Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Stability of Isomorphous Substituted SOD
2.1.1. Distribution of Al Atoms in SOD
2.1.2. Structural Stability of Al-Substituted SOD
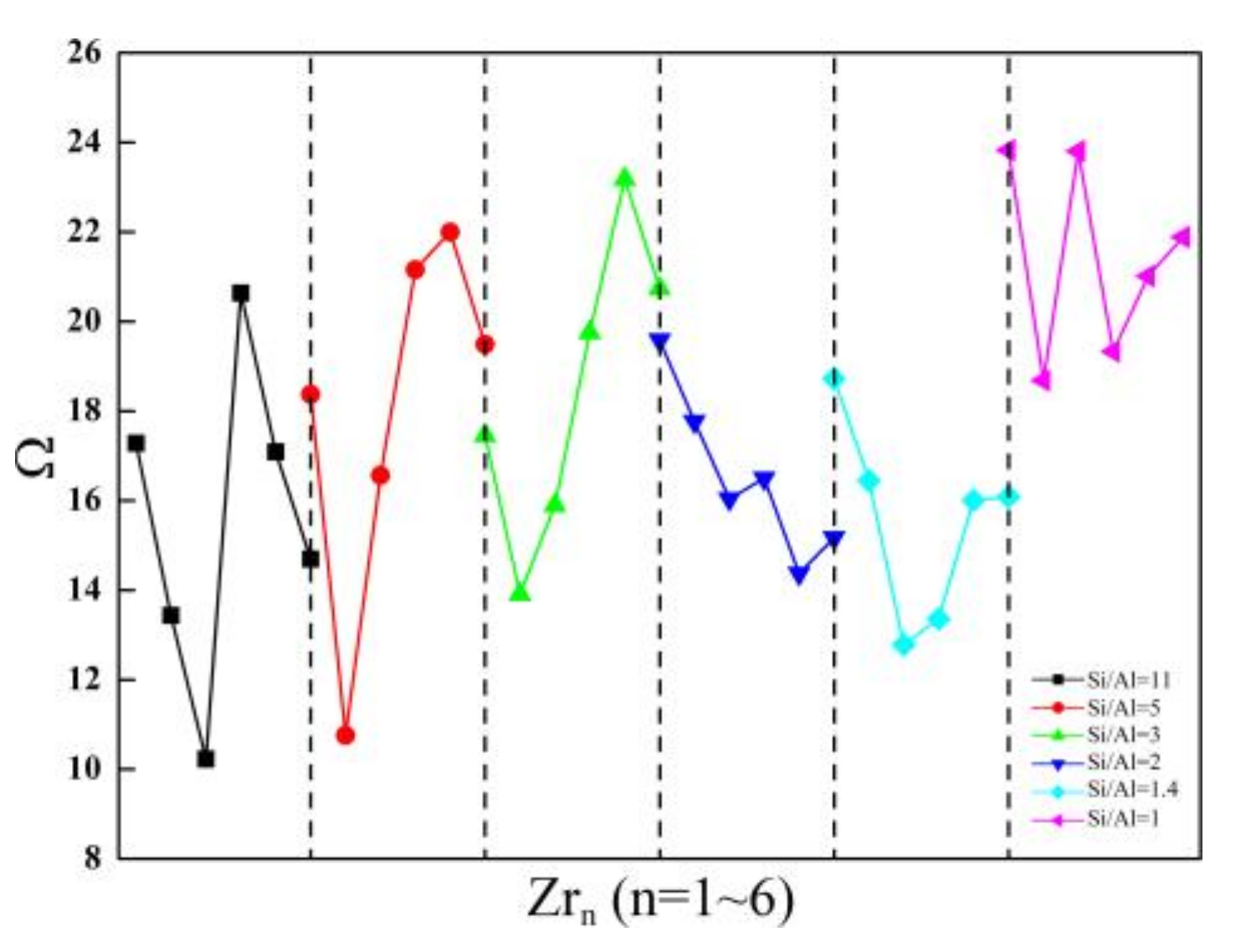
2.2. Distribution of Zr and Structural Stability of Zr-Substituted SOD
2.2.1. Stable Location of Zr atoms in SOD Framework of Si/Al Equals Eleven
2.2.2. Structural Stability of Zr-Substituted SOD
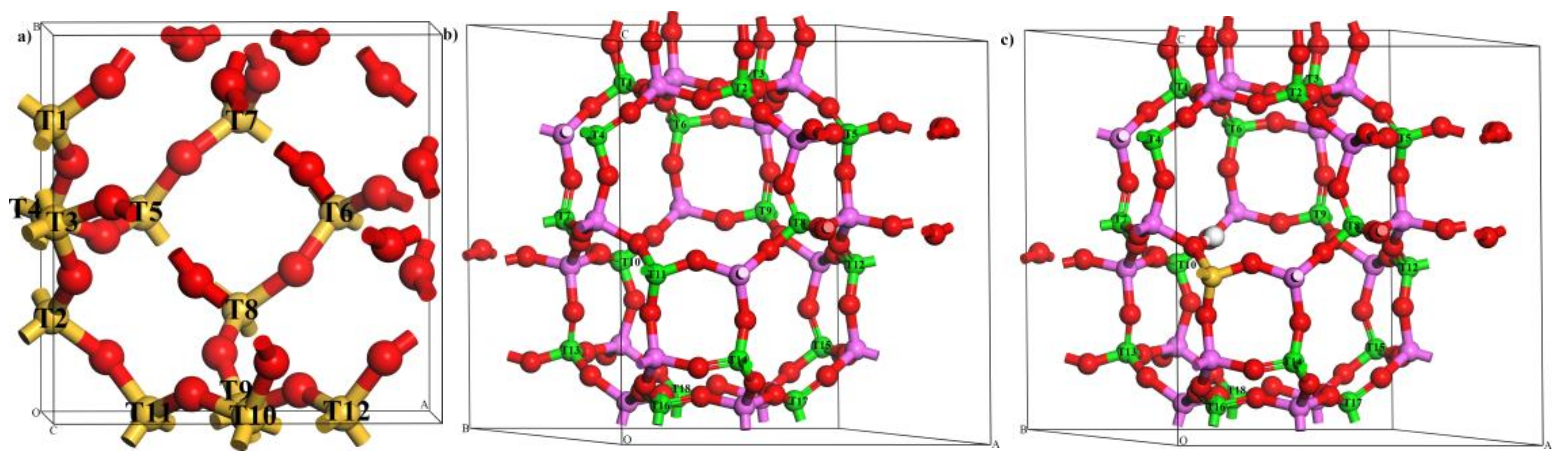
2.3. Distribution of Zr and Structural Stability of Zr-Substituted AlPO-34 and SAPO-34
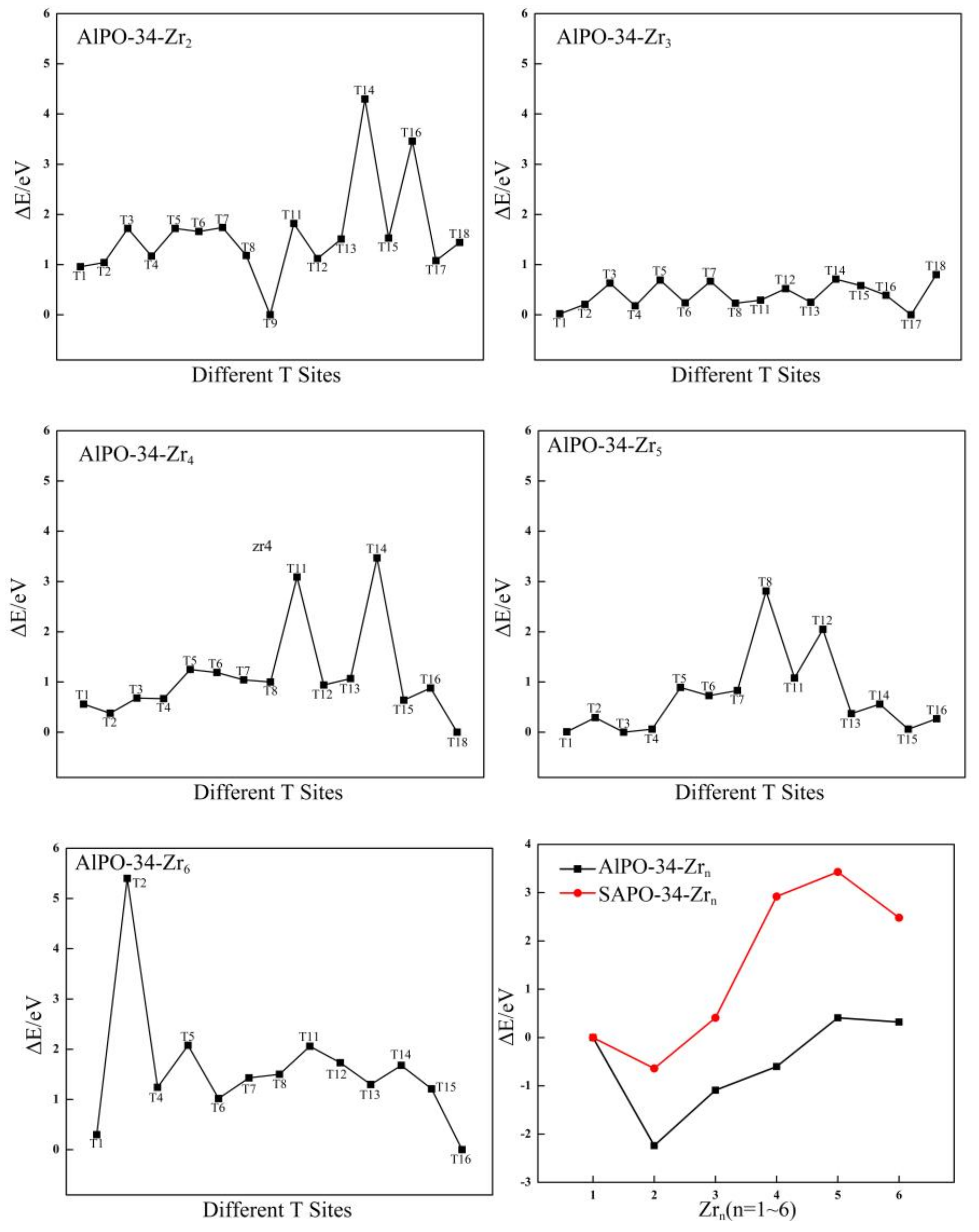
2.3.1. Stable Location of Zr Atoms in AlPO-34 Framework
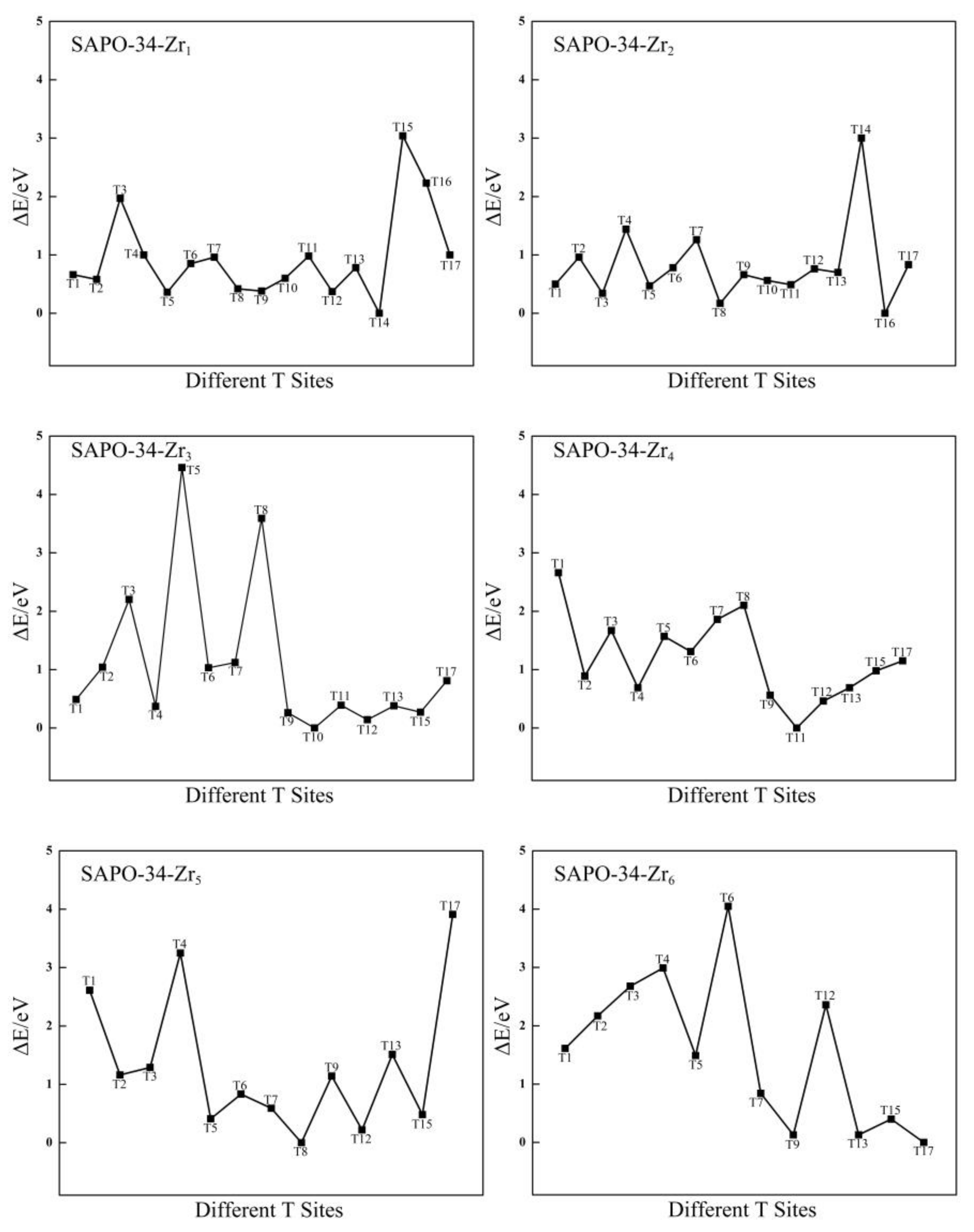
2.3.2. Stable Location of Zr Atoms in SAPO-34 Framework
2.3.3. Structural Stability of Zr-Substituted AlPO-34 and SAPO-34
2.4. Structural Distortion of the Most Stable Zr-Substituted Frameworks
2.4.1. Structural Distortion of the Most Stable Zr-Substituted SOD Framework
2.4.2. Structural Distortion of the Most Stable Zr-Substituted AlPO-34 and SAPO-34 Frameworks
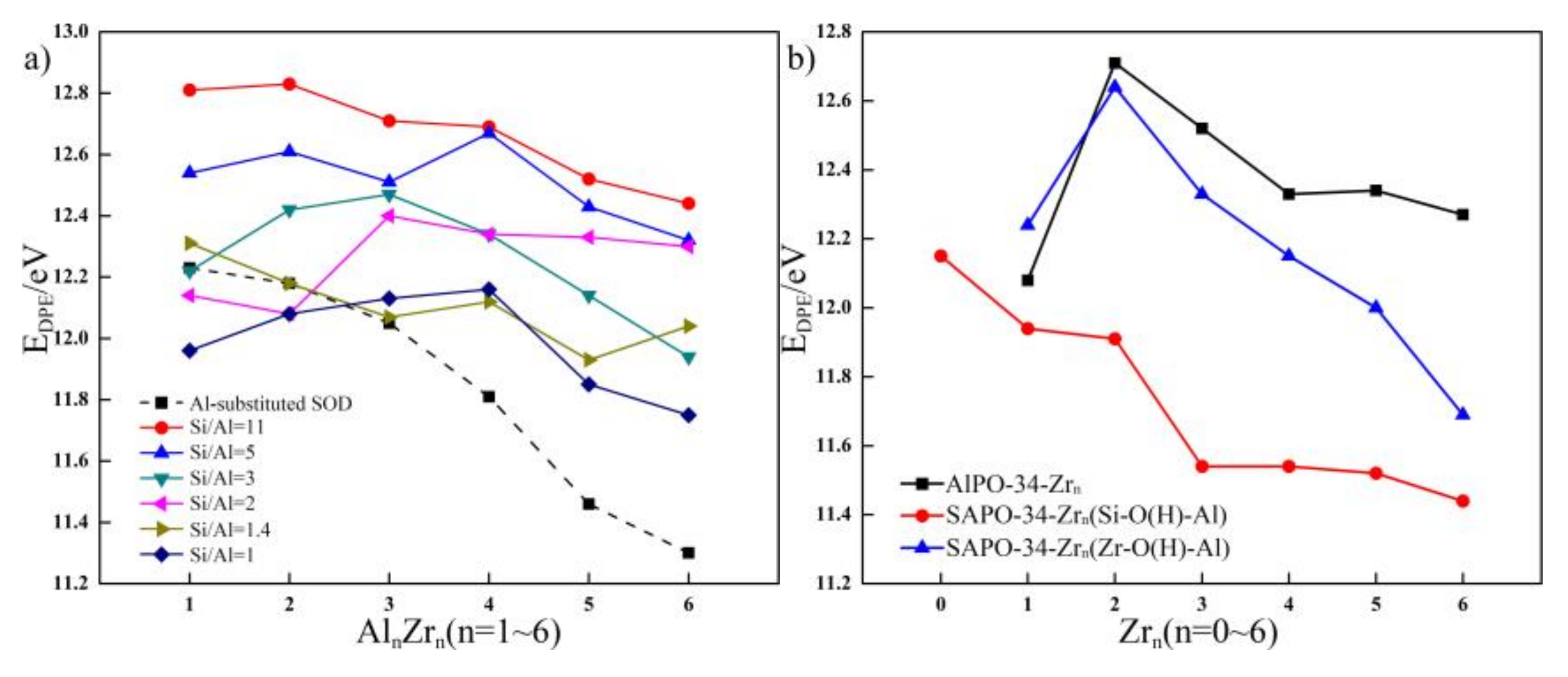
2.5. Brönsted Acidity of the Most Stable Zr-Substituted SOD, AlPO-34, and SAPO-34
2.5.1. Brönsted Acidity of the Zr-Substituted SOD with Si/Al Ratio from Eleven to One
2.5.2. Brönsted Acidity of the Zr-Substituted AlPO-34 and SAPO-34
3. Computational Details
3.1. Model
3.2. Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Structure, Stability, and Lewis Acidity of Mono and Double Ti, Zr, and Sn Framework Substitutions in BEA Zeolites: A Periodic Density Functional Theory Study. J. Phys. Chem. C 2013, 117, 3976–3986. [Google Scholar] [CrossRef]
- Zones, S.I. Translating new materials discoveries in zeolite research to commercial manufacture. Micropor. Mesopor. Mater. 2011, 144, 1–8. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, D.; Xu, L.; Chang, F.; He, Y.; Meng, S.; Su, B.-l.; Liu, Z. Synthesis, characterization and catalytic performance of metal-incorporated SAPO-34 for chloromethane transformation to light olefins. Catal. Today 2008, 131, 262–269. [Google Scholar] [CrossRef]
- Dubois, D.R.; Obrzut, D.L.; Liu, J.; Thundimadathil, J.; Adekkanattu, P.M.; Guin, J.A.; Punnoose, A.; Seehra, M.S. Conversion of methanol to olefins over cobalt-, manganese- and nickel-incorporated SAPO-34 molecular sieves. Fuel Process. Technol. 2003, 83, 203–218. [Google Scholar] [CrossRef]
- Van Niekerk, M.J.; Fletcher, J.C.Q.; O’Connor, C.T. Effect of catalyst modification on the conversion of methanol to light olefins over SAPO-34. Appl. Catal. A 1996, 138, 135–145. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, L.; Feng, G.; Wang, X.; Zhang, R.; Liu, J. Trivalent ions modification for high-silica mordenite: A first principles study. Appl. Surf. Sci. 2018, 433, 627–638. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Lin, M.; Peng, X.; Zhu, B.; Shu, X. Confirmation of the isomorphous substitution by Sn atoms in the framework positions of MFI-typed zeolite. Catal. Today 2018, 316, 193–198. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Jiao, W.; Wang, J.; Fan, W. Recent experimental and theoretical studies on Al siting/acid site distribution in zeolite framework. Curr. Opin. Chem. Eng. 2019, 23, 146–154. [Google Scholar] [CrossRef]
- Losch, P.; Joshi, H.R.; Vozniuk, O.; Grünert, A.; Ochoa-Hernández, C.; Jabraoui, H.; Badawi, M.; Schmidt, W. Proton Mobility, Intrinsic Acid Strength, and Acid Site Location in Zeolites Revealed by Varying Temperature Infrared Spectroscopy and Density Functional Theory Studies. J. Am. Chem. Soc. 2018, 140, 17790–17799. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef]
- Sedighi, M.; Ghasemi, M.; Sadeqzadeh, M.; Hadi, M. Thorough study of the effect of metal-incorporated SAPO-34 molecular sieves on catalytic performances in MTO process. Powder Technol. 2016, 291, 131–139. [Google Scholar] [CrossRef]
- Sena, F.C.; de Souza, B.F.; de Almeida, N.C.; Cardoso, J.S.; Fernandes, L.D. Influence of framework composition over SAPO-34 and MeAPSO-34 acidity. Appl. Catal. A 2011, 406, 59–62. [Google Scholar] [CrossRef]
- Yuan, S.P.; Wang, J.G.; Li, Y.W.; Jiao, H. Brønsted Acidity of Isomorphously Substituted ZSM-5 by B, Al, Ga, and Fe. Density Functional Investigations. J. Phys. Chem. A 2002, 106, 8167–8172. [Google Scholar] [CrossRef]
- Tielens, F.; Shishido, T.; Dzwigaj, S. What Do Tantalum Framework Sites Look Like in Zeolites? A Combined Theoretical and Experimental Investigation. J. Phys. Chem. C 2010, 114, 9923–9930. [Google Scholar] [CrossRef]
- Ko, Y.S.; Ahn, W.S. Synthesis and characterization of tantalum silicalite molecular sieves with MFI structure. Micropor. Mesopor. Mater. 1999, 30, 283–291. [Google Scholar] [CrossRef]
- Ziolek, M.; Nowak, I.; Kilos, B.; Sobczak, I.; Decyk, P.; Trejda, M.; Volta, J.C. Template synthesis and characterisation of MCM-41 mesoporous molecular sieves containing various transition metal elements—TME (Cu, Fe, Nb, V, Mo). J. Phys. Chem. Solids 2004, 65, 571–581. [Google Scholar] [CrossRef]
- Prakash, A.M.; Kevan, L. Synthesis of Niobium Silicate Molecular Sieves of the MFI Structure: Evidence for Framework Incorporation of the Niobium Ion. J. Am. Chem. Soc. 1998, 120, 13148–13155. [Google Scholar] [CrossRef]
- Wierzchowski, P.T.; Zatorski, L.W. Aldol condensation in gaseous phase by zeolite catalysts. Catal. Lett. 1991, 9, 411–414. [Google Scholar] [CrossRef]
- Rocha, J.; Brandao, P.; Phillippou, A.; Anderson, M.W. Synthesis and characterisation of a novel microporous niobium silicate catalyst. Chem. Commun. 1998, 2687–2688. [Google Scholar] [CrossRef]
- Sobczak, I.; Decyk, P.; Ziolek, M.; Daturi, M.; Lavalley, J.-C.; Kevan, L.; Prakash, A.M. Physicochemical Properties and Catalytic Activity of Cu–NbZSM-5—A Comparative Study with Cu–AlZSM-5. J. Catal. 2002, 207, 101–112. [Google Scholar] [CrossRef]
- Tielens, F. Exploring the reactivity of framework vanadium, niobium, and tantalum sites in zeolitic materials using DFT reactivity descriptors. J. Comput. Chem. 2009, 30, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M. State of the art of Lewis acid-containing zeolites: Lessons from fine chemistry to new biomass transformation processes. Dalton Trans. 2014, 43, 4197–4208. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer–Villiger oxidations. Nature 2001, 412, 423. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. PNAS 2010, 107, 6164–6168. [Google Scholar] [CrossRef]
- Sastre, G.; Corma, A. Relation between structure and Lewis acidity of Ti-Beta and TS-1 zeolites: A quantum-chemical study. Chem. Phys. Lett. 1999, 302, 447–453. [Google Scholar] [CrossRef]
- Damin, A.; Bordiga, S.; Zecchina, A.; Lamberti, C. Reactivity of Ti(IV) sites in Ti-zeolites: An embedded cluster approach. J. Chem. Phys. 2002, 117, 226–237. [Google Scholar] [CrossRef]
- Damin, A.; Bonino, F.; Ricchiardi, G.; Bordiga, S.; Zecchina, A.; Lamberti, C. Effect of NH3 Adsorption on the Structural and Vibrational Properties of TS-1. J. Phys. Chem. B 2002, 106, 7524–7526. [Google Scholar] [CrossRef]
- Damin, A.; Bordiga, S.; Zecchina, A.; Doll, K.; Lamberti, C. Ti-chabazite as a model system of Ti(IV) in Ti-zeolites: A periodic approach. J. Chem. Phys. 2003, 118, 10183–10194. [Google Scholar] [CrossRef]
- Shetty, S.; Kulkarni, B.S.; Kanhere, D.G.; Goursot, A.; Pal, S. A Comparative Study of Structural, Acidic and Hydrophilic Properties of Sn−BEA with Ti−BEA Using Periodic Density Functional Theory. J. Phys. Chem. B 2008, 112, 2573–2579. [Google Scholar] [CrossRef]
- Kulkarni, B.S.; Krishnamurty, S.; Pal, S. Probing Lewis acidity and reactivity of Sn- and Ti-beta zeolite using industrially important moieties: A periodic density functional study. J. Mol. Catal. A: Chem. 2010, 329, 36–43. [Google Scholar] [CrossRef]
- Yang, G.; Zhuang, J.; Ma, D.; Lan, X.; Zhou, L.; Liu, X.; Han, X.; Bao, X. A joint experimental–theoretical study on trimethylphosphine adsorption on the Lewis acidic sites present in TS-1 zeolite. J. Mol. Struct. 2008, 882, 24–29. [Google Scholar] [CrossRef]
- Yang , G.; Zhou, L.; Liu, X.; Han, X.; Bao, X. Density Functional Calculations on the Distribution, Acidity, and Catalysis of TiIV and TiIII Ions in MCM-22 Zeolite. Chem. Eur. J. 2011, 17, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Jardillier, N.; Berthomieu, D.; Goursot, A.; Reveles, J.U.; Köster, A.M. Theoretical Study of CuIY Zeolite: Structure and Electronic Properties. J. Phys. Chem. B 2006, 110, 18440–18446. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Goursot, A.; Coq, B.; Delahay, G. Theoretical Study of the Dissociation of N2O in a Transition Metal Ion-Catalyzed Reaction. J. Phys. Chem. B 2004, 108, 8823–8829. [Google Scholar] [CrossRef]
- Löwenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Neurock, M. Perspectives on the first principles elucidation and the design of active sites. J. Catal. 2003, 216, 73–88. [Google Scholar] [CrossRef]
- Kühl, G.H. The coordination of aluminum and silicon in zeolites as studied by x-ray spectrometry. J. Phys. Chem. Solids 1977, 38, 1259–1263. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The Strength of Brønsted Acid Sites in Microporous Aluminosilicates. Acs Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M.; Pazhohniya, Z.; Aghamohammadi, S. One-pot hydrothermal synthesis of nanostructured ZrAPSO-34 powder: Effect of Zr-loading on physicochemical properties and catalytic performance in conversion of methanol to ethylene and propylene. Micropor. Mesopor. Mater. 2016, 226, 331–343. [Google Scholar] [CrossRef]
- Alonso, A.; Valle, B.; Atutxa, A.; Gayubo Ana, G.; Aguayo, A. Development of Alternative Catalysts Based on HZSM-5 Zeolite for the BTO Process. Int. J. Chem. React. Eng. 2007, 5, A61. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 17 March 2017).
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
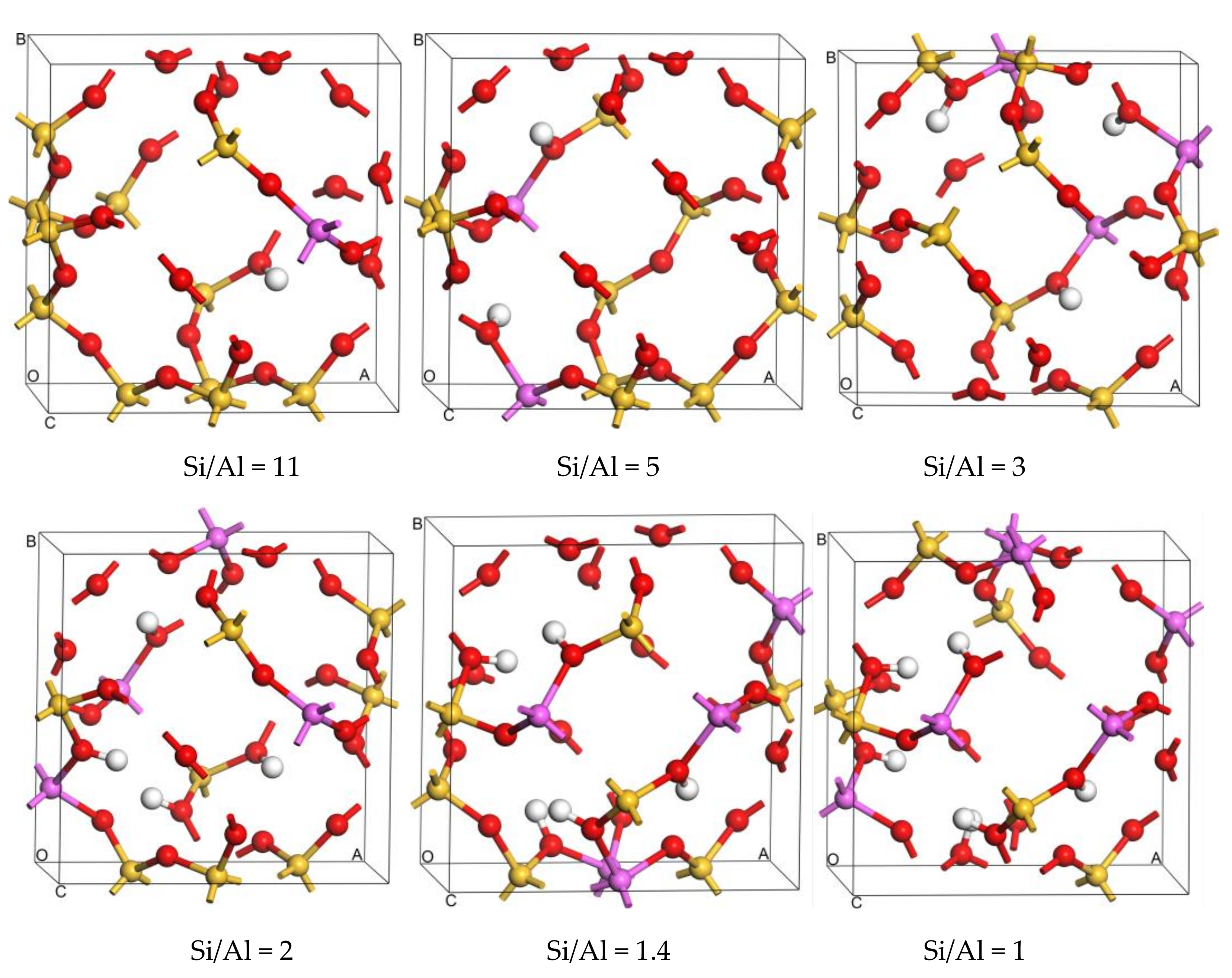
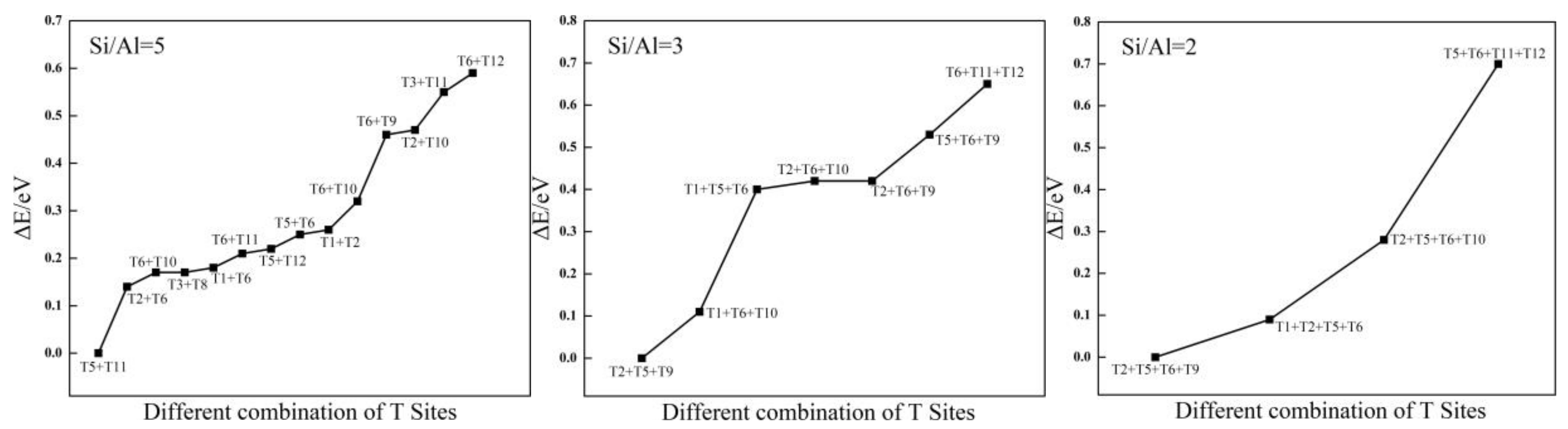
| Framework | T Sites | Eav-sub/eV |
|---|---|---|
| Si/Al = 11 | --- | 0.17 |
| Si/Al = 5 | T5 + T11 | −0.63 |
| Si/Al = 3 | T2 + T5 +T9 | −0.85 |
| Si/Al = 2 | T2 + T5 +T6 + T9 | −0.94 |
| Si/Al = 1.4 | T1 + T2 + T6 + T9 + T10 | −0.92 |
| Si/Al = 1 | --- | −0.96 |
| Zr Content | Si/Al = 11 | Si/Al = 5 | Si/Al = 3 | Si/Al = 2 | Si/Al = 1.4 | Si/Al = 1 |
|---|---|---|---|---|---|---|
| 0 | −9.35 | −9.33 | −9.39 | −9.40 | −9.34 | −9.36 |
| 1 | −9.51 | −9.30 | −9.27 | −9.22 | −9.11 | −9.15 |
| 2 | −9.32 | −9.38 | −9.35 | −9.33 | −9.13 | −9.28 |
| 3 | −9.30 | −9.45 | −9.39 | −9.38 | −9.38 | −9.27 |
| 4 | −9.33 | −9.44 | −9.46 | −9.48 | −9.38 | −9.32 |
| 5 | −9.31 | −9.51 | −9.52 | −9.51 | −9.41 | −9.39 |
| 6 | −9.44 | −9.57 | −9.63 | −9.54 | −9.47 | −9.42 |
| Zr Content | Si/Al = 11 | Si/Al = 5 | Si/Al = 3 | Si/Al = 2 | Si/Al = 1.4 | Si/Al = 1 |
|---|---|---|---|---|---|---|
| Eav-sub | Eav-sub | Eav-sub | Eav-sub | Eav-sub | Eav-sub | |
| 1 | −0.56 | −0.65 | −0.83 | −0.83 | −2.10 | −2.22 |
| 2 | −0.80 | −0.81 | −0.80 | −0.87 | −1.46 | −1.55 |
| 3 | −0.62 | −0.73 | −0.84 | −0.90 | −1.07 | −1.40 |
| 4 | −0.74 | −0.79 | −0.70 | −0.87 | −0.96 | −0.99 |
| 5 | −0.75 | −0.71 | −0.88 | −0.75 | −0.79 | −0.85 |
| 6 | −0.68 | −0.74 | −0.77 | −0.62 | −0.79 | −0.87 |
| Zr Content | AlPO-34 | SAPO-34 |
|---|---|---|
| Eav-sub | Eav-sub | |
| 0 | --- | 2.801 |
| 1 | 3.72 | 0.83 |
| 2 | 0.74 | 0.10 |
| 3 | 0.88 | 0.41 |
| 4 | 0.78 | 0.94 |
| 5 | 0.83 | 0.85 |
| 6 | 0.67 | 0.55 |
| Zr Content | AlPO-34 | SAPO-34 |
|---|---|---|
| 0 | −9.12 | −9.17 |
| 1 | −9.01 | −8.95 |
| 2 | −9.11 | −9.05 |
| 3 | −9.15 | −9.11 |
| 4 | −9.20 | −9.21 |
| 5 | −9.22 | −9.26 |
| 6 | −9.27 | −9.31 |
| Framework | ΘAl/° | ΩSi→Al |
|---|---|---|
| Si/Al = 11 | 6.18 | 5.79 |
| Si/Al = 5 | 5.23 | 4.75 |
| Si/Al = 3 | 4.70 | 4.17 |
| Si/Al = 2 | 5.66 | 5.22 |
| Si/Al = 1.4 | 6.46 | 6.11 |
| Si/Al = 1 | 5.99 | 5.59 |
| Framework | ΘZr/° | ΩP⟶Zr |
| AlPO-34-Zr1 | 3.06 | 1.51 |
| AlPO-34-Zr2 | 12.66 | 9.38 |
| AlPO-34-Zr3 | 11.28 | 8.25 |
| AlPO-34-Zr4 | 13.07 | 9.71 |
| AlPO-34-Zr5 | 14.43 | 10.83 |
| AlPO-34-Zr6 | 13.27 | 9.88 |
| Framework | ΘZr/° | ΩP⟶Zr |
| SAPO-34 | 6.01(ΘSi) | 3.93(ΩP⟶Si) |
| SAPO-34-Zr1 | 5.85 | 3.80 |
| SAPO-34-Zr2 | 13.53 | 10.09 |
| SAPO-34-Zr3 | 13.17 | 9.80 |
| SAPO-34-Zr4 | 12.52 | 9.26 |
| SAPO-34-Zr5 | 12.18 | 8.98 |
| SAPO-34-Zr6 | 13.55 | 10.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xing, B.; Wang, B.; Li, R. Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks. Molecules 2019, 24, 4466. https://doi.org/10.3390/molecules24244466
Li D, Xing B, Wang B, Li R. Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks. Molecules. 2019; 24(24):4466. https://doi.org/10.3390/molecules24244466
Chicago/Turabian StyleLi, Duichun, Bin Xing, Baojun Wang, and Ruifeng Li. 2019. "Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks" Molecules 24, no. 24: 4466. https://doi.org/10.3390/molecules24244466
APA StyleLi, D., Xing, B., Wang, B., & Li, R. (2019). Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks. Molecules, 24(24), 4466. https://doi.org/10.3390/molecules24244466






