Housefly Pupae-Derived Antioxidant Peptides Exerting Neuroprotective Effects on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells
Abstract
:1. Introduction
2. Results and Discussion
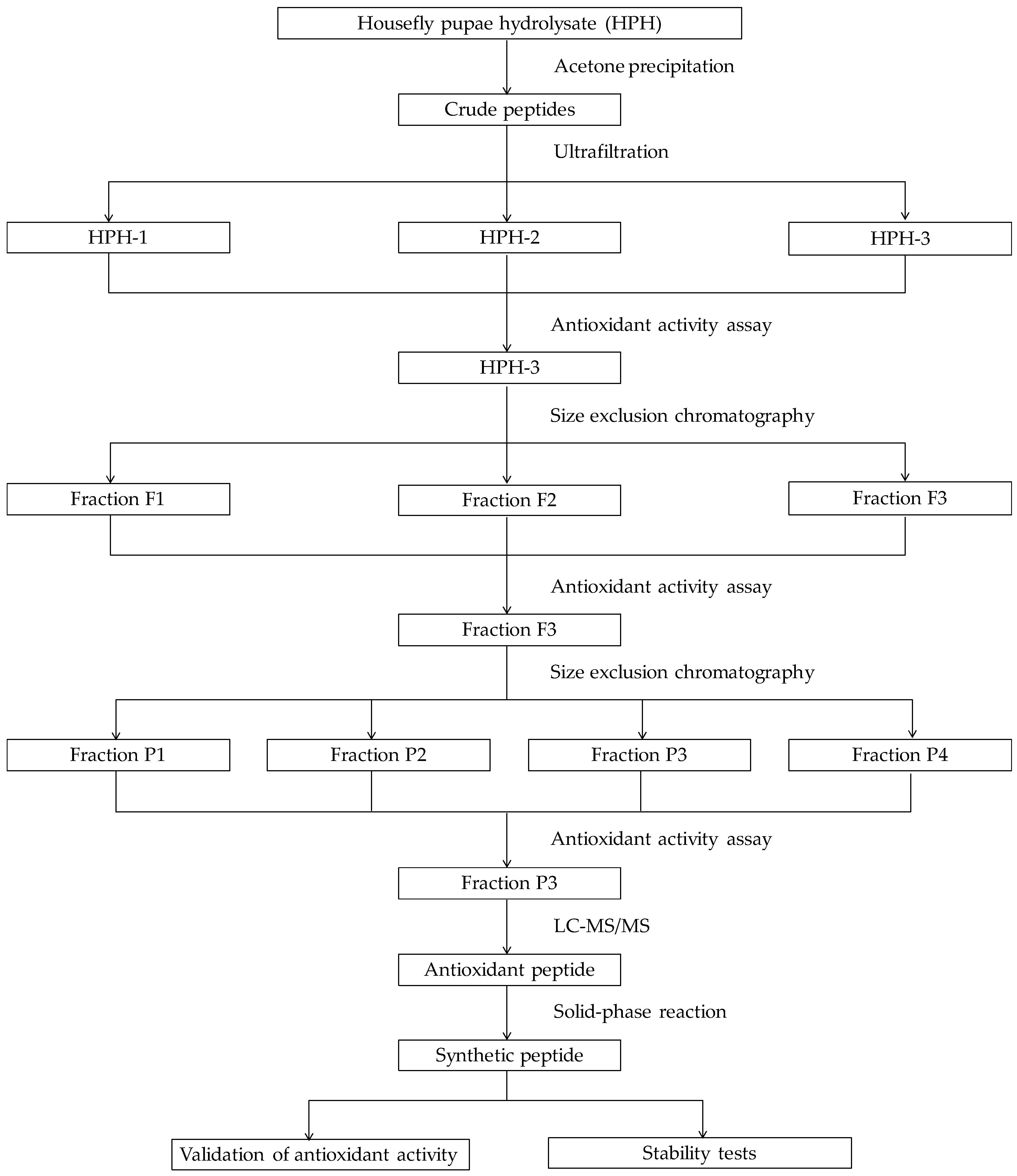
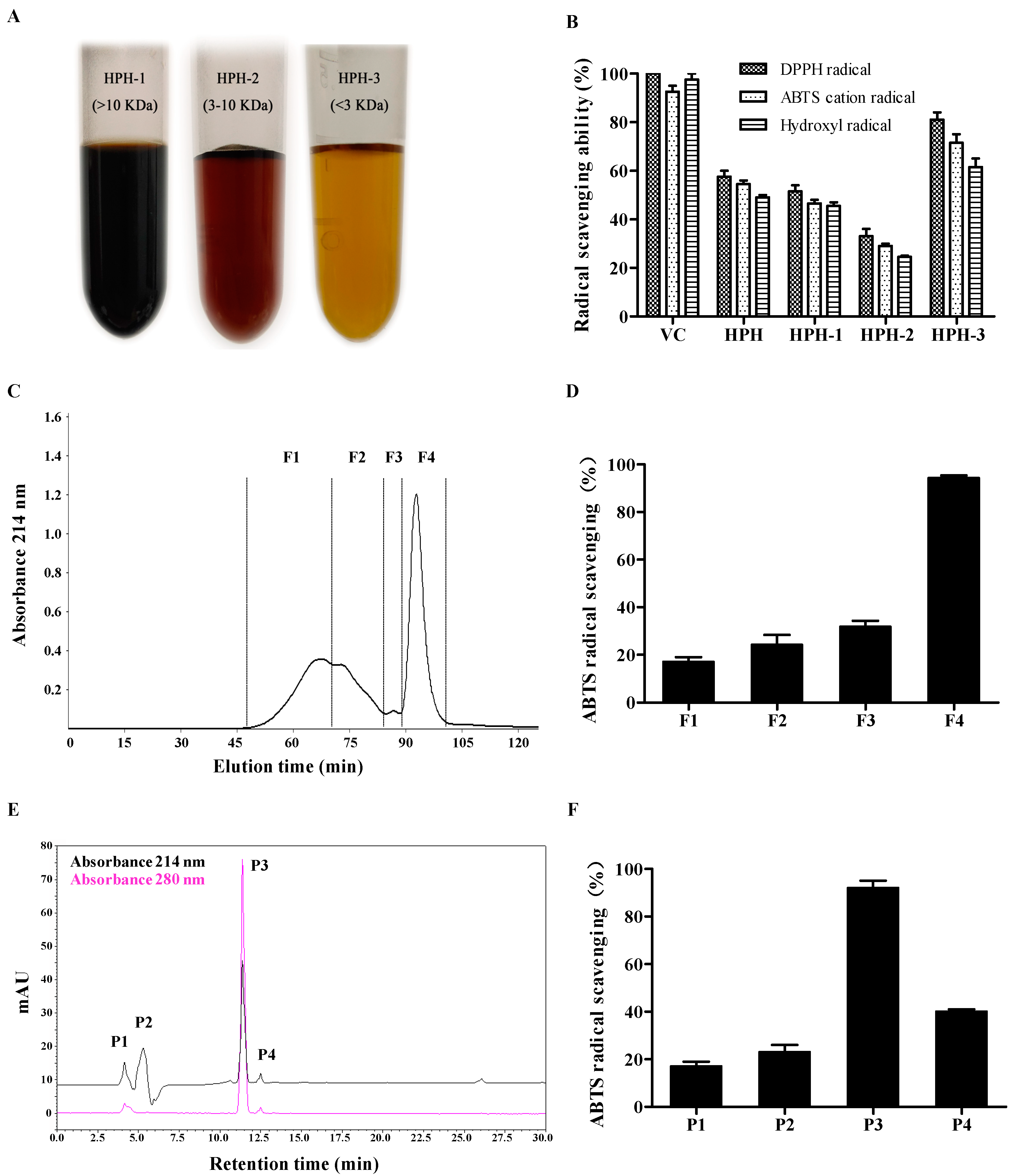
2.1. Purification of Antioxidative Peptides
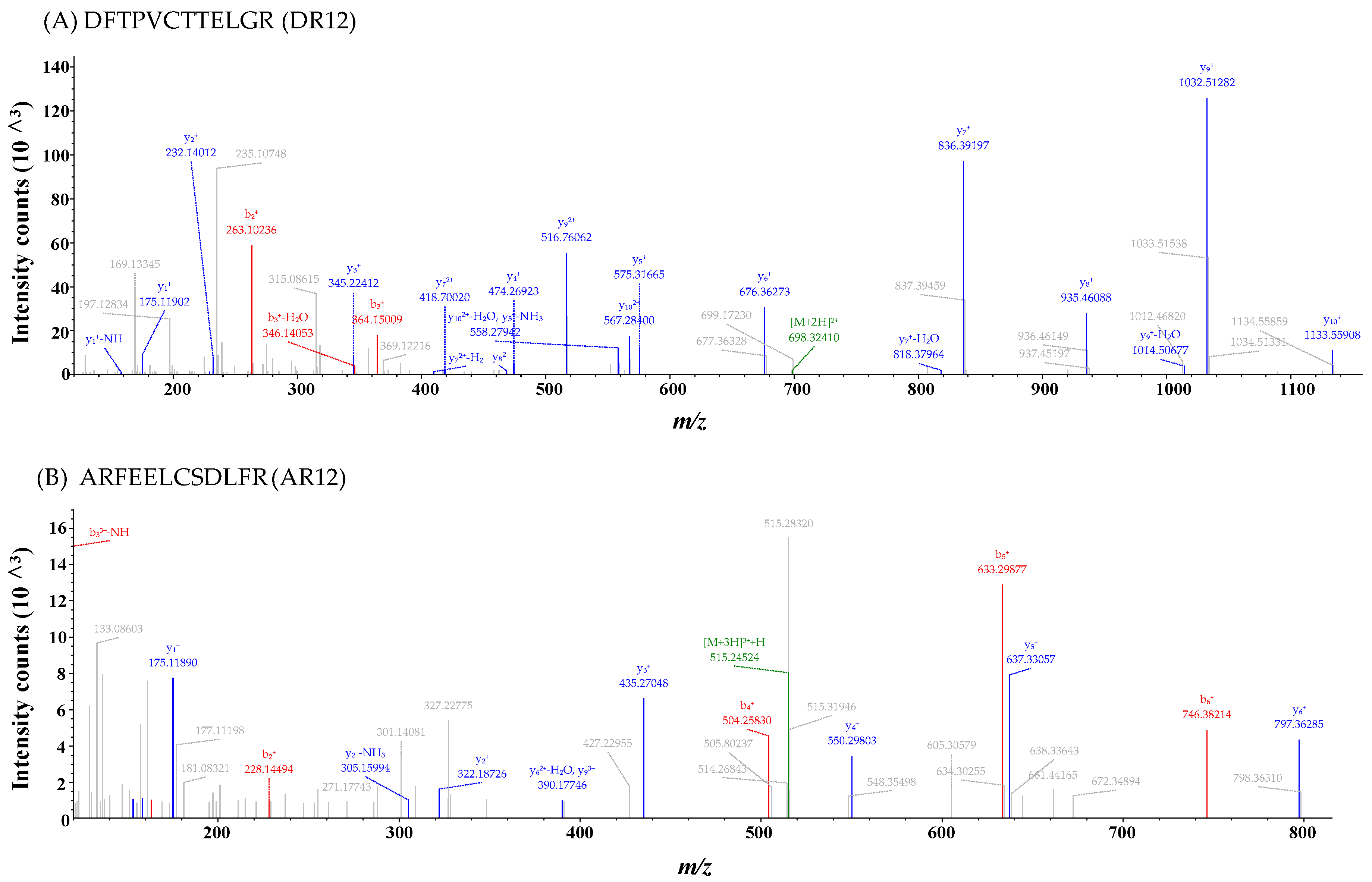
2.2. Identification of Antioxidant Peptides
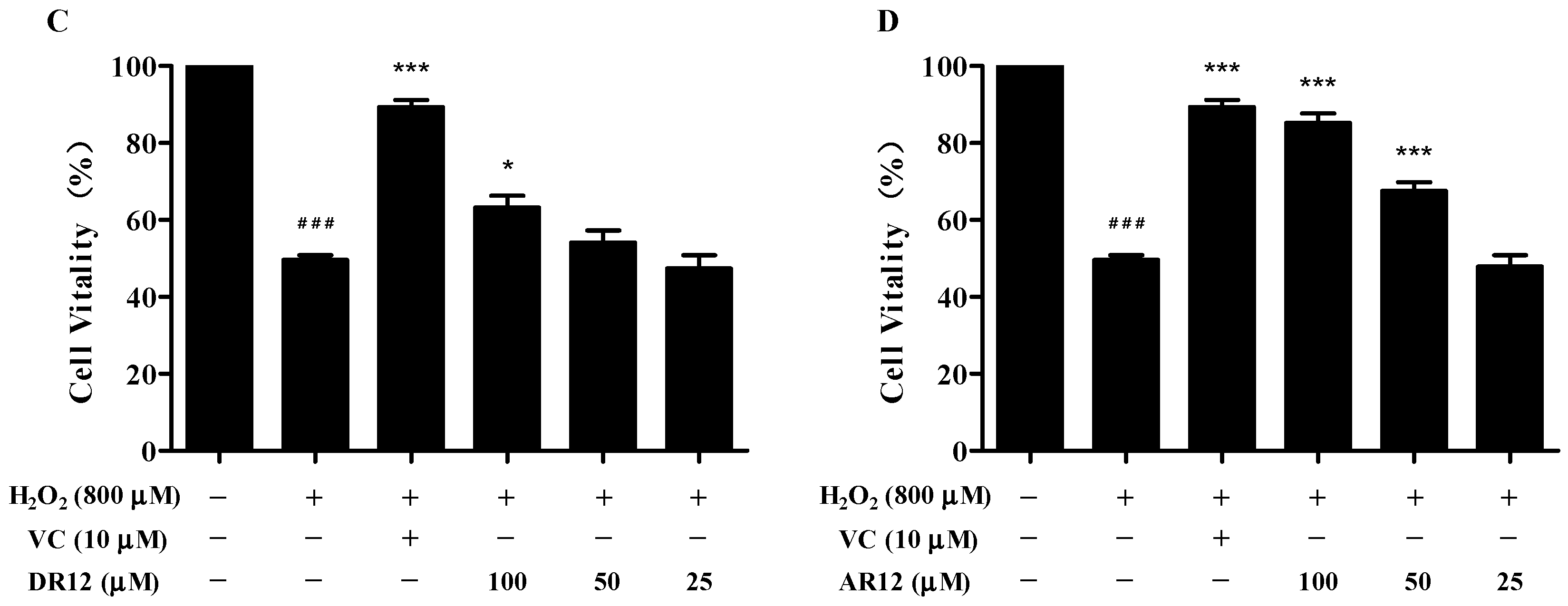
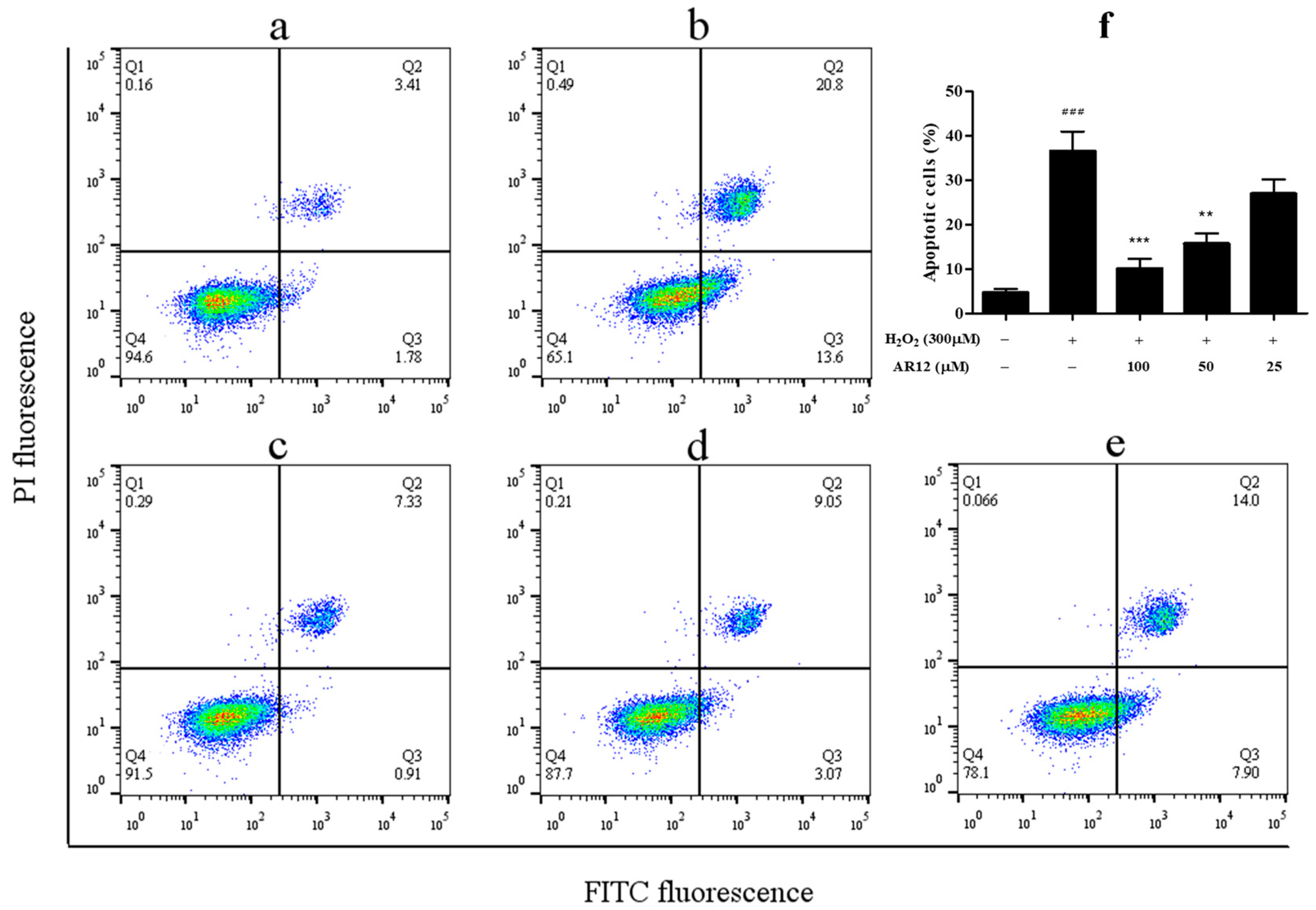
2.3. Neuroprotective Effect of Peptides on H2O2-Induced Cell Injury in PC-12 Cells
2.4. AR12 Protected H2O2-Induced PC12 Cells Damage through Reducing Intracellular ROS
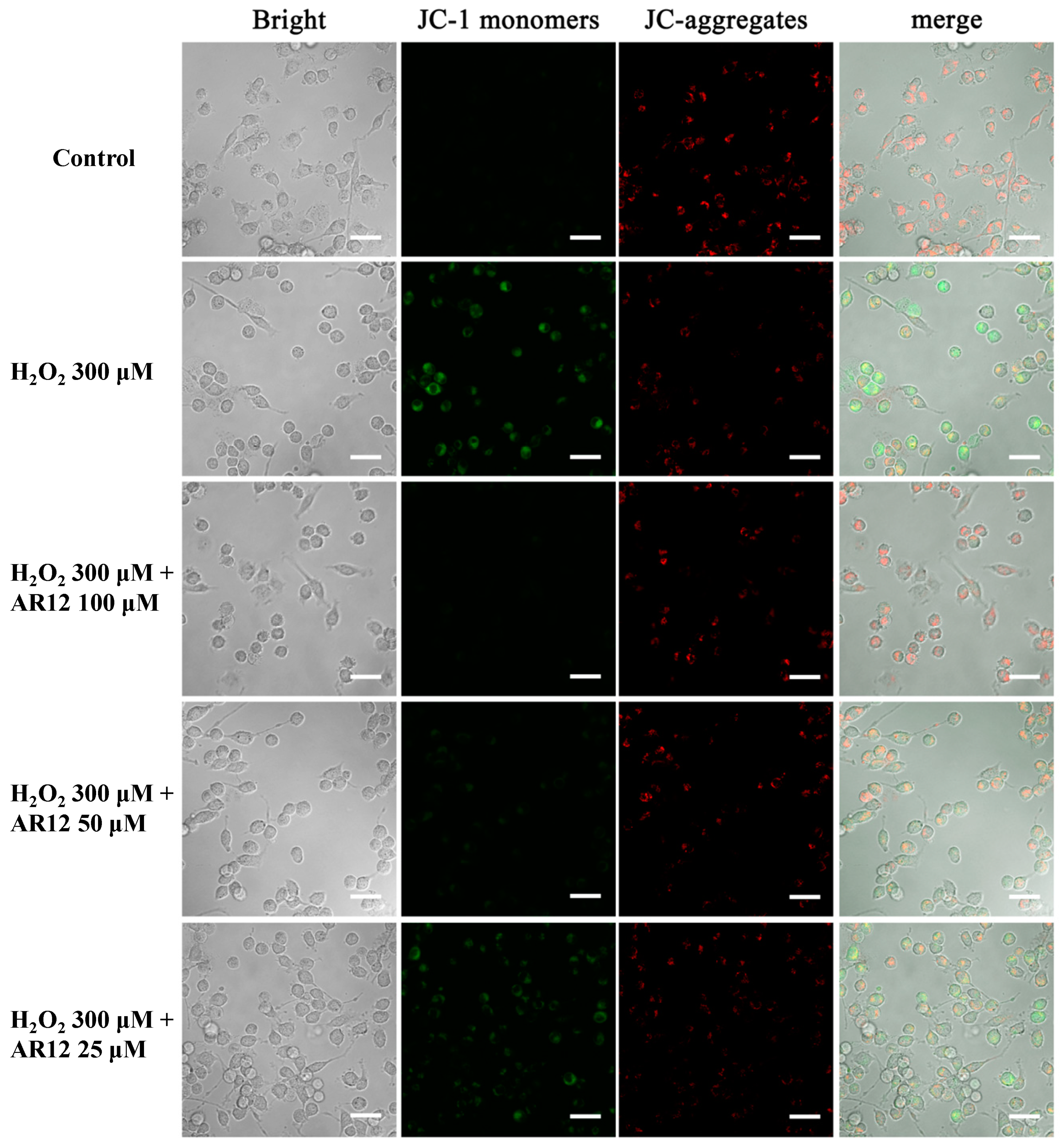
2.5. AR12 Protected H2O2-Induced PC12 Cells Damage through Recovering the Loss of Mitochondrial Membrane Potential (MMP)
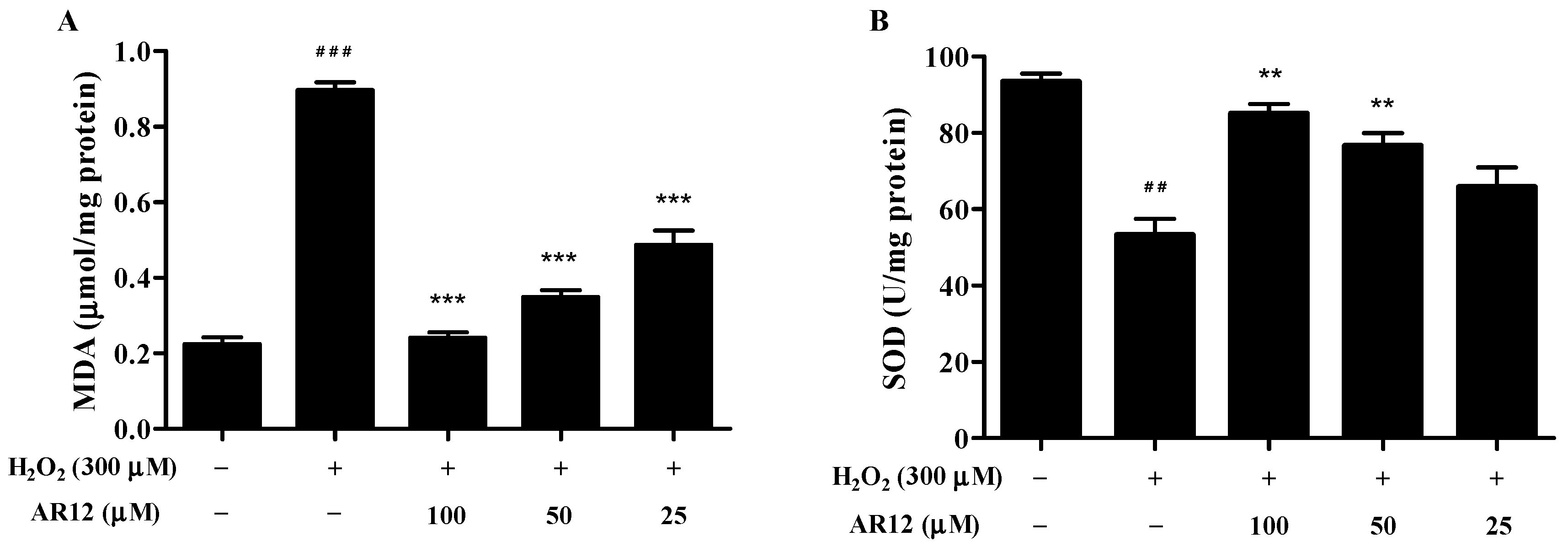
2.6. Effects of AR12 on Malonaldehyde (MDA) and Superoxide Dismutase (SOD) in H2O2-Induced PC12 Cells
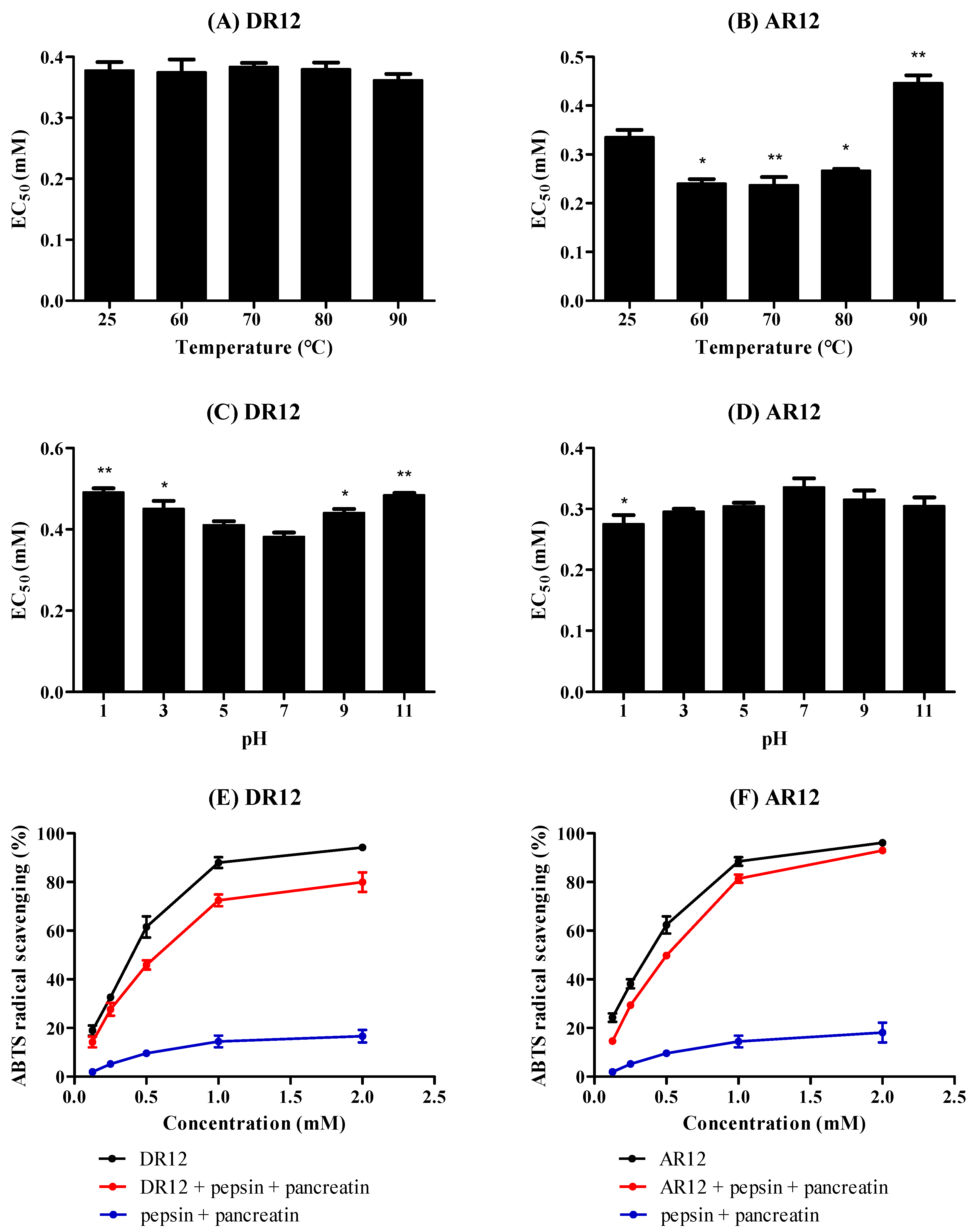
2.7. Effects of Thermal, pH and Simulated GI Digestion on Peptides
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of HPH
3.3. Peptides Separation by Membrane Ultrafiltration
3.4. Purification by Size Exclusion Chromatography
3.5. Purification by RP-HPLC
3.6. Identification of Antioxidant Peptides by LC-MS/MS and Peptide Synthesis
3.7. Radical Scavenging Activity Assay
3.8. Cell Culture and Viability Analysis
3.9. Apoptosis Assay
3.10. Intracellular ROS Level Measurement
3.11. Measurement of MMP
3.12. Measurement of Intracellular MDA Content and SOD Activity
3.13. Stability of Synthetic Peptides against Heat, pH, and Simulated GI Digestion
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Nikliński, J.; Žarković, N.; Žarković, K.; Waeg, G.; Łuczaj, W.; Charkiewicz, R.; Skrzydlewska, E. Lipid mediators involved in the oxidative stress and antioxidant defence of human lung cancer cells. Redox Biol. 2016, 9, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Zujko, M.E.; Witkowska, A.M.; Górska, M.; Wilk, J.; Kretowski, A. Reduced intake of dietary antioxidants can impair antioxidant status in type 2 diabetes patients. Pol. Arch. Med. Wewn. 2014, 124, 599–607. [Google Scholar] [CrossRef]
- Lakshmi, S.V.V.; Padmaja, G.; Kuppusamy, P.; Kutala, V.K. Oxidative stress in cardiovascular disease. Indian J. Biochem. Biophys. 2009, 46, 421–440. [Google Scholar]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017, 71, 92–96. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial dysfunction in neural injury. Front. Neurosci. 2019, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chai, T.T.; Law, Y.C.; Wong, F.C.; Kim, S.K. Enzyme-Assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Lim, W.; Park, S.; Bae, H.; You, S.; Song, G. Synthetic phenolic antioxidant propyl gallate induces male infertility through disruption of calcium homeostasis and mitochondrial function. Environ. Pollut. 2019, 248, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Zhang, W.G.; Zhou, G.H.; Xu, X.L.; Kang, Z.L.; Yin, Y. Isolation and identification of antioxidant peptides from Jinhua ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Huang, J.C.; Chen, Y.L.; Huang, M.; Zhou, G.H. Identification and Characterization of Antioxidant Peptides from Enzymatic Hydrolysates of Duck Meat. J. Agric. Food Chem. 2015, 63, 3437–3444. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Ren, Y.; Wang, E.; Shi, L.; Wu, X.; Wu, H. Novel Antioxidant Peptides Purified from Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates with Hemolysis Inhibition Ability and Cellular Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef]
- Chai, T.T.; Tong, S.R.; Law, Y.C.; Ismail, N.I.M.; Manan, F.A.; Wong, F.C. Anti-Oxidative, metal chelating and radical scavenging effects of protein hydrolysates from blue-spotted stingray. Trop. J. Pharm. Res. 2015, 14, 1349–1355. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Q.; Peng, Q.; Yu, X.; Yin, X.; Liang, M.; Ma, C.W.; Huang, Z.; Jia, W. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef]
- Shen, J.; Chen, J.; Yang, D.; Zhao, Z.; Tang, C.; Zhang, R.; Yang, W.; Niu, Y. Antidiarrheal effects of a thermostable protein fraction obtained from the larvae of Musca domestica. Biomed. Pharmacother. 2019, 115, 108813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, P.; Qin, Q.; Zhang, H.; Wu, Y. Protective effect of polypeptides from larva of housefly (Musca domestica) on hydrogen peroxide-induced oxidative damage in HepG2 cells. Food Chem. Toxicol. 2013, 60, 385–390. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, X.; Jiao, M.; Anoopkumar-Dukie, S.; Zeng, Y.; Mei, H. Housefly (Musca domestica) larvae powder, preventing oxidative stress injury via regulation of UCP4 and CyclinD1 and modulation of JNK and P38 signaling in APP/PS1 mice. Food Funct. 2019, 10, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yang, W.; Zhang, S.; Zhao, Z.; He, G.; Yang, D. Composition analysis of Musca Domestica pupa and preparation of antioxidant peptides. Feed Ind. 2019, 40, 30–37. [Google Scholar]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Ong, M.G.L.; Pang, M.J.; Wong, S.J.; Teh, L.K.; Chai, T.T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.S.; Ahn, C.B.; Je, J.Y. Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J. Funct. Foods 2016, 20, 88–95. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Kimata, J.; Shigeri, Y.; Yoshida, Y.; Niki, E.; Kinumi, T. Artificial oxidation of cysteine residues in peroxiredoxin 6 detected by two-dimensional gel electrophoresis and capillary liquid chromatography-electrospray mass spectrometry. Mass Spectrom. Lett. 2012, 3, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Sierra, H.; Cordova, M.; Chen, C.S.J.; Rajadhyaksha, M. Confocal imaging-guided laser ablation of basal cell carcinomas: An ex vivo study. J. Investig. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, C.W.; Cheng, N.L.; Chang, W.M.; Tseng, T.L.; Lai, Y.K. Transactivation of hsp70-1/2 in geldanamycin-treated human non-small cell lung cancer H460 cells: Involvement of intracellular calcium and protein kinase C. J. Cell. Biochem. 2005, 94, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, B.; Kuhla, S.; Rudolph, P.E.; Albrecht, D.; Metges, C.C. Proteomics analysis of hypothalamic response to energy restriction in dairy cows. Proteomics 2007, 7, 3602–3617. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J. Research Progress in Structure-Activity Relationship of Bioactive Peptides. J. Med. Food 2014, 18, 147–156. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Lavrovsky, Y.; Chatterjee, B.; Clark, R.A.; Roy, A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000, 35, 521–532. [Google Scholar] [CrossRef]
- Nielsen, P.M. Improved Method for Determining Protein Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Zhou, J.; Kong, L.; Fang, N.; Mao, B.; Ai, H. Synthesis and functional characterization of MAF-1A peptide derived from the larvae of housefly, musca domestica (Diptera: Muscidae). J. Med. Entomol. 2016, 53, 1467–1472. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Kishimura, H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one- and two-step hydrolysis. J. Food Sci. Technol. 2015, 52, 3336–3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.Z.; Zhang, W.G.; Kang, Z.L.; Zhou, G.H.; Xu, X.L. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014, 96, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Zhang, S.; Yang, W.; Zhao, Z.; Yang, D. Housefly Pupae-Derived Antioxidant Peptides Exerting Neuroprotective Effects on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells. Molecules 2019, 24, 4486. https://doi.org/10.3390/molecules24244486
Sun T, Zhang S, Yang W, Zhao Z, Yang D. Housefly Pupae-Derived Antioxidant Peptides Exerting Neuroprotective Effects on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells. Molecules. 2019; 24(24):4486. https://doi.org/10.3390/molecules24244486
Chicago/Turabian StyleSun, Tingting, Sichen Zhang, Wenzhe Yang, Zhimin Zhao, and Depo Yang. 2019. "Housefly Pupae-Derived Antioxidant Peptides Exerting Neuroprotective Effects on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells" Molecules 24, no. 24: 4486. https://doi.org/10.3390/molecules24244486
APA StyleSun, T., Zhang, S., Yang, W., Zhao, Z., & Yang, D. (2019). Housefly Pupae-Derived Antioxidant Peptides Exerting Neuroprotective Effects on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells. Molecules, 24(24), 4486. https://doi.org/10.3390/molecules24244486




