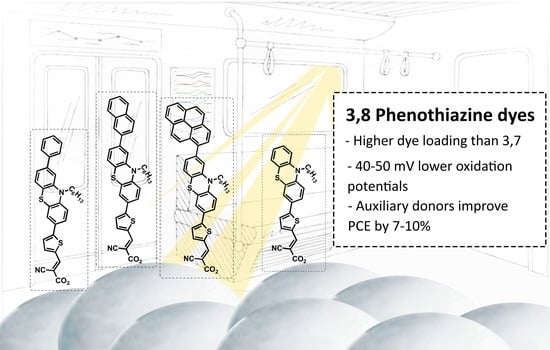
Effect of Auxiliary Donors on 3,8-Phenothiazine Dyes for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
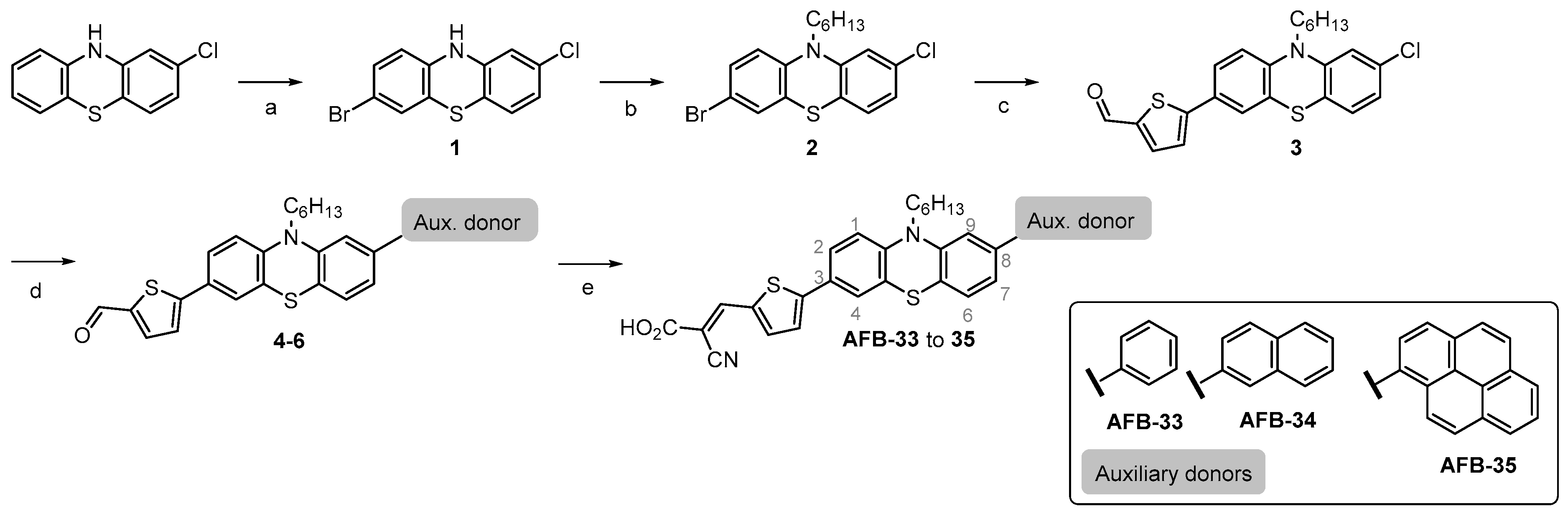
2.1. Dye Synthesis
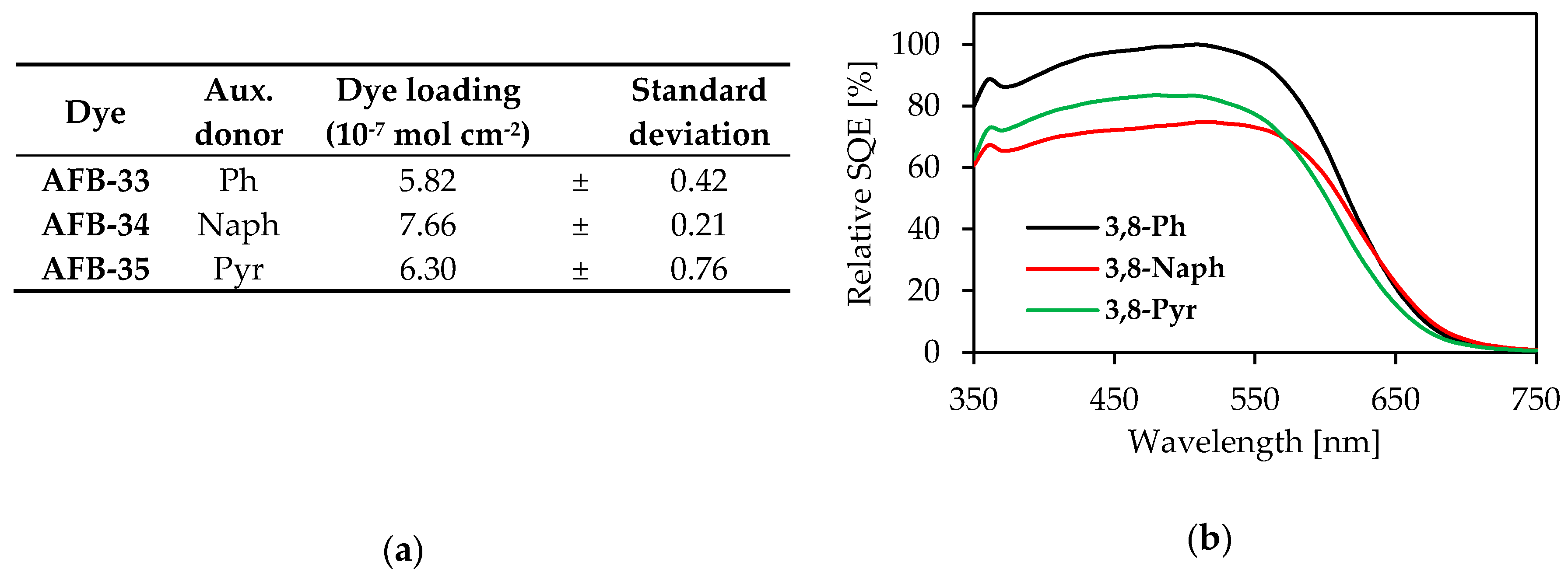
2.2. Photophysical Properties
2.3. Electrochemical Properties
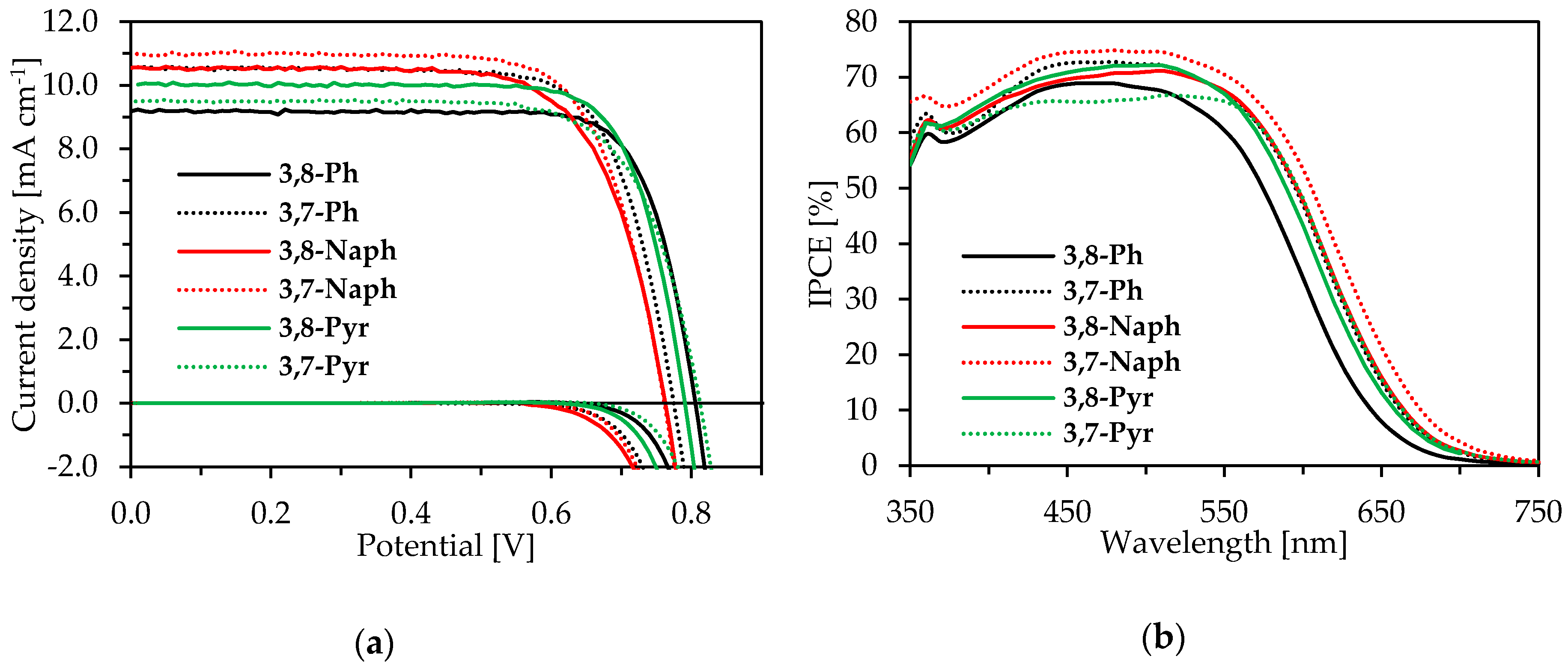
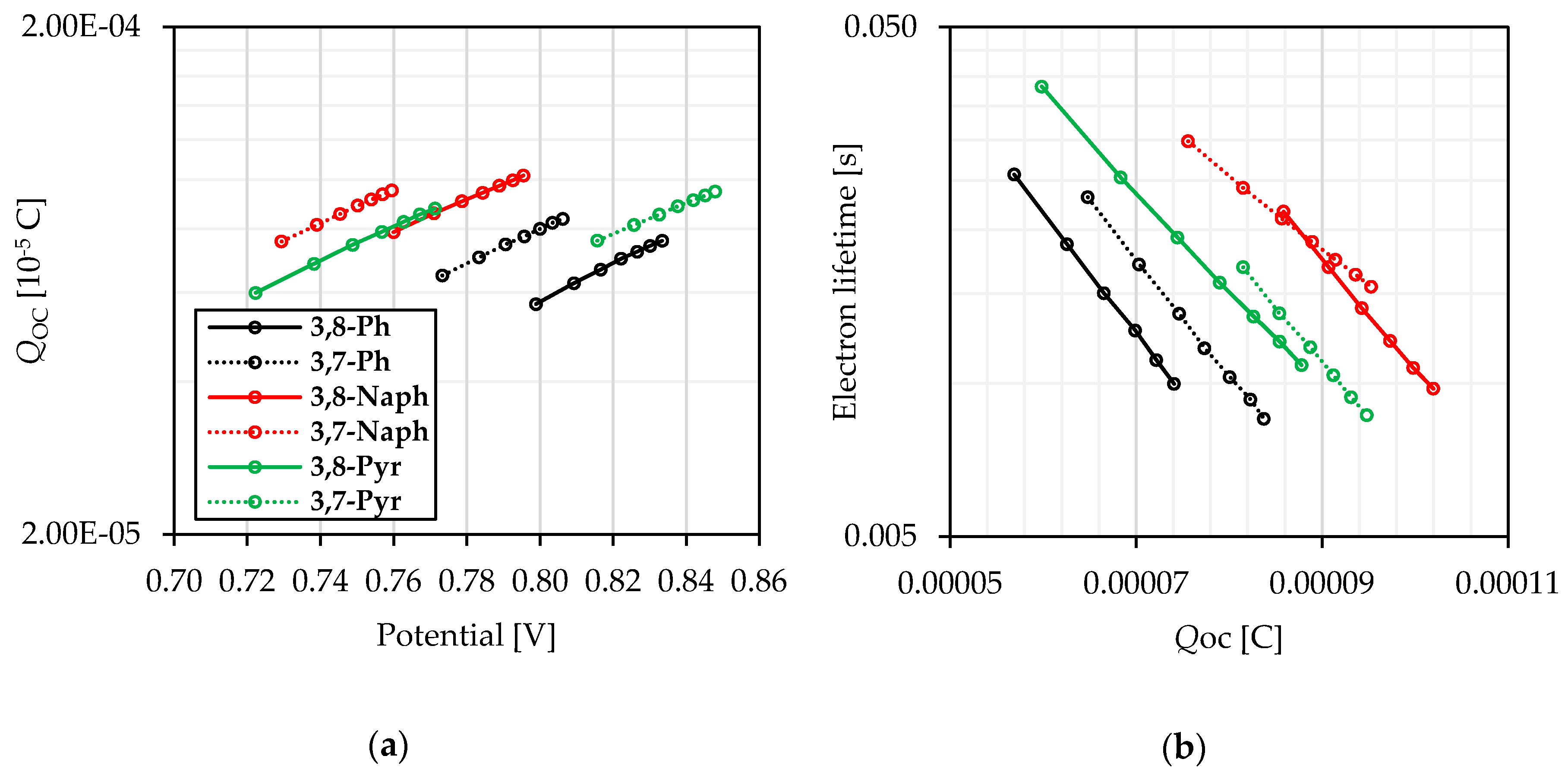
2.4. Photovoltaic Properties
3. Conclusions
4. Experimental
4.1. Synthesis
4.2. DSSC Fabrication
4.3. Device Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in Regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Fischer, M.K.; Baüerle, P. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-i.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A. Triphenylamine based dyes for dye sensitized solar cells: A review. Solar Energy 2016, 123, 127–144. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Dye aggregation in dye-sensitized solar cells. J. Mater. Chem. A. 2017, 5, 19541–19559. [Google Scholar] [CrossRef] [Green Version]
- Robertson, N. Optimizing dyes for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2006, 45, 2338–2345. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Liao, J.Y.; Zhou, S.M.; Shen, Y.; Liu, J.M.; Kuang, D.B.; Su, C.Y. Effect of Hydrocarbon Chain Length of Disubstituted Triphenyl-amine-Based Organic Dyes on Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 22002–22008. [Google Scholar] [CrossRef]
- Gabrielsson, E.; Ellis, H.; Feldt, S.; Tian, H.; Boschloo, G.; Hagfeldt, A.; Sun, L. Convergent/Divergent Synthesis of a Linker-Varied Series of Dyes for Dye-Sensitized Solar Cells Based on the D35 Donor. Adv. Energy Mater. 2013, 3, 1647–1656. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Thomas, K.R.J.; Lee, C.P.; Li, C.T.; Ho, K.C. Organic Dyes Containing Carbazole as Donor and π-Linker: Optical, Electrochemical, and Photovoltaic Properties. ACS Appl. Mater. Interfaces 2014, 6, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyomen, T.; Unno, M.; Hanaya, M. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Seo, K.D.; Choi, W.S.; Hong, J.Y.; Kim, H.K. Near-IR organic sensitizers containing squaraine and phenothiazine units for dye-sensitized solar cells. Dyes Pigm. 2015, 113, 18–26. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, D.; Cao, Y.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Grätzel, M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef]
- Buene, A.F.; Hagfeldt, A.; Hoff, B.H. A comprehensive experimental study of five fundamental phenothiazine geometries increasing the diversity of the phenothiazine dye class for dye-sensitized solar cells. Dyes Pigm. 2019, 169, 66–72. [Google Scholar] [CrossRef]
- Buene, A.F.; Ose, E.E.; Zakariassen, A.G.; Hagfeldt, A.; Hoff, B.H. Auxiliary donors for phenothiazine sensitizers for dye-sensitized solar cells – how important are they really? J. Mater. Chem. A 2019, 7, 7581–7590. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Pan, Y.; Li, L.; Hagfeldt, A.; Sun, L. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 2007, 3741–3743. [Google Scholar] [CrossRef]
- Luo, J.S.; Wan, Z.Q.; Jia, C.Y. Recent advances in phenothiazine-based dyes for dye-sensitized solar cells. Chin. Chem. Lett. 2016, 27, 1304–1318. [Google Scholar] [CrossRef]
- Huang, Z.S.; Meier, H.; Cao, D. Phenothiazine-based dyes for efficient dye-sensitized solar cells. J. Mater. Chem. C. 2016, 4, 2404–2426. [Google Scholar] [CrossRef]
- Kim, S.H.; Sakong, C.; Chang, J.B.; Kim, B.; Ko, M.J.; Kim, D.H.; Hong, K.S.; Kim, J.P. The effect of N-substitution and ethylthio substitution on the performance of phenothiazine donors in dye-sensitized solar cells. Dyes Pigm. 2013, 97, 262–271. [Google Scholar] [CrossRef]
- Marszalek, M.; Nagane, S.; Ichake, A.; Humphry-Baker, R.; Paul, V.; Zakeeruddin, S.M.; Grätzel, M. Tuning spectral properties of phenothiazine based donor-π-acceptor dyes for efficient dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 889–894. [Google Scholar] [CrossRef]
- Li, J.; Wu, W.; Yang, J.; Tang, J.; Long, Y.; Hua, J. Effect of chenodeoxycholic acid (CDCA) additive on phenothiazine dyes sensitized photovoltaic performance. Sci. China Chem. 2011, 54, 699–706. [Google Scholar] [CrossRef]
- Dryza, V.; Bieske, E.J. Does the triphenylamine-based D35 dye sensitizer form aggregates on metal-oxide surfaces? J. Photochem. Photobiol. A 2015, 302, 35–41. [Google Scholar] [CrossRef]
- Agrawal, S.; Pastore, M.; Marotta, G.; Reddy, M.A.; Chandrasekharam, M.; De Angelis, F. Optical Properties and Aggregation of Phenothiazine-Based Dye-Sensitizers for Solar Cells Applications: A Combined Experimental and Computational Investigation. J. Phys. Chem. C 2013, 117, 9613–9622. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Chang, S.; Huang, D.D.; Zhou, X.; Zhu, X.J.; Zhao, J.Z.; Chen, T.; Wong, W.Y.; Wong, W.K. Significant Improvement of Dye-Sensitized Solar Cell Performance Using Simple Phenothiazine-Based Dyes. Chem. Mater. 2013, 25, 2146–2153. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Kumbhar, H.S.; Shankarling, G.S. Solvatochromic fluorescence properties of phenothiazine-based dyes involving thiazolo[4,5-b]quinoxaline and benzo[e]indole as strong acceptors. Spectrochim. Acta A 2017, 174, 154–163. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
Sample Availability: No available. |
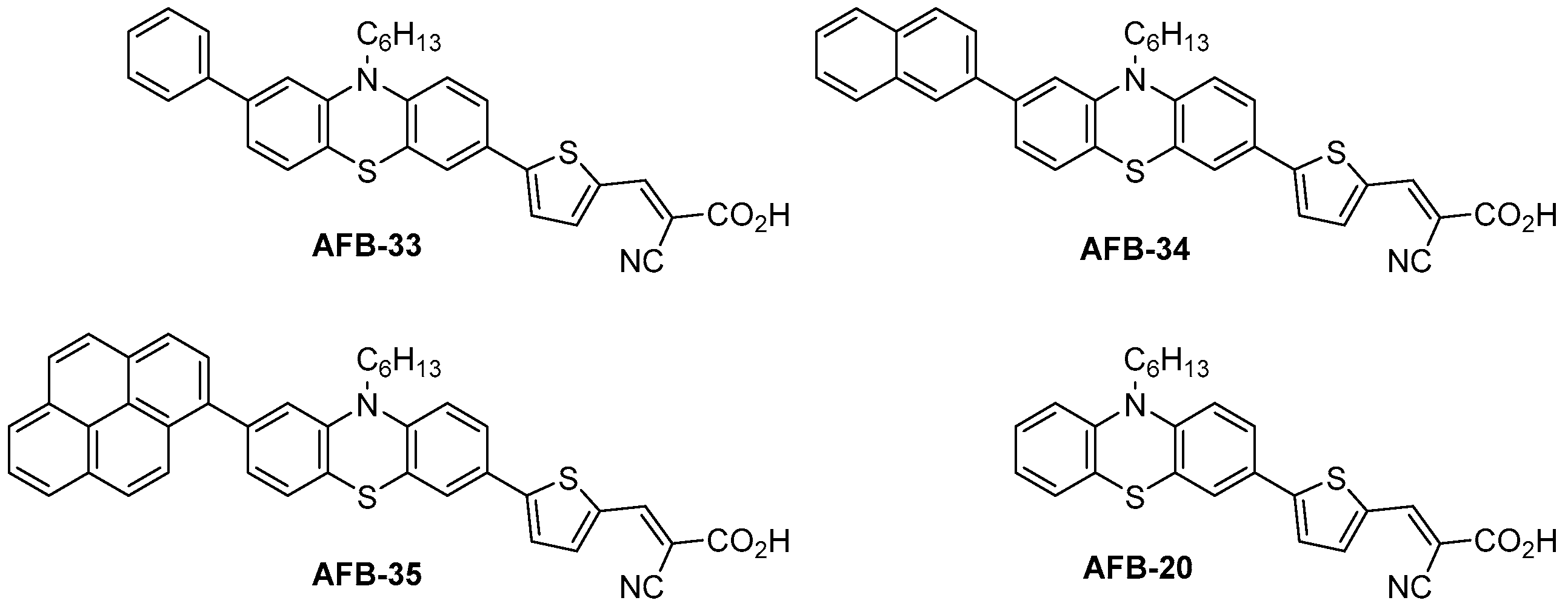

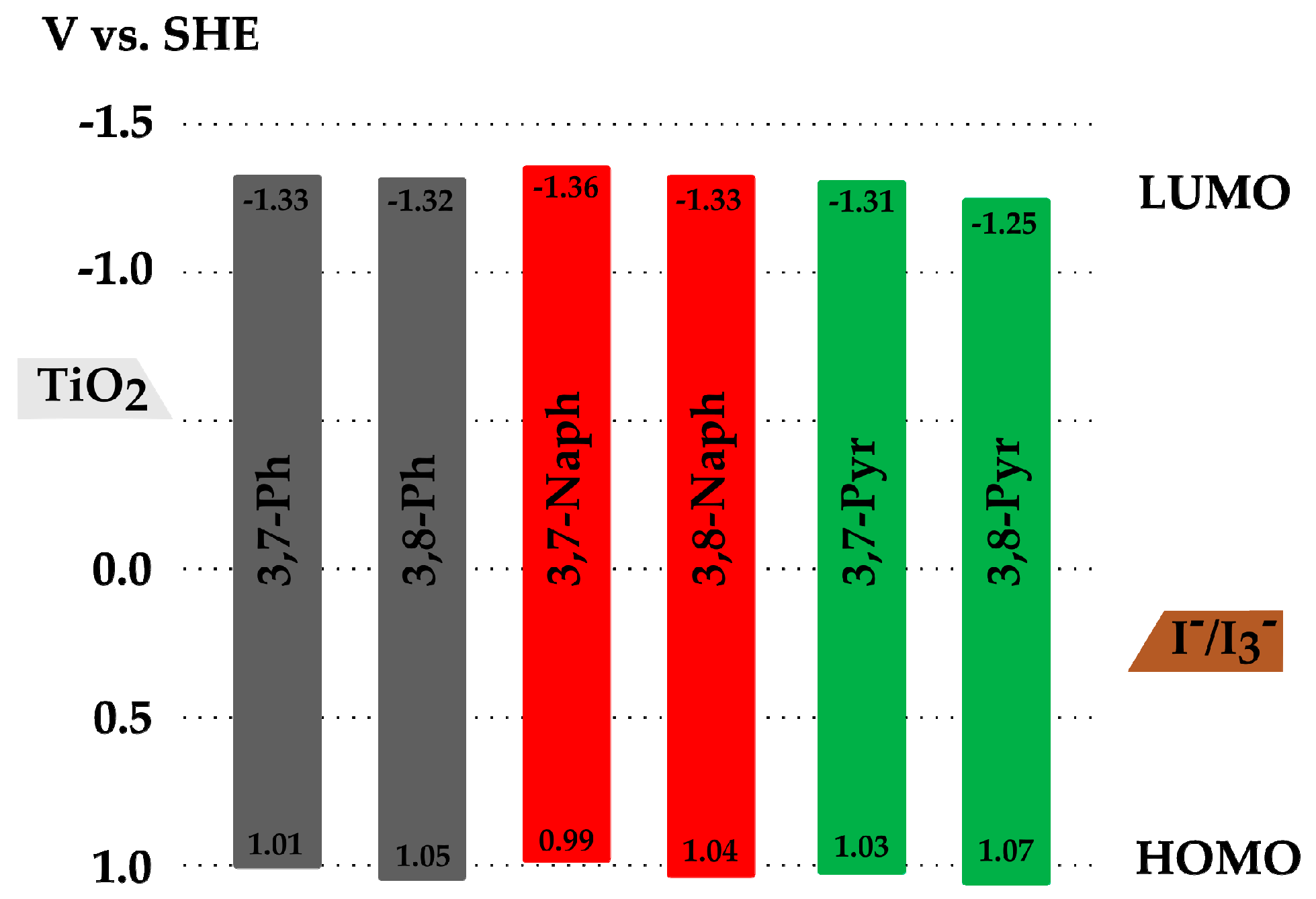
| Dye | Aux. Donor (Geometry) | λabs (nm) 1 | ε (M−1 cm−1) | Em. (nm) 2 | λabs on TiO2 (nm) 3 | E0-0 (eV) 4 | Eox (V) 5 | ELUMO (V) 6 |
|---|---|---|---|---|---|---|---|---|
| AFB-20 7 | H | 435 | 24,400 | 618 | 430 | 2.38 | 1.08 | −1.30 |
| AFB-8 | Ph (3,7) | 441 | 23,800 | 623 | 438 | 2.34 | 1.01 | −1.33 |
| AFB-33 | Ph (3,8) | 433 | 22,500 | 621 | 433 8 | 2.37 | 1.05 | −1.32 |
| AFB-16 | Naph (3,7) | 456 | 19,800 | 610 | 442 | 2.35 | 0.99 | −1.36 |
| AFB-34 | Naph (3,8) | 434 | 21,100 | 615 | 440 8 | 2.37 | 1.04 | −1.33 |
| AFB-19 | Pyr (3,7) | 440 | 24,100 | 622 | 441 8 | 2.34 | 1.03 | −1.31 |
| AFB-35 | Pyr (3,8) | 451 | 23,400 | 634 | 439 8 | 2.32 | 1.07 | −1.25 |
| Dye | Aux. Donor (Geometry) | CDCA (eq.) | IPCE JSC (mA cm−2) 1 | JSC (mA cm−2) | VOC (mV) | FF | PCE (%) |
|---|---|---|---|---|---|---|---|
| AFB-33 | Ph (3,8) | 10 | 8.64 | 9.67 ± 0.29 | 790 ± 14 | 0.75 ± 0.04 | 5.77 ± 0.20 |
| 0 | 9.80 | 9.95 ± 0.19 | 779 ± 13 | 0.73 ± 0.01 | 5.70 ± 0.15 | ||
| AFB-34 | Naph (3,8) | 10 | 9.87 | 10.55 ± 0.17 | 770 ± 8 | 0.70 ± 0.01 | 5.72 ± 0.08 |
| 0 | 10.22 | 10.25 ± 0.08 | 768 ± 1 | 0.69 ± 0.02 | 5.46 ± 0.12 | ||
| AFB-35 | Pyr (3,8) | 10 | 9.67 | 10.10 ± 0.39 | 771 ± 18 | 0.75 ± 0.01 | 5.88 ± 0.24 |
| 0 | 8.63 | 9.90 ± 0.01 | 771 ± 3 | 0.71 ± 0.02 | 5.51 ± 0.14 | ||
| AFB-8 | Ph (3,7) | 10 | 9.91 | 10.31 ± 0.16 | 781 ± 7 | 0.74 ± 0.01 | 5.99 ± 0.12 |
| AFB-16 | Naph (3,7) | 10 | 10.75 | 11.27 ± 0.20 | 754 ± 10 | 0.71 ± 0.03 | 6.08 ± 0.21 |
| AFB-19 | Pyr (3,7) | 10 | 9.54 | 9.43 ± 0.25 | 810 ± 3 | 0.74 ± 0.01 | 5.68 ± 0.13 |
| AFB-20 2 | H | 10 | 8.40 | 9.13 ± 0.20 | 778 ± 7 | 0.76 ± 0.01 | 5.34 ± 0.11 |
| N719 | - | 10 | - | 12.85 | 805 | 0.76 | 7.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buene, A.F.; Christensen, M.; Hoff, B.H. Effect of Auxiliary Donors on 3,8-Phenothiazine Dyes for Dye-Sensitized Solar Cells. Molecules 2019, 24, 4485. https://doi.org/10.3390/molecules24244485
Buene AF, Christensen M, Hoff BH. Effect of Auxiliary Donors on 3,8-Phenothiazine Dyes for Dye-Sensitized Solar Cells. Molecules. 2019; 24(24):4485. https://doi.org/10.3390/molecules24244485
Chicago/Turabian StyleBuene, Audun Formo, Mats Christensen, and Bård Helge Hoff. 2019. "Effect of Auxiliary Donors on 3,8-Phenothiazine Dyes for Dye-Sensitized Solar Cells" Molecules 24, no. 24: 4485. https://doi.org/10.3390/molecules24244485
APA StyleBuene, A. F., Christensen, M., & Hoff, B. H. (2019). Effect of Auxiliary Donors on 3,8-Phenothiazine Dyes for Dye-Sensitized Solar Cells. Molecules, 24(24), 4485. https://doi.org/10.3390/molecules24244485