Soil Behaviour of the Veterinary Drugs Lincomycin, Monensin, and Roxarsone and Their Toxicity on Environmental Organisms
Abstract
:1. Introduction
2. Results & Discussion
2.1. Adsorption-Desorption Test
2.2. Soil Mobility Test
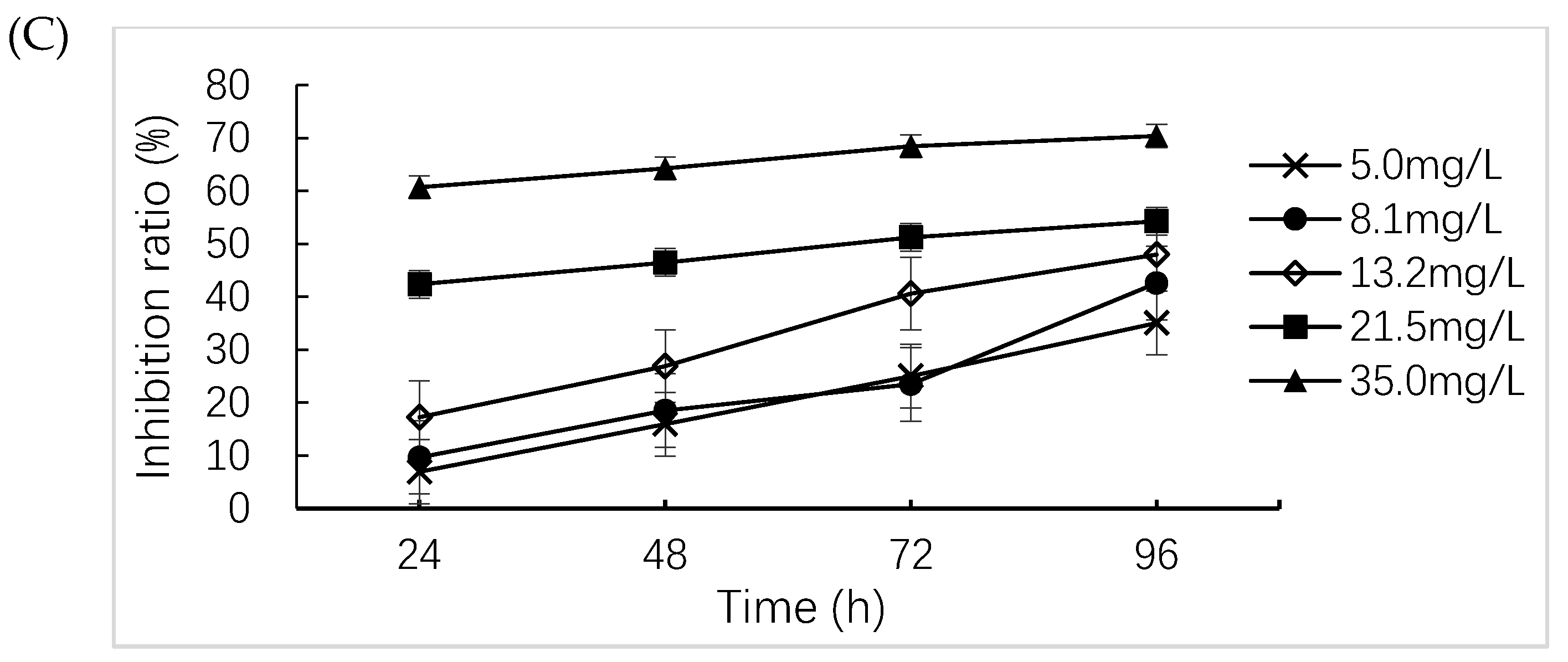
2.3. Algae Growth Inhibition Test
2.4. Plant Sensitivity Test
2.4.1. Lincomycin
2.4.2. Monensin
2.4.3. Roxarsone
2.5. Daphnia Activity Inhibition Test
2.6. Acute Toxicity Tests of Zebrafish
2.7. Acute Toxicity Tests of Earthworm
2.8. Acute Toxicity Test on French Giant Quail
2.9. Summary of Results
3. Materials and Methods
3.1. Chemicals, Test Soils and Organisms
3.2. Adsorption-Desorption and Soil Mobility
3.2.1. Adsorption-Desorption Test
3.2.2. Soil Mobility Test
3.2.3. Soil Drug Extraction
3.2.4. Drug Detection
Lincomycin
Monensin
Roxarsone
3.3. Ecotoxicology Tests
3.3.1. Algae Growth Inhibition Test
3.3.2. Plant Sensitivity Test
3.3.3. Growth Inhibition Test of Daphnia Activity
3.3.4. Acute Toxicity Test of Zebrafish
3.3.5. Acute Toxicity Test of Earthworms
3.3.6. Acute Toxicity Test of Quails
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Mellon, M.; Benbrook, C.; Benbrook, L.K. Hogging it: Estimates of Antimicrobial Abuse in Livestock; Union of Concerned Scientists: Cambridge, MA, USA, 2001. [Google Scholar]
- Kong, W.D.; Zhu, Y.G.; Liang, Y.C. Uptake of oxytetracycline and its phytotoxicity to alfalfa (Medicago sativa L.). Environ. Pollut. 2007, 147, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total. Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.; Dominguez, C.; Perez-Babace, L. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Chen, C.M.; Wei, J.T. Analysis of veterinary drug residue monitoring results for commercial livestock products in Taiwan between 2011 and 2015. J. Food Drug Anal. 2018, 26, 565–571. [Google Scholar] [CrossRef]
- Aus, D.B.T.; Weber, F.-A.; Bergmann, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar]
- Wei, R.; Ge, F.; Zhang, L. Occurrence of 13 veterinary drugs in animal manure-amended soils in Eastern China. Chemosphere 2016, 144, 2377–2383. [Google Scholar] [CrossRef]
- Paltiel, O.; Fedorova, G.; Tadmor, G. Human exposure to wastewater-derived pharmaceuticals in fresh produce: A randomized controlled trial focusing on carbamazepine. Environ. Sci. Technol. 2016, 50, 4476–4482. [Google Scholar] [CrossRef]
- Chen, W.R.; Ding, Y.; Johnston, C.T. Reaction of lincosamide antibiotics with manganese oxide in aqueous solution. Environ. Sci. Technol. 2010, 44, 4486–4492. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Wang, J. Degradation of lincomycin in aqueous solution with hydrothermal treatment: Kinetics, pathway, and toxicity evaluation. Chem. Eng. J. 2018, 343, 138–145. [Google Scholar] [CrossRef]
- Jwad, S.M.; Abbas, B.; Jaffat, H.S. Study of the protective effect of vitamin C plus E on lincomycin-induced hepatotoxicity and nephrotoxicity. Res. J. Pharm. Technol. 2015, 8, 177. [Google Scholar] [CrossRef]
- Migliore, L.; Civitareale, C.; Brambilla, G. Toxicity of several important agricultural antibiotics to Artemia. Water Res. 1997, 31, 1801–1806. [Google Scholar] [CrossRef]
- Hagenbuch, I.M.; Pinckney, J.L. Toxic effect of the combined antibiotics ciprofloxacin, lincomycin, and tylosin on two species of marine diatoms. Water Res. 2012, 46, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.; Flores, C.; Caixach, J. Evaluation of antibiotic mobility in soil associated with swine-slurry soil amendment under cropping conditions. Environ. Sci. Pollut. Res. 2014, 21, 12336–12344. [Google Scholar] [CrossRef] [PubMed]
- Arikan, O.A.; Mulbry, W.; Rice, C. The fate and effect of monensin during anaerobic digestion of dairy manure under mesophilic conditions. PLoS ONE. 2018, 13, e0192080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizek, S.; Hrzenjak, R.; Kalcher, G.T. Does monensin in chicken manure from poultry farms pose a threat to soil invertebrates? Chemosphere 2011, 83, 517–523. [Google Scholar] [CrossRef]
- Storteboom, H.N.; Kim, S.C.; Doesken, K.C. Response of antibiotics and resistance genes to high-intensity and low-intensity manure management. J. Environ. Qual. 2007, 36, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Sassman, S.A.; Lee, L.S. Sorption and degradation in soils of veterinary ionophore antibiotics: Monensin and lasalocid. Environ. Toxicol. Chem. 2010, 26, 1614–1621. [Google Scholar] [CrossRef]
- Wang, Y. Study on the Ecotoxicological Effects of Monensin on Earthworms. Master’s Thesis, Hainan University, Hainan, China, 2010. [Google Scholar]
- Capleton, A.C.; Courage, C.; Rumsby, P. Prioritising veterinary medicines according to their potential indirect human exposure and toxicity profile. Toxicol. Lett. 2006, 163, 213–223. [Google Scholar] [CrossRef]
- Garbarino, J.R.; Bednar, A.J.; Rutherford, D.W. Environmental fate of roxarsone in poultry litter. Part, I. Degradation of roxarsone during composting. Environ. Sci. Technol. 2003, 37, 1509–1514. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, M.; Thunders, M. Effect of enrofloxacin and roxarsone on CYP450s in pig. Res. Vet. Sci. 2018, 117, 97–98. [Google Scholar] [CrossRef]
- Zhang, Y.M. Ecotoxicological Study on the Residue of Roxarsone. Master’s Thesis, Yangzhou University, Yangzhou, China, 2007. [Google Scholar]
- Makris, K.C.; Salazar, J.; Quazi, S. Controlling the fate of roxarsone and inorganic arsenic in poultry litter. J. Environ. Qual. 2008, 37, 963–971. [Google Scholar] [CrossRef]
- Brown, B.L.; Slaughter, A.D.; Schreiber, M.E. Controls on roxarsone transport in agricultural watersheds. Appl. Geochem. 2005, 20, 123–133. [Google Scholar] [CrossRef]
- Zhang, W. A study on Ecological Toxicity and Environmental Behavior of Ethanamizuril. Master’s Thesis, Shanghai Jiaotong University, Shanghai, China, 2018. [Google Scholar]
- Rutherford, D.W.; Bednar, A.J.; Garbarino, J.R. Environmental fate of roxarsone in poultry liner. Part II. Mobility of arsenic in soils amended with chicken manure. Environ. Sci. Technol. 2003, 37, 1515–1520. [Google Scholar] [CrossRef]
- Jiang, C.A.; Zhai, X.F.; Wang, Y. Adsorption of ascorbic acid and roxarsone additives in different soils. J. South. China Agric. Univ. 2013, 34, 272–276. [Google Scholar]
- National Standards of People’s Republic of China. In GB/T31270-2014 (Test Guidelines on Environmental Safety Assessment for Chemical Pesticides); Standardization Administration of the People’s Republic of China: Beijing, China, 2014.
- Petrovic, A.M.; Larsson-Kovach, I. Effect of maturing turfgrass soils on the leaching of the herbicide mecoprop. Chemosphere 1996, 33, 585–593. [Google Scholar] [CrossRef]
- Fiolka, M.J. Activity and immunodetection of lysozyme in earthworm Dendrobaena veneta (Annelida). J. Invertebr. Pathol. 2012, 109, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Srivastava, G. Adsorption and movement of carbofuran in four different soils varying in physical and chemical properties. Adsorpt. Sci. Technol. 2009, 27, 193–203. [Google Scholar] [CrossRef]
- Duan, Y.; Bao, N.; Li, J. Fate characteristics of florasulam in soil environment. Guizhou Agric. Sci. 2018, 46, 66–70. [Google Scholar]
- Zhang, X.H.; Wan, T.; Cheng, W. Effects of quinolones and sulfonamides on the growth of green algae. J. Water Resour. Water Engin. 2018, 29, 115–120. [Google Scholar]
- Peng, Q.C.; Song, J.M.; Li, X.G. Biogeochemical characteristics and ecological risk assessment of pharmaceutically active compounds (PhACs) in the surface seawaters of Jiaozhou Bay, North China. Environ. Pollut. 2001, 255, 113247. [Google Scholar] [CrossRef]
- Bergmann, T. Synergy of light and nutrients on the photosynthetic efficiency of phytoplankton populations from the Neuse River Estuary, North Carolina. J. Plankton Res. 2002, 24, 923–933. [Google Scholar] [CrossRef]
- Hoagand, R.E. Herbicidal properties of the antibiotic monensin. J. Sci.Food Agric. 1996, 70, 373–379. [Google Scholar] [CrossRef]
- National standards of People’s Republic of China. In GB/T 27861-2011 (Chemicals-Fish Acute Toxicity Test); Standardization Administration of the People’s Republic of China: Beijing, China, 2011.
- Liu, P.; Wang, M.Z.; Zhong, W.X. Stress-responsive genes (hsp70 and mt) and genotoxicity elicited by roxarsone exposure in Carassius auratus. Environ. Toxicol. Pharmacol. 2018, 62, 132–139. [Google Scholar]
- Cai, M.T.; Hou, G.Q.; Xi, H. Combined toxicity of co-exposure of typical antibiotic and heavy metal copper on freshwater green algae and zebrafish. J. Zhejiang Shuren Univ. 2018, 18, 11–15. [Google Scholar]
- Wenshyg, C.; Kuolung, C.; Bi, Y. Effects of roxarsone on performance, toxicity, tissue accumulation and residue of eggs and excreta in laying hens. J. Sci. Food Agric. 1997, 74, 229–236. [Google Scholar]
- Otvos, L., Jr. Antibacterial peptides and proteins with multiple cellular targets. J. Pept Sci. 2005, 11, 697–706. [Google Scholar] [CrossRef]
- Wang, F.M.; Chen, Z.L.; Zhang, L. Arsenic uptake and accumulation in rice (Oryza sativa L.) at different growth stages following soil incorporation of roxarsone and arsanilic acid. Plant. Soil. 2006, 285, 359–367. [Google Scholar] [CrossRef]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563–564, 366–376. [Google Scholar] [CrossRef]
- National standards of People’s Republic of China. In GB/T 21851-2008 (Chemicals-Adsorption-desorption Using a Batch Equilibrium Method), GB/T 21805-2008 (Chemicals-Alga Growth Inhibition Test), GB/T 21830-2008 (Chemicals-Daphnia sp., Acute Immobilisation Test); Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- OECD Guidelines for the Testing of Chemicals, No.207 “Earthworm, Acute Toxicity Tests”; Organisation for Economic Cooperation and Development: Paris, France, 1984.
- Helling, C.S.; Turner, B.C. Pesticide mobility: Determination by soil thin-layer chromatography. Science 1968, 162, 562. [Google Scholar] [CrossRef]
- Yan, J. Determination of the content of lincomycin hydrochloride injection by spectrophotometry. Shanghai Med. Pharm. J. 1994, 12, 33–34. [Google Scholar]
- Zeng, Z.G.; Liu, B.; Chen, Y.H. Determination of monensin by spectrophotometry. Chin. J. Vet. Drug 2007, 41, 19–21. [Google Scholar]
- Zhang, F.F.; Wang, W.; Yuan, S.J. Biodegradation and speciation of roxarsone in an anaerobic granular sludge system and its impacts. J. Hazard. Mater. 2014, 279, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.R.; Qian, Q.M.; Sun, C. Comparative study on the toxic effects of lindane and chlorpyrifos on freshwater algae. Ecol. Environ. 2014, 3, 472–476. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Drug | Soil | Initial Drug Concentration (mg/L) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Lincomycin | Laterite soil | 66.67 | 30.56 | 35.19 | 19.44 | 0 |
| Podzol soil | 97.22 | 54.17 | 54.63 | 0 | 0 | |
| Black soil | 100.0 | 100.0 | 86.11 | 16.67 | 1.111 | |
| Monensin | Laterite soil | 56.16 | 93.68 | 55.26 | 90.92 | 93.66 |
| Podzol soil | 100.0 | 92.60 | 87.50 | 89.99 | 88.25 | |
| Black soil | 100.0 | 93.21 | 94.12 | 95.19 | 98.78 | |
| Roxarsone | Laterite soil | 0 | 0 | 0 | 0 | 0 |
| Podzol soil | 0 | 0.8080 | 0 | 0 | 0 | |
| Black soil | 18.80 | 38.18 | 97.41 | 51.95 | 42.00 | |
| Drug | Soil | Lg Kd | 1/n | R2 |
|---|---|---|---|---|
| Lincomycin | Podzol soil | 1.5672 | 0.1116 | 0.2043 |
| Laterite soil | 1.8235 | 0.0935 | 0.5282 | |
| Black soil | 1.7091 | −1.2543 | 0.7329 | |
| Monensin | Podzol soil | 1.5194 | −0.1842 | 0.0355 |
| Laterite soil | 2.7601 | 0.6117 | 0.8910 | |
| Black soil | 1.8502 | −0.3915 | 0.2411 | |
| Roxarsone | Black soil | 1.2855 | 1.9393 | 0.8759 |
| Drug | Soil | Drug Content in Each Moving Distance (%) | Rf | |||||
|---|---|---|---|---|---|---|---|---|
| 0–3 cm | 3–6 cm | 6–9 cm | 9–12 cm | 12–15 cm | 15–18 cm | |||
| Lincomycin | Laterite soil | 9.250 | 27.74 | 19.99 | 10.54 | 12.09 | 20.40 | 0.4995 |
| Black soil | 16.11 | 15.63 | 15.01 | 14.66 | 17.07 | 21.51 | 0.5258 | |
| Monensin | Laterite soil | 8.154 | 0 | 0 | 4.154 | 0 | 87.69 | 0.8348 |
| Black soil | 0 | 0 | 0 | 0 | 68.02 | 31.98 | 0.8033 | |
| Roxarsone | Laterite soil | 17.51 | 20.93 | 17.96 | 20.98 | 13.31 | 9.310 | 0.4493 |
| Black soil | 12.45 | 12.99 | 10.85 | 13.10 | 26.86 | 23.74 | 0.5835 | |
| Monensin | Concentration (mg/L) | 25 | 50 | 75 | 100 | 125 | 0 | Methanol control | |
| Plants Emerged | 7 d | 0.3 ± 0.6 1 | 0 | 0 | 0 | 0 | 8 | 6 | |
| 14 d | 0.3 ± 0.6 | 0.3 ± 0.6 | 1.0 ± 1.7 | 0.3 ± 0.6 | 0 | 10 | 5 | ||
| Lincomycin | Concentration (mg/L) | 6 | 12 | 18 | 24 | 30 | 0 | Methanol control | |
| Plants Emerged | 7 d | 9.0 ± 1.0 | 8.0 ± 2.0 | 6.7 ± 2.1 | 6.7 ± 1.5 | 7.3 ± 0.6 | 8 | / | |
| 14 d | 7.7 ± 2.1 | 8.0 ± 2.6 | 5.3 ± 3.5 | 6.3 ± 0.6 | 7 ± 1.0 | 10 | / | ||
| Roxarsone | Concentration (mg/L) | 5 | 25 | 45 | 65 | 80 | 0 | Methanol control | |
| Plants Emerged | 7 d | 9.3 ± 0.6 | 8.0 ± 1.7 | 9.7 ± 0.6 | 9.0 ± 1.0 | 6.3 ± 0.6 | 8 | / | |
| 14 d | 6.0 ± 3.0 * | 4.3 ± 1.5 * | 3.3 ± 2.3 * | 2.7 ± 2.1 * | 1.7 ± 0.6 * | 10 | / | ||
| Lincomycin | Concentration (mg/L) | 6 | 12 | 18 | 24 | 30 | 0 |
| Total Gross Dry Weight (g) | 0.0033 ± 0.0008 | 0.0046 ± 0.0012 | 0.0040 ± 0.0030 | 0.0031 ± 0.0011 | 0.0074 ± 0.0007 | 0.0045 | |
| Max. Plant Height (cm) | 2.5 ± 0.5 | 2.7 ± 1.0 | 3.0 ± 2.8 | 2.9 ± 1.4 | 4.2 ± 1.3 | 3.6 | |
| Roxarsone | Concentration (mg/L) | 5 | 25 | 45 | 65 | 80 | 0 |
| Total Gross Dry Weight (g) | 0.0038 ± 0.0045 | 0.0010 ± 0.0005 | 0.0011 ± 0.0012 | 0.0019 ± 0.0005 | 0.0005 ± 0.0004 | 0.0045 | |
| Max. Plant Height (cm) | 1.7 ± 0.3 | 0. 8± 0.3 | 1.0 ± 0.5 | 1.8 ± 1.0 | 0.4 ± 0.1 | 3.6 |
| Monensin | Concentration (mg/L) | 9.5 | 14.7 | 20.0 | 22.7 | 35.0 | 0 |
| Daphnia inhibited | 0 | 1.0 ± 0 | 2.3 ± 0.6 | 4.0 ± 1.0 | 8.0 ± 0 | 0 | |
| Roxarsone | Concentration (mg/L) | 250.0 | 268.9 | 289.3 | 311.1 | 334.7 | 0 |
| Daphnia inhibited | 0 | 1.3 ± 0.6 | 3.3 ± 0.6 | 5.7 ± 0.6 | 8.0 ± 0 | 0 |
| Drug Concentration (mg/L) | 0 | 250 | 300 | 350 | 400 | 450 |
| Mortality | 0 | 0 | 2 | 4 | 6 | 7 |
| Monensin | Concentration (mg/L) | 4 | 4.34 | 4.7 | 5.1 | 5.53 | 0 |
| Mortality | 0 | 1 | 4 | 6 | 8 | 0 | |
| Roxarsone | Concentration (mg/L) | 400 | 423 | 447 | 473 | 500 | 0 |
| Mortality | 0 | 1 | 3 | 6 | 8 | 0 |
| Monensin | Dose (g/kg Dry Soil) | CK 1 | 200 | 258 | 332 | 427 | 550 | |
| Average Mortality | 7 d | 0 | 0 | 0 | 1 | 3 | 5 | |
| 14 d | 0 | 0 | 2 | 4 | 7 | 10 | ||
| Roxarsone | Dose (g/kg Dry Soil) | CK | 4 | 5.03 | 6.33 | 7.95 | 10 | |
| Average Mortality | 7 d | 0 | 0 | 0 | 2 | 5 | 10 | |
| 14 d | 0 | 0 | 3 | 6 | 10 | 10 | ||
| Monensin | Drug Dose (mg/kg) | 0 | 80 | 400 | 800 | 1100 | 1500 | |
| Mortality | Male | 0 | 0 | 1 | 3 | 3 | 5 | |
| Female | 0 | 0 | 2 | 2 | 3 | 4 | ||
| Roxarsone | Drug Dose (mg/kg) | 0 | 30 | 52.7 | 100 | 162.3 | 284.8 | |
| Mortality | Male | 0 | 0 | 1 | 2 | 4 | 5 | |
| Female | 0 | 0 | 1 | 2 | 3 | 5 | ||
| Species | Roxarsone | Monensin | Lincomycin | |||
|---|---|---|---|---|---|---|
| Data | Category | Data | Category | Data | Category | |
| S. obliquus EC50 (96 h) mg/L | 13.15 | low | 1.085 | medium | 0.813 | medium |
| A. thaliana EC50 (14 d) mg/kg dry soil | 32.18 | low | <25 | / | 35.40 | low |
| E. fetida LC50 (14 d) g/kg dry soil | 5.74 | low | 0.346 | low | >15 | low |
| D. rerio LC50 (96 h) mg/L | 452.7 | low | 4.76 | medium | >2800 | low |
| D. magna EC50 (48 h) mg/L | 292.6 | low | 21.1 | low | >400 | low |
| C. coturnix LD50 (7 d) mg/kg | 103.9 | medium | 672.8 | low | >2000 | low |
| Test Soil | pH | Organic Matter (g/kg) | Electrical Conductance (μS/cm) | Total P (g/kg) | NH4+-N (mg/kg) | Total K (g/kg) |
|---|---|---|---|---|---|---|
| Podzol soil | 6.876 | 26.00 | 180.3 | 0.958 | 12.50 | 2.53 |
| Laterite soil | 6.710 | 3.62 | 58.5 | 0.070 | 7.13 | 11.40 |
| Black soil | 5.257 | 263.69 | 605.5 | 1.980 | 19.74 | 6.97 |
| Soil | Lincomycin | Monensin | Roxarsone | |||
|---|---|---|---|---|---|---|
| Ratio | Time (h) | Ratio | Time (h) | Ratio | Time (h) | |
| Podzol soil | 50:1 | 24 | 100:1 | 48 | 50:1 | 24 |
| Laterite soil | 50:1 | 24 | 20:1 | 24 | 50:1 | 48 |
| Black soil | 50:1 | 24 | 50:1 | 24 | 50:1 | 48 |
| Drug | Podzol Soil | Laterite Soil | Black Soil | |||
|---|---|---|---|---|---|---|
| Recovery | RSD | Recovery | RSD | Recovery | RSD | |
| Lincomycin | 73.2–97.4 | 0.801–3.10 | 71.7–108 | 1.21–4.83 | 78.1–98.6 | 0.344–2.12 |
| Monensin | 84.6–91.8 | 1.98–4.62 | 81.0–111 | 0.620–4.62 | 90.3–106 | 1.72–5.10 |
| Roxarsone | 89.9–92.6 | 1.15–3.18 | 91.2–99.3 | 1.34–1.69 | 79.4–96.7 | 1.36–3.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wu, Y.; Wang, Y.; Qiu, J.; Li, Y. Soil Behaviour of the Veterinary Drugs Lincomycin, Monensin, and Roxarsone and Their Toxicity on Environmental Organisms. Molecules 2019, 24, 4465. https://doi.org/10.3390/molecules24244465
Li P, Wu Y, Wang Y, Qiu J, Li Y. Soil Behaviour of the Veterinary Drugs Lincomycin, Monensin, and Roxarsone and Their Toxicity on Environmental Organisms. Molecules. 2019; 24(24):4465. https://doi.org/10.3390/molecules24244465
Chicago/Turabian StyleLi, Peiyi, Yizhao Wu, Yali Wang, Jiangping Qiu, and Yinsheng Li. 2019. "Soil Behaviour of the Veterinary Drugs Lincomycin, Monensin, and Roxarsone and Their Toxicity on Environmental Organisms" Molecules 24, no. 24: 4465. https://doi.org/10.3390/molecules24244465






