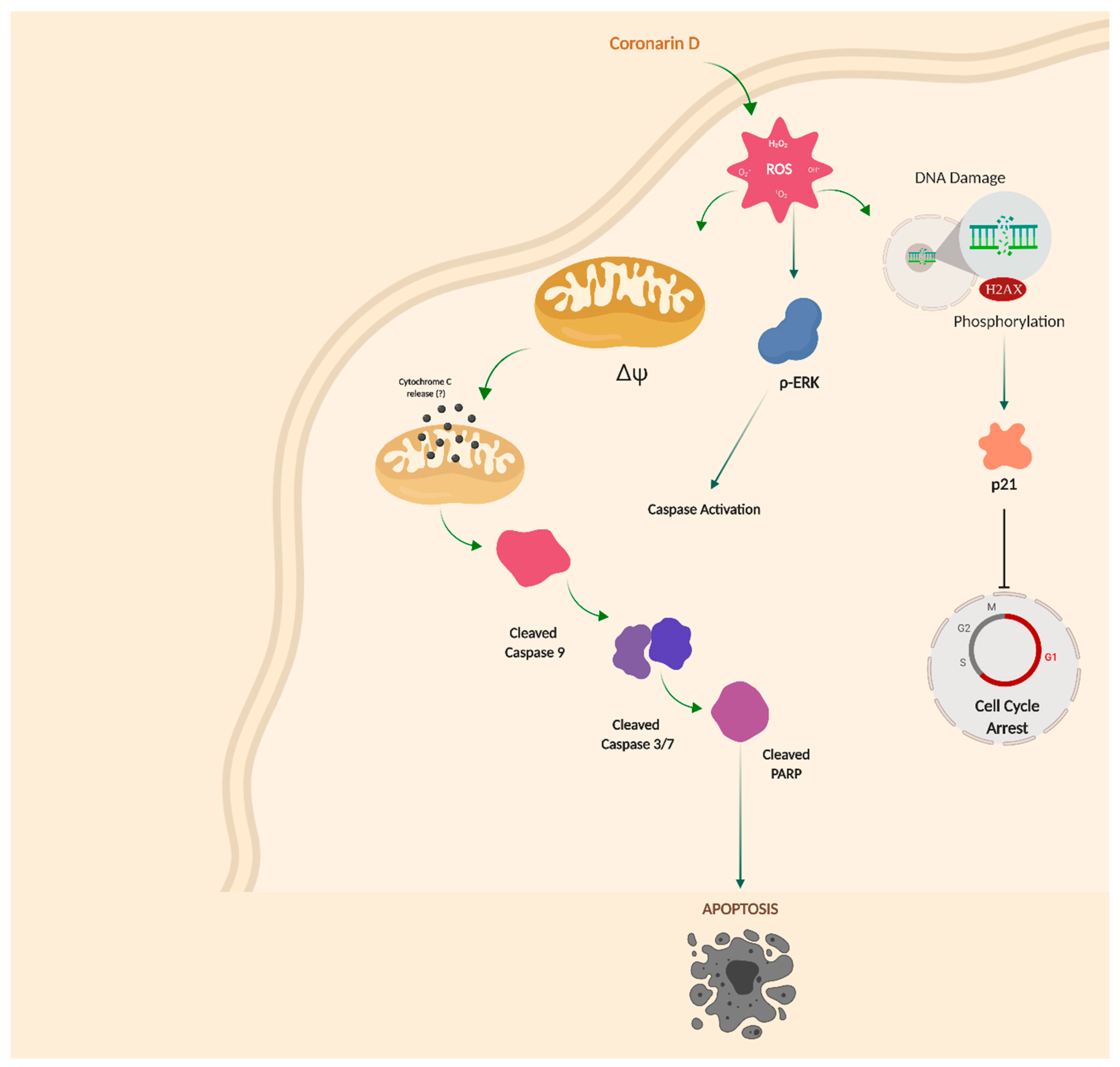
3. Discussion
4. Materials and Methods
4.1. Chemicals and Equipments
4.2. Isolation of Coronarin D Obtained from Hedychium Coronarium
4.3. In Vitro Anticancer Activity Assay
4.3.1. Cell Culture
4.3.2. Antiproliferative Activity
4.4. Cell Cycle Analysis
4.5. Phosphatidylserine (PS) Externalization Assay
4.6. Detection of Activated Multicaspases
4.7. Mitochondrial Membrane Potential Assay
4.8. Measurement of Hydrogen Peroxide (H2O2) Generation
4.9. Western Blotting Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz–Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro. Oncol. 2018, 1, 20–86. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, L.S.; Sun, D.; Pedraza, A.M.; Vera, E.; Wang, Z.; Burns, D.K. Cell–of–origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat. Neurosci. 2019, 18, 545–555. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 21, 405–417. [Google Scholar] [CrossRef]
- Sanai, N.; Alvarez–Buylla, A.; Berger, M.S. Neural Stem Cells and the Origin of Gliomas. N. Engl. J. Med. 2005, 25, 811–822. [Google Scholar] [CrossRef]
- Jones, C.; Perryman, L.; Hargrave, D. Paediatric and adult malignant glioma: Close relatives or distant cousins? Nat. Rev. Clin. Oncol. 2012, 29, 400–413. [Google Scholar] [CrossRef]
- Diwanji, T.P.; Engelman, A.; Snider, J.W.; Mohindra, P. Epidemiology, diagnosis, and optimal management of glioma in adolescents and young adults. Adolesc. Health Med. Ther. 2017, 8, 99–113. [Google Scholar] [CrossRef]
- Kesari, S. Understanding Glioblastoma Tumor Biology: The Potential to Improve Current Diagnosis and Treatments. Semin. Oncol. 2011, 38, S2–10. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 31, 492–507. [Google Scholar] [CrossRef]
- Strobel, H.; Baisch, T.; Fitzel, R.; Schilberg, K.; Siegelin, M.D.; Karpel–Massler, G. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Panosyan, E.H.; Lasky, J.L.; Lin, H.J.; Lai, A.; Hai, Y.; Guo, X. Clinical aggressiveness of malignant gliomas is linked to augmented metabolism of amino acids. J. Neurooncol. 2016, 128, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death. Differ. 2018, 23, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K. Phytochemistry and pharmacology of ornamental gingers, Hedychium coronarium and Alpinia purpurata: A review. J. Integr. Med. 2015, 13, 368–379. [Google Scholar] [CrossRef]
- Zenni, R.D.; Ziller, S.R. An overview of invasive plants in Brazil. Rev. Bras. Botânica. 2011, 34, 431–446. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Ferreira, K.C.; de Oliveira, A.L.M.; Lião, L.M.; Caliari, M.; Júnior, M.S.S. Extraction, characterization and technological properties of white garland–lily starch. Int. J. Biol. Macromol. 2019, 135, 422–428. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Sahoo, A.; Kar, B.; Patnaik, J. Hedychium coronarium extract arrests cell cycle progression, induces apoptosis, and impairs migration and invasion in HeLa cervical cancer cells. Cancer Manag. Res. 2019, 11, 483–500. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Sakamoto, Y.; Toguchida, I.; Yoshikawa, M. Labdane–type diterpenes with inhibitory effects on increase in vascular permeability and nitric oxide production from Hedychium coronarium. Bioorg. Med. Chem. 2002, 10, 2527–2534. [Google Scholar] [CrossRef]
- Kiem, P.V.; Anh, H.L.T.; Nhiem, N.X.; Van Minh, C.; Thuy, N.T.K.; Yen, P.H. Labdane–Type Diterpenoids from the Rhizomes of Hedychium coronarium Inhibit Lipopolysaccharide–Stimulated Production of Pro–inflammatory Cytokines in Bone Marrow–Derived Dendritic Cells. Chem. Pharm. Bull. 2012, 60, 246–250. [Google Scholar] [CrossRef]
- Morikawa, T.; Matsuda, H.; Sakamoto, Y.; Ueda, K.; Yoshikawa, M. New farnesane–type sesquiterpenes, hedychiols A and B 8,9–diacetate, and inhibitors of degranulation in RBL–2H3 cells from the rhizome of Hedychium coronarium. Chem. Pharm. Bull. 2002, 50, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Reuk–ngam, N.; Chimnoi, N.; Khunnawutmanotham, N.; Techasakul, S. Antimicrobial Activity of Coronarin D and Its Synergistic Potential with Antibiotics. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suresh, G.; Prabhakar Reddy, P.; Suresh Babu, K.; Shaik, T.B.; Kalivendi, S.V. Two new cytotoxic labdane diterpenes from the rhizomes of Hedychium coronarium. Bioorg. Med. Chem. Lett. 2010, 20, 7544–7548. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Ichikawa, H.; Anand, P.; Mohankumar, C.J.; Hema, P.S.; Nair, M.S. Coronarin D, a labdane diterpene, inhibits both constitutive and inducible nuclear factor–κB pathway activation, leading to potentiation of apoptosis, inhibition of invasion, and suppression of osteoclastogenesis. Mol. Cancer Ther. 2008, 7, 3306–3317. [Google Scholar] [CrossRef]
- Lin, H.; Hsieh, M.; Hsieh, Y. Coronarin D induces apoptotic cell death through the JNK pathway in human hepatocellular carcinoma. Environ. Toxico. 2018, 33, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Hsieh, M.; Lin, J.; Lin, G.C.C.; Chuang, Y.L.Y.; Chen, Y.H.M. Coronarin D induces human oral cancer cell apoptosis though upregulate JNK1/2 signaling pathway. Environ. Toxicol. 2019, 34, 513–520. [Google Scholar] [CrossRef]
- Chen, J.; Hsieh, M.; Lin, S.; Lin, C.; Lo, Y.; Chuang, Y. Coronarin D induces reactive oxygen species–mediated cell death in human nasopharyngeal cancer cells through inhibition of p38 MAPK and activation of JNK. Oncotarget 2017, 8, 108006–1080019. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, H.; Katou, I.; Takeya, K.; Cavalheiro, A.; de Oliveira, R.; Ishige, M.; Motidome, M. Cytotoxic Diterpenes from the Rhizomes of Hedychium coronarium. Planta Med. 1988, 24, 311–315. [Google Scholar] [CrossRef]
- PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Coronarin–D (accessed on 30 September 2019).
- Longato, G.B.; Fiorito, G.F.; Vendramini–Costa, D.B.; de Oliveira Sousa, I.M.; Tinti, S.V.; Ruiz, A.L.T.G. Different cell death responses induced by eupomatenoid–5 in MCF–7 and 786–0 tumor cell lines. Toxicol. Vitr. 2015, 29, 1026–1033. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 14, 400–414. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fragkos, M.; Jurvansuu, J.; Beard, P. H2AX Is Required for Cell Cycle Arrest via the p53/p21 Pathway. Mol. Cell Biol. 2009, 15, 2828–2840. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 15, 749–762. [Google Scholar] [CrossRef]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, C.R.; Long, J.; Laidler, P. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mtor pathways in controlling growth and sensitivity to therapy–implications for cancer and aging. Aging 2011, 3, 192–222. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK–induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Bacus, S.S.; Gudkov, A.V.; Lowe, M.; Lyass, L.; Yung, Y.; Komarov, A.P. Taxol–induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 2001, 12, 147–155. [Google Scholar] [CrossRef]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B. ERK Activation Mediates Cell Cycle Arrest and Apoptosis after DNA Damage Independently of p53. J. Biol. Chem. 2002, 12, 12710–12717. [Google Scholar] [CrossRef]
- Tan, B.-J.; Chiu, G.N.C. Role of oxidative stress, endoplasmic reticulum stress and ERK activation in triptolide–induced apoptosis. Int. J. Oncol. 2013, 42, 1605–1612. [Google Scholar] [CrossRef]
- Ramachandiran, S.; Huang, Q.; Dong, J.; Lau, S.S.; Monks, T.J. Mitogen–Activated Protein Kinases Contribute to Reactive Oxygen Species–Induced Cell Death in Renal Proximal Tubule Epithelial Cells. Chem. Res. Toxicol. 2002, 15, 1635–1642. [Google Scholar] [CrossRef]
- Zhuang, S.; Yan, Y.; Daubert, R.A.; Han, J.; Schnellmann, R.G. ERK promotes hydrogen peroxide–induced apoptosis through caspase–3 activation and inhibition of Akt in renal epithelial cells. Am. J. Physiol. 2007, 292, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK Activation in Cisplatin–induced Apoptosis. J. Biol. Chem. 2000, 15, 39435–39443. [Google Scholar] [CrossRef] [PubMed]
- Ricks, T.K.; Chiu, H.-J.; Ison, G.; Kim, G.; McKee, A.E.; Kluetz, P. Successes and Challenges of PARP Inhibitors in Cancer Therapy. Front. Oncol. 2015, 5, 222. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, M.; Li, J.; Lai, F.; Lee, H.; Hsu, Y. Punicalagin induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta. Pharmacol. Sin. 2013, 30, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zheng, L.; Dong, D.; Xu, L.; Yin, L.; Xu, Y. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: A potential new drug for treatment of glioblastoma multiforme. Food Chem. Toxicol. 2013, 59, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Dimas, K.; Demetzos, C.; Vaos, V.; Ioannidis, P.; Trangas, T. Labdane type diterpenes down–regulate the expression of c–myc protein, but not of bcl–2, in human leukemia T–cells undergoing apoptosis. Leuk Res. 2001, 25, 449–454. [Google Scholar] [CrossRef]
- Mahaira, L.G.; Tsimplouli, C.; Sakellaridis, N.; Alevizopoulos, K.; Demetzos, C.; Han, Z. The labdane diterpene sclareol (labd–14–ene–8, 13–diol) induces apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53–independent mechanism of action. Eur. J. Pharmacol. 2011, 666, 173–182. [Google Scholar] [CrossRef]
- Yang, S.H.; Li, S.; Lu, G.; Xue, H.; Kim, D.H.; Zhu, J.-J. Metformin treatment reduces temozolomide resistance of glioblastoma cells. Oncotarget 2016, 7, 48. [Google Scholar] [CrossRef][Green Version]
- Silva–Oliveira, R.J.; Melendez, M.; Martinho, O.; Zanon, M.F.; de Souza Viana, L.; Carvalho, A.L. AKT can modulate the in vitro response of HNSCC cells to irreversible EGFR inhibitors. Oncotarget 2017, 8, 53288–53301. [Google Scholar]
Sample Availability: Samples of the compound Coronarin D are not available from the authors. |

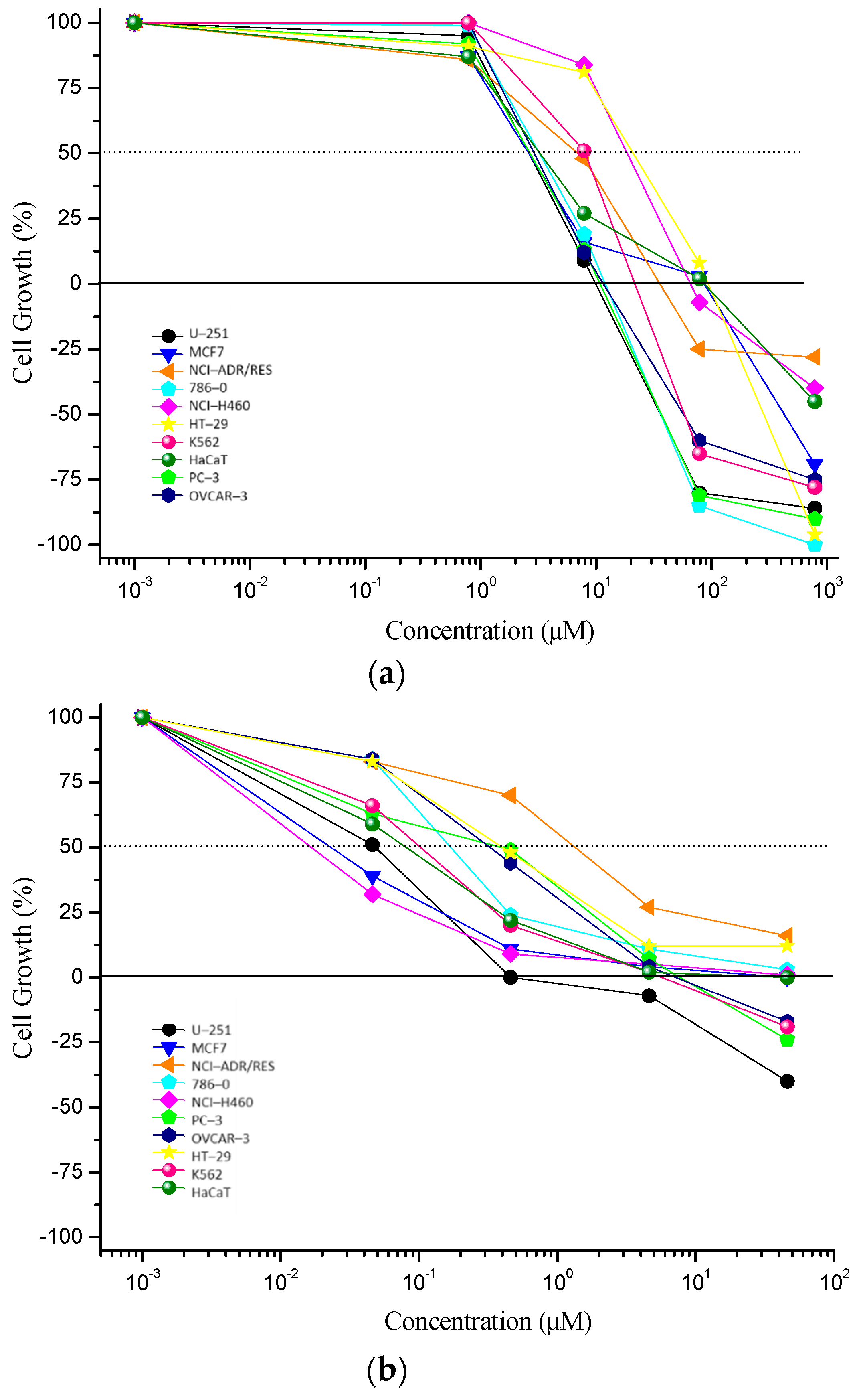



| Cell Lines | Coronarin D | Doxorubicin Hydrochloride |
|---|---|---|
| TGI (μM) | TGI (μM) | |
| U–251 | 18.6 ± 0.5 | 2.4 ± 0.4 |
| MCF7 | 105.0 ± 5.1 | 11.6 ± 1.8 |
| NCI–ADR/RES | 550.1 ± 79.9 | >43.1 * |
| 786–0 | 36.4 ± 4.2 | 17.8 ± 3.4 |
| NCI–H460 | 640.8 ± 11.3 | 26.7 ± 1.8 |
| PC–3 | 17.1 ± 0.6 | 21.4 ± 4.2 |
| OVCAR–3 | 41.6 ± 2.1 | 19.2 ± 1.9 |
| HT–29 | 534.1 ± 31.8 | >43.1 * |
| K562 | 56.6 ± 2.4 | 10.0 ± 1.2 |
| HaCaT | 12.9 ± 1.7 | 1.1 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Franco, Y.E.; Y. Okubo, M.; D. Torre, A.; P. Paiva, P.; N. Rosa, M.; O. Silva, V.A.; M. Reis, R.; T. G. Ruiz, A.L.; M. Imamura, P.; de Carvalho, J.E.; et al. Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line. Molecules 2019, 24, 4498. https://doi.org/10.3390/molecules24244498
M. Franco YE, Y. Okubo M, D. Torre A, P. Paiva P, N. Rosa M, O. Silva VA, M. Reis R, T. G. Ruiz AL, M. Imamura P, de Carvalho JE, et al. Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line. Molecules. 2019; 24(24):4498. https://doi.org/10.3390/molecules24244498
Chicago/Turabian StyleM. Franco, Yollanda E., Marcia Y. Okubo, Adriana D. Torre, Paula P. Paiva, Marcela N. Rosa, Viviane A. O. Silva, Rui M. Reis, Ana L. T. G. Ruiz, Paulo M. Imamura, João E. de Carvalho, and et al. 2019. "Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line" Molecules 24, no. 24: 4498. https://doi.org/10.3390/molecules24244498
APA StyleM. Franco, Y. E., Y. Okubo, M., D. Torre, A., P. Paiva, P., N. Rosa, M., O. Silva, V. A., M. Reis, R., T. G. Ruiz, A. L., M. Imamura, P., de Carvalho, J. E., & B. Longato, G. (2019). Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line. Molecules, 24(24), 4498. https://doi.org/10.3390/molecules24244498








