Insights into Mechanisms of Electrochemical Drug Degradation in Their Mixtures in the Split-Flow Reactor
Abstract
1. Introduction
2. Results and Discussion
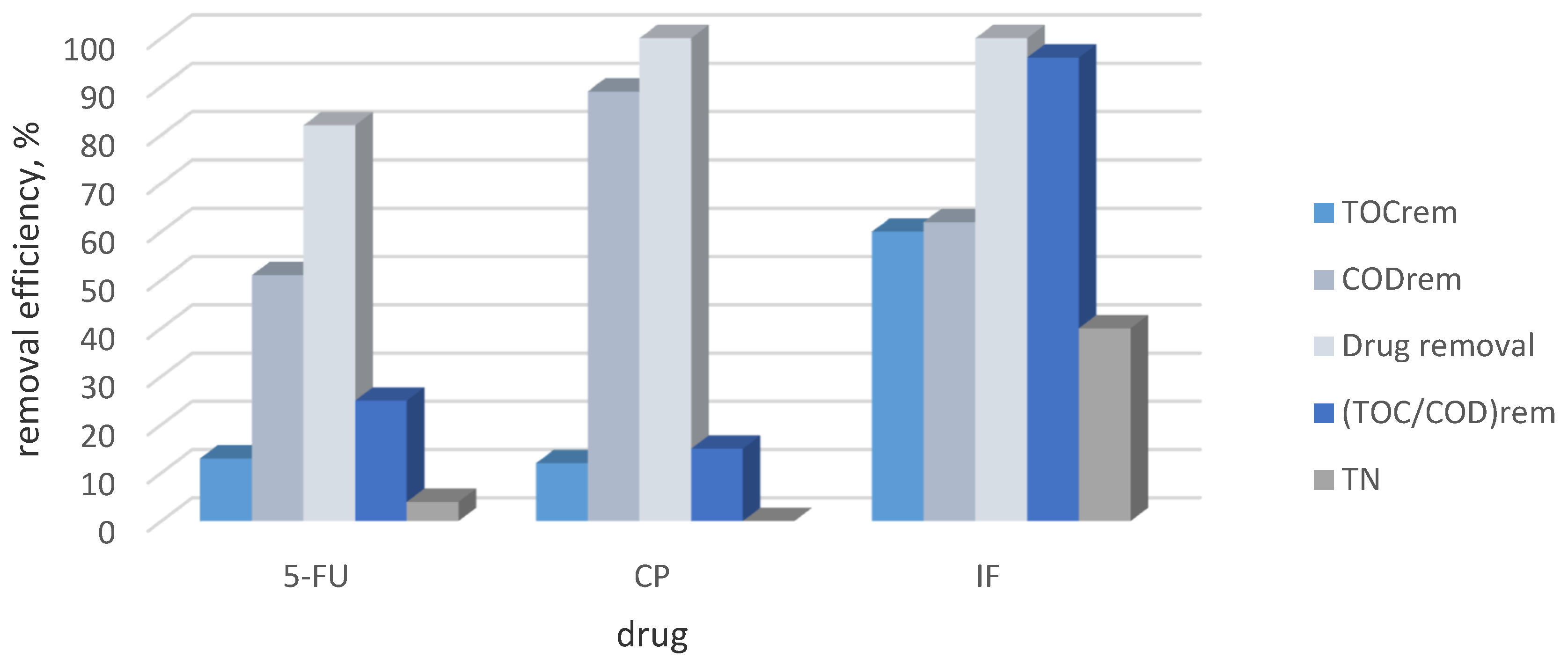
2.1. Comparison of Electrochemical Decomposition in Recirculating Split-Flow Batch Reactor of IF, CF and 5-FU in Single Drug Solution
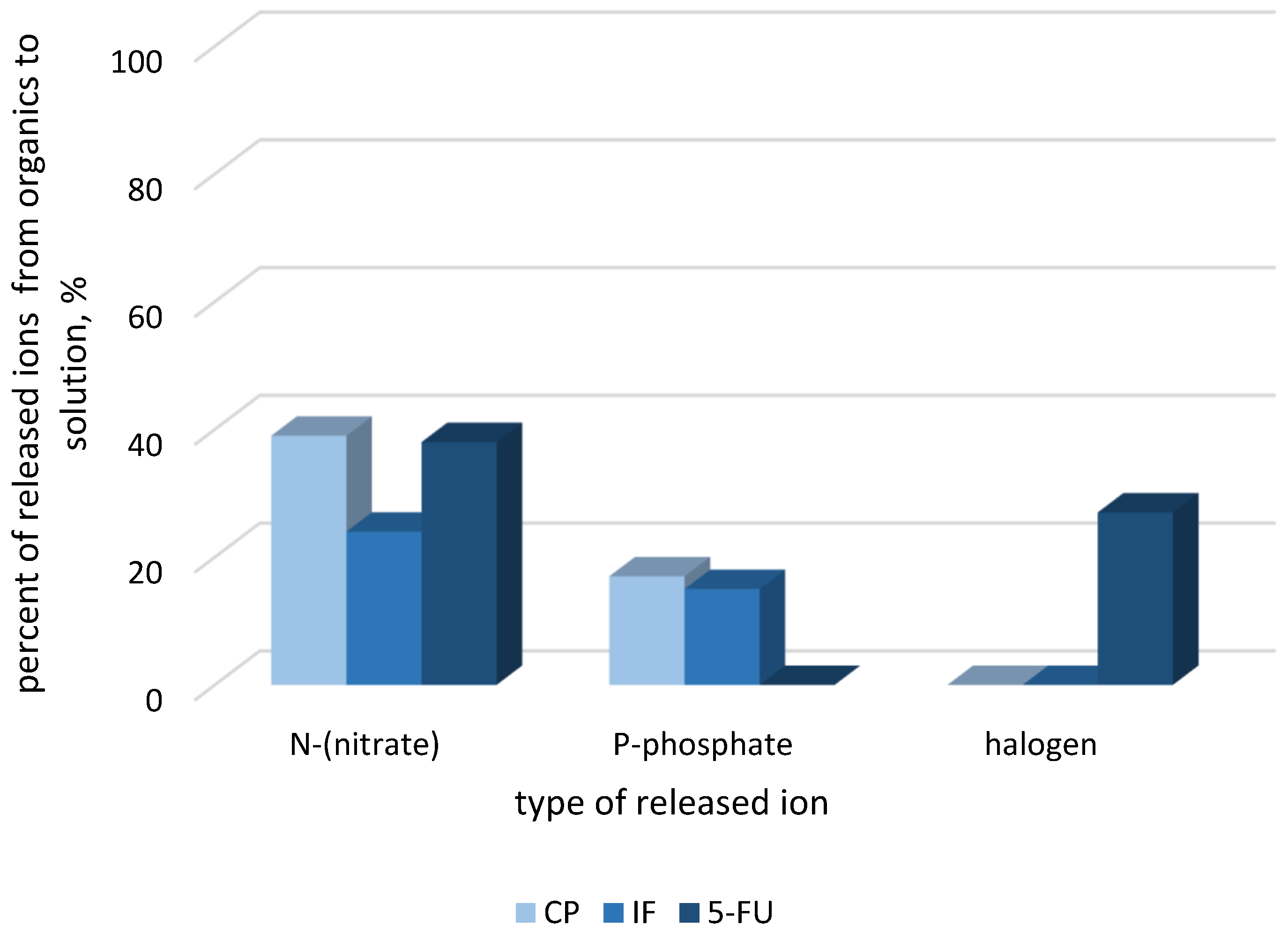
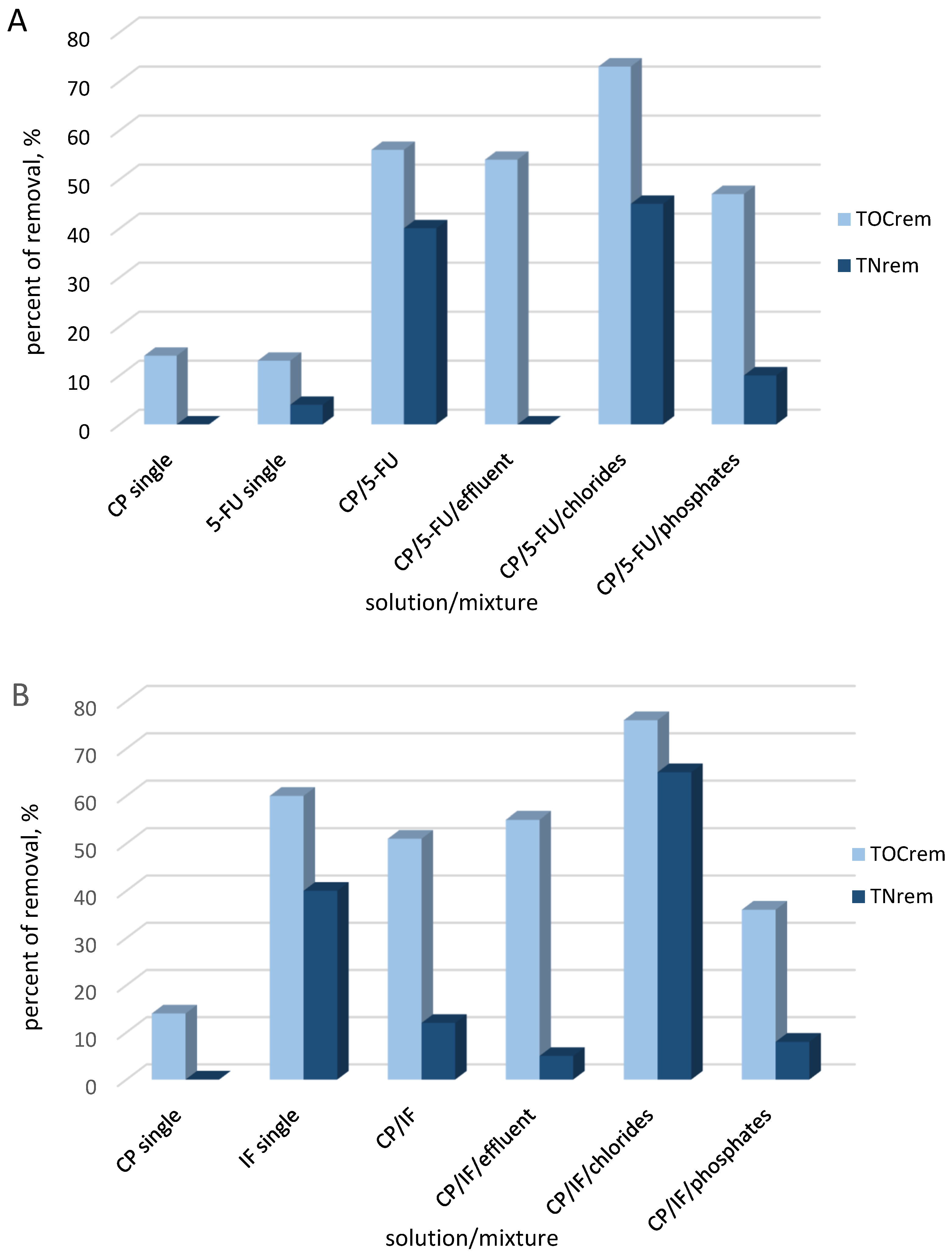
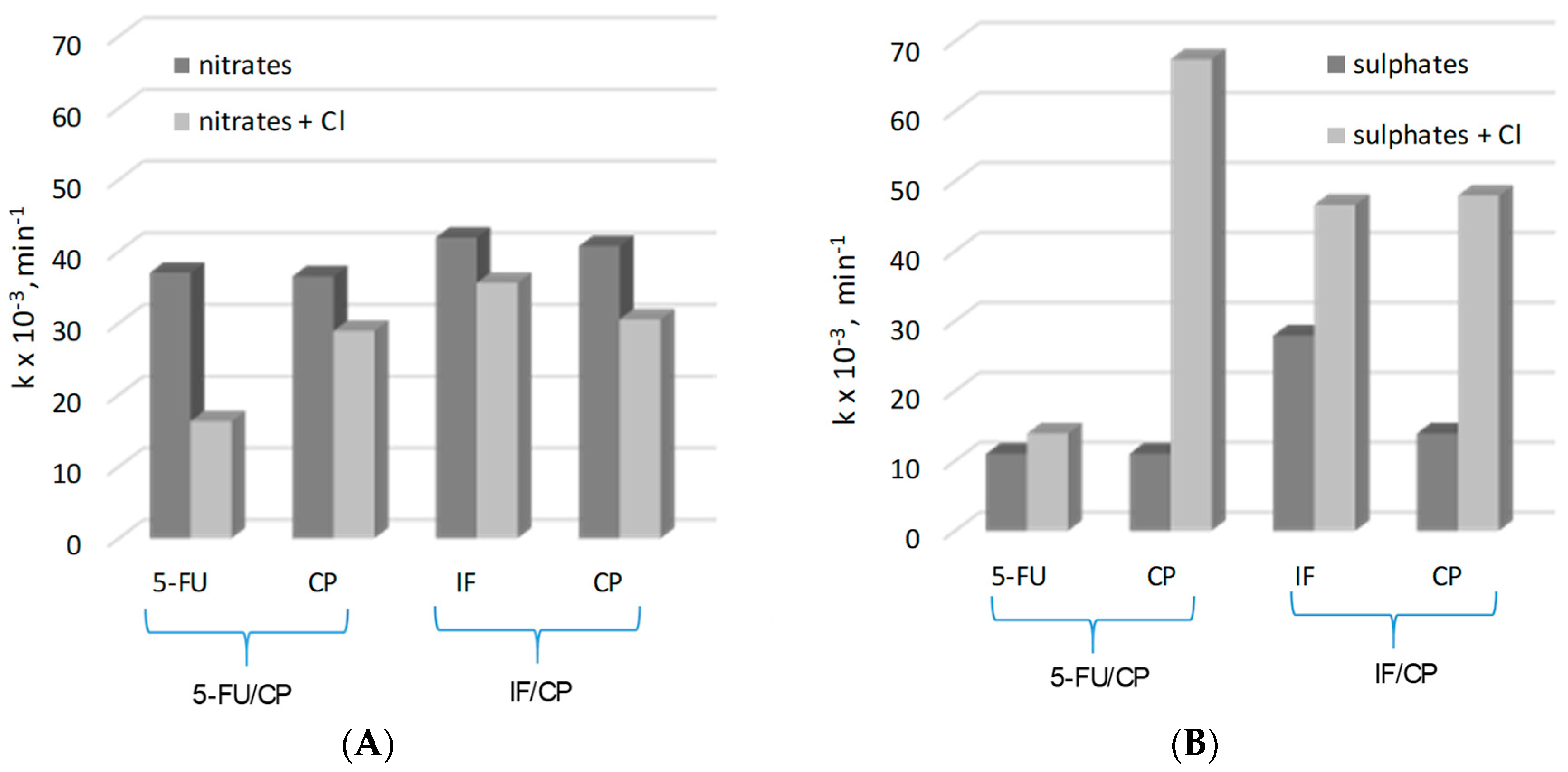
2.2. Decomposition of Cytostatic Drugs in Their Mixtures
2.2.1. Decomposition of CP and 5-FU Mixture
2.2.2. IF/CP Mixture Decomposition
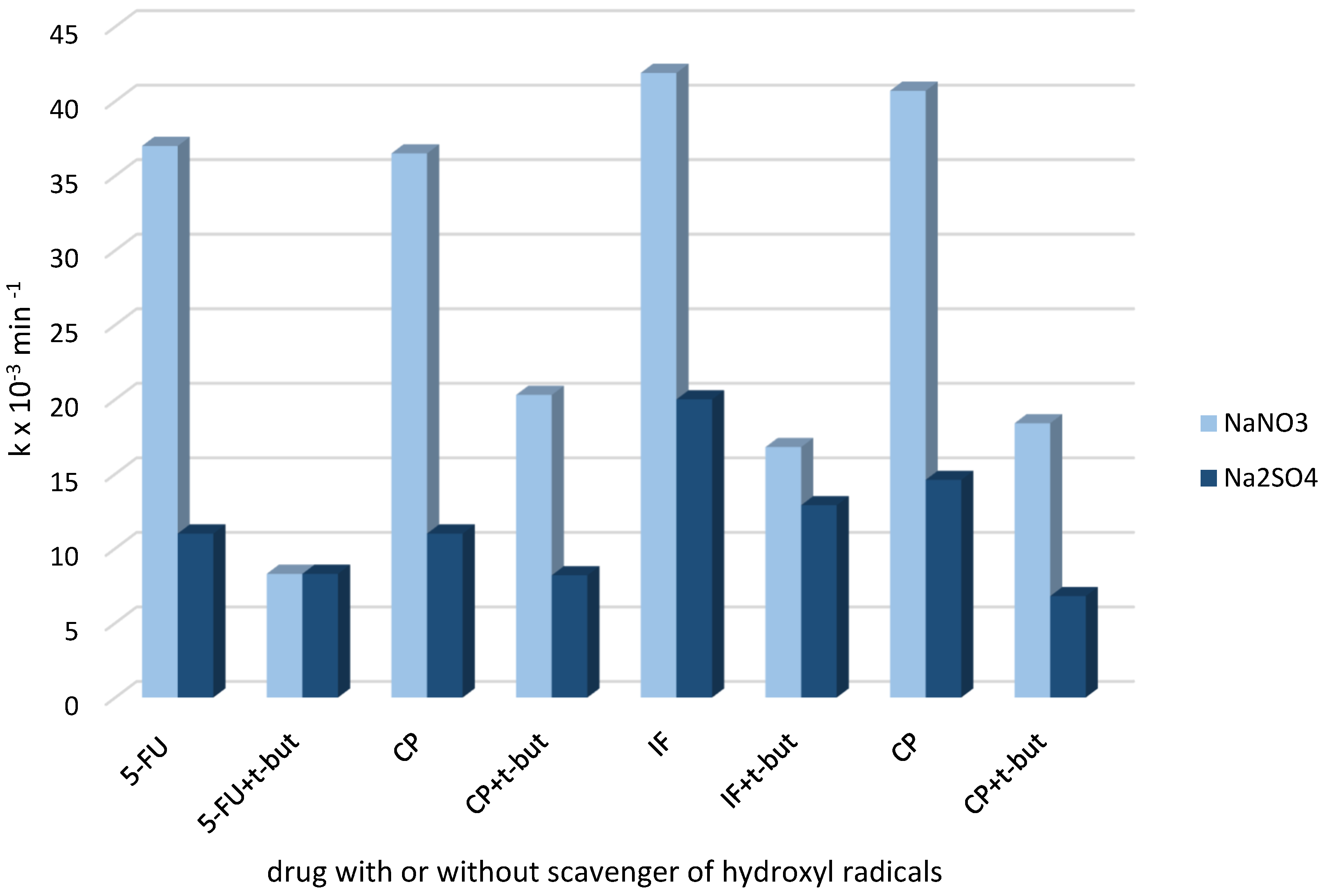
2.3. Active Species Participated in Cytostatic Drugs Removal
2.4. Identification of CP and IF Intermediates in Mixture in Na2SO4
3. Materials and Methods
3.1. Chemicals
3.2. Voltammetric Measurements
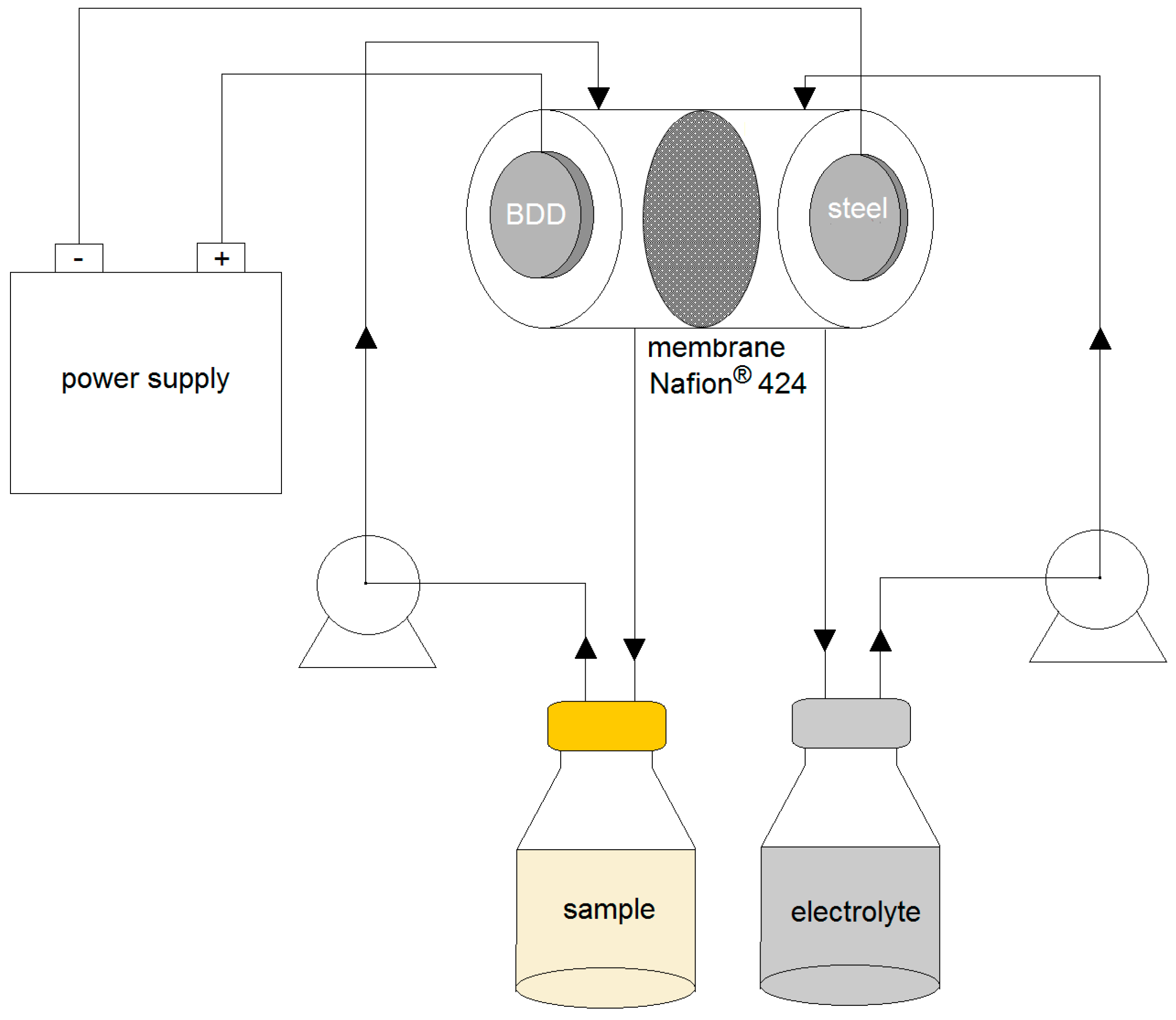
3.3. Electrochemical Systems
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huitema, A.D.R.; Tibben, M.M.; Kerbusch, T.; Jantien Kettenes-Van Den Bosch, J.; Rodenhuis, S.; Beijnen, J.H. Simple and selective determination of the cyclophosphamide metabolite phosphoramide mustard in human plasma using high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 345–355. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska, A.; Fiszka Borzyszkowska, A.; Ofiarska, A.; Siedlecka, E.M. Removal of cytostatic drugs by AOPs: A review of applied processes in the context of green technology. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1282–1335. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Medina, A.; Sans, C.; Lacorte, S. Biological and photochemical degradation of cytostatic drugs under laboratory conditions. J. Hazard. Mater. 2017, 323, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.S.; Golder, A.K. Decomposition of drug mixture in Fenton and photo-Fenton processes: Comparison to singly treatment, evolution of inorganic ions and toxicity assay. Chemosphere 2015, 127, 254–261. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Stepnowski, P. Decomposition rates of methyl tert-butyl ether and its by-products by the Fenton system in saline wastewaters. Sep. Purif. Technol. 2006, 52, 317–324. [Google Scholar] [CrossRef]
- Lai, W.W.-P.; Lin, H.H.-H.; Lin, A.Y.-C. TiO2 photocatalytic degradation and transformation of oxazaphosphorine drugs in an aqueous environment. J. Hazard. Mater. 2015, 287, 133–141. [Google Scholar] [CrossRef]
- Yu-Chen Lin, A.; Han-Fang Hsueh, J.; Andy Hong, P.K. Removal of antineoplastic drugs cyclophosphamide, ifosfamide, and 5-fluorouracil and a vasodilator drug pentoxifylline from wastewaters by ozonation. Environ. Sci. Pollut. Res. 2015, 22, 508–515. [Google Scholar]
- Siedlecka, E.M.; Stepnowski, P. Effect of chlorides and sulfates on the performance of a Fe3+/H2O2 Fenton-like system in the degradation of MTBE and its by-products. Water Environ. Res. 2007, 79, 2318–2324. [Google Scholar] [CrossRef]
- Fabiańska, A.; Ofiarska, A.; Fiszka-Borzyszkowska, A.; Stepnowski, P.; Siedlecka, E.M. Electrodegradation of ifosfamide and cyclophosphamide at BDD electrode: Decomposition pathway and its kinetics. Chem. Eng. J. 2015, 276, 274–282. [Google Scholar] [CrossRef]
- Ofiarska, A.; Pieczyńska, A.; Fiszka-Borzyszkowska, A.; Stepnowski, P.; Siedlecka, E.M. Pt-TiO2-assisted photocatalytic degradation of the cytostatic drugs ifosfamide and cyclophosphamide under artificial sunlight. Chem. Eng. J. 2016, 285, 417–427. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Fabiańska, A.; Stolte, S.; Nienstedt, A.; Ossowski, T.; Stepnowski, P.; Thöming, J. Electrocatalytic oxidation of 1-butyl-3-methylimidazolium chloride: Effect of the electrode material. Int. J. Electrochem. Sci. 2013, 8, 5560–5574. [Google Scholar]
- Pieczyńska, A.; Ofiarska, A.; Fiszka-Borzyszkowska, A.; Białk-Bielińska, A.; Stepnowski, P.; Stolte, S.; Siedlecka, E.M. A comparative study of electrochemical degradation of imidazolium and pyridinium ionic liquids: A reaction pathway and ecotoxicity evaluation. Sep. Purif. Technol. 2015, 156, 522–534. [Google Scholar] [CrossRef]
- Coria, G.; Nava, J.L.; Carreño, G. Electrooxidation of diclofenac in synthetic pharmaceutical wastewater using an electrochemical reactor equipped with a boron doped diamond electrode. Mex. J. Chem. Soc. 2014, 58, 303–308. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of estrone using a boron-doped diamond anode in a filter-press reactor. Electrochim. Acta 2016, 197, 186–193. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hirose, J.; Sano, K.; Hiro, N.; Ijiri, Y.; Takiuchi, H.; Tamai, H.; Takenaka, H.; Tanaka, K.; Nakano, T. Evaluation of an electrolysis apparatus for inactivating antineoplastic in clinical wastewater. Chemosphere 2008, 72, 659–665. [Google Scholar] [CrossRef]
- Queiroza, N.M.P.; Sirésb, I.; Zantac, C.L.P.S.; Tonholoc, J.; Brillas, E. Removal of the drug procaine from acidic aqueous solutions using aflowreactor with a boron-doped diamond anode. Sep. Purif. Technol. 2019, 216, 65–73. [Google Scholar] [CrossRef]
- Radjenovic, J.; Petrovic, M. Sulfate-mediated electrooxidation of X-ray contrast media on boron-doped diamond anode. Water Res. 2016, 94, 128–135. [Google Scholar] [CrossRef]
- Ochoa-Chavez, A.S.; Pieczyńska, A.; Fiszka Borzyszkowska, A.; Espinoza-Montero, P.J.; Siedlecka, E.M. Electrochemical degradation of 5-FU using a flow reactor with BDD electrode: Chomparison of two electrochemical systems. Chemosphere 2018, 201, 816–825. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Mostafa, E.; Baltruschat, H. Could NOx be released during mineralization of pollutants containing nitrogen by hydroxyl radical? As certaining the release of N-volatile species. Appl. Catal. B Environ. 2017, 207, 376–384. [Google Scholar] [CrossRef]
- Weiss, E.; Groenen-Serrano, K.; Savall, A.; Comninellis, C. A kinetic study of the electrochemical oxidation of maleic acid on boron doped diamond. J. App. Electrochem. 2007, 37, 41–47. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. Investigation of the Anodic Oxidation of Acetic Acid on Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 2008, 155, 27–32. [Google Scholar] [CrossRef]
- Da Costa, T.F.; Santos, J.E.L.; da Silva, D.R.; Martinez-Huitle, C.A. BDD-Electrolysis of Oxalic Acid in Diluted Acidic Solutions. J. Braz. Chem. Soc. 2019, 30, 1541–1547. [Google Scholar] [CrossRef]
- Sánchez-Carretero, A.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrochemical production of perchlorates using conductive diamond electrolysis. Chem. Eng. J. 2011, 166, 710–714. [Google Scholar] [CrossRef]
- Mostafa, E.; Reinsberg, P.; Garcia-Segura, S.; Baltruschat, H. Chlorine species evolution during electrochlorination on boron-doped diamond anodes: In-situ electrogeneration of Cl2, Cl2O and ClO2. Electrochimica Acta 2018, 281, 831–840. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Liu, Y.; Zhang, L.; Feng, L. Modelling study on the effects of chloride on the degradation of bezafibrate and carbamazepine in sulfate radical-based advanced oxidation processes: Conversion of reactive radicals. Chem. Eng. J. 2019, 258, 1332–1341. [Google Scholar] [CrossRef]
- Cañizares, P.; Sáez, C.; Sánchez-Carretero, A.; Rodrigo, M.A. Synthesis of novel oxidants by electrochemical technology. J. Appl. Electrochem. 2009, 39, 2143–2149. [Google Scholar] [CrossRef]
- Burgos-Castillo, R.C.; Sirés, I.; Sillanpää, M.; Brillas, E. Application of electrochemical advanced oxidation to bisphenol A degradation in water. Effect of sulfate and chloride ions. Chemosphere 2018, 194, 812–820. [Google Scholar] [CrossRef]
- Pérez, G.; Saiz, J.; Ibañez, R.; Urtiaga, A.; Ortiz, I. Assessment of the formation of inorganic oxidation by-products during the electrocatalytic treatment of ammonium from landfill leachates. Water Res. 2012, 46, 2579–2590. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Mostafa, E.; Baltruschat, H. Electrogeneration of inorganic chloramines on boron-doped diamond anodes during electrochemical oxidation of ammonium chloride, urea and synthetic urine matrix. Water Res. 2019, 160, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Mahdi Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.S.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hazime, R.; Nguyen, Q.H.; Ferronato, C.; Slavador, A.; Jaber, F.; Chovelon, J.-M. Comparative study of imazalil degradation in three systems: UV/TiO2, UV/K2S2O8 and UV/TiO2/K2SO8. App. Catal. B 2014, 144, 286–291. [Google Scholar] [CrossRef]
- Cho, K.; Qu, Y.; Kwon, D.; Zhang, H.; Cid, C.A.; Aryanfar, A.; Hoffmann, M.R. Effects of anodic potential and chloride ion on overall reactivity in electrochemical reactors designed for solar-powered wastewater treatment. Environ. Sci. Technol. 2014, 48, 2377–2384. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Ofiarska, A.; Fiszka Borzyszkowska, A.; Białk-Bielińska, A.; Stepnowski, P.; Pieczyńska, A. Cytostati drug removal using electrochemical oxidation with BDD electrode: Degradation pathway and toxicity. Water Res. 2018, 144, 235–245. [Google Scholar] [CrossRef]
- Česen, M.; Kosjek, T.; Busetti, F.; Kompare, B.; Heath, E. Human metabolites and transformation products of cyclophosphamide and ifosfamide: Analysis, occurrence and formation during abiotic treatments. Environ. Sci. Pollut. Res. 2016, 23, 11209–11223. [Google Scholar] [CrossRef]
- Struck, R.F.; Kirk, M.C.; Mellett, L.B.; el Dareer, S.; Hill, D.L. Urinary metabolites of the antitumor agent cyclophosphamide. Mol Pharmacol. 1971, 7, 519–529. [Google Scholar]
- El-Ghenymy, A.; Rodríguez, R.M.; Arias, C.; Centellas, F.; Garrido, J.A.; Cabot, P.L.; Brillas, E. Electro-Fenton and photoelectro-Fenton degradation of the antimicrobial sulfamethazine using a boron-doped diamond anode and an airdiffusion cathode. J. Electroanal. Chem. 2013, 701, 7–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Single Drug Solution | C = 50 mg L−1 | C = 25 mg L−1 | ||||
| kapp × 10−3 min−1 | R2 | kapp × 10−3 min−1 | R2 | |||
| 5-FU | 3.8 | 0.99 | 8.9 | 0.98 | ||
| CP | 10.4 | 0.99 | 13.5 | 0.99 | ||
| IF | 19.1 | 0.99 | 27.7 | 0.99 | ||
| Mixture | C = 25 mg L−1 1:1 | C = 25 mg L−1 1:1 + 10 mg L−1 Phosphates | C = 25 mg L−1 1:1 + 100 mg L−1 Chlorides | |||
| kapp × 10−3 min−1 | R2 | kapp × 10−3 min−1 | R2 | kapp × 10−3 min−1 | R2 | |
| 5-FU/CP | ||||||
| 5-FU | 11.0 | 0.96 | 10.5 | 0.97 | 13.6 | 0.98 |
| CP | 11.0 | 0.99 | 10.8 | 0.96 | 67.4 | 0.99 |
| CP/IF | ||||||
| CP | 13.9 | 0.98 | 13.0 | 0.99 | 47.4 | 0.95 |
| IF | 27.9 | 0.97 | 27.7 | 0.98 | 46.6 | 0.99 |
| Drug | Structure of Intermediate | Molecular ion (Fragmentation Ions) | |
|---|---|---|---|
Ifosfamide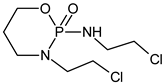 | Cyclophosphamide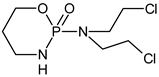 | ||
| ✓ | 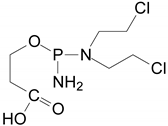 | [M + H]+ = 293 (275, 227) | |
| ✓ | ✓ |  | [M + H]+ = 277 |
| ✓ | ✓ | 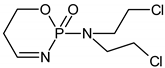 | [M + H]+ = 259 (140) |
| ✓ | ✓ | 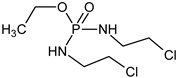 | [M + H]+ = 249 (164) |
| ✓ | 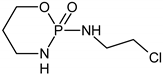 | [M + H]+ = 199 (171; 79) | |
| ✓ | ✓ | ? | [M + H]+ = 165 |
| Parameter | Units | Value |
|---|---|---|
| pH | 6.8 | |
| COD | mgO2/L | 62 |
| N-NH4+ | mgN/L | 0.225 |
| NO3− | mgN/L | 4.463 |
| Cl− | mg/L | 62 |
| PO43− | mgP/L | 0.159 |
| SO42− | mg/L | 89 |
| Conductivity | µS/cm | 435 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieczyńska, A.; Ochoa-Chavez, S.A.; Wilczewska, P.; Bielicka-Giełdoń, A.; Siedlecka, E.M. Insights into Mechanisms of Electrochemical Drug Degradation in Their Mixtures in the Split-Flow Reactor. Molecules 2019, 24, 4356. https://doi.org/10.3390/molecules24234356
Pieczyńska A, Ochoa-Chavez SA, Wilczewska P, Bielicka-Giełdoń A, Siedlecka EM. Insights into Mechanisms of Electrochemical Drug Degradation in Their Mixtures in the Split-Flow Reactor. Molecules. 2019; 24(23):4356. https://doi.org/10.3390/molecules24234356
Chicago/Turabian StylePieczyńska, Aleksandra, Stalin Andres Ochoa-Chavez, Patrycja Wilczewska, Aleksandra Bielicka-Giełdoń, and Ewa M. Siedlecka. 2019. "Insights into Mechanisms of Electrochemical Drug Degradation in Their Mixtures in the Split-Flow Reactor" Molecules 24, no. 23: 4356. https://doi.org/10.3390/molecules24234356
APA StylePieczyńska, A., Ochoa-Chavez, S. A., Wilczewska, P., Bielicka-Giełdoń, A., & Siedlecka, E. M. (2019). Insights into Mechanisms of Electrochemical Drug Degradation in Their Mixtures in the Split-Flow Reactor. Molecules, 24(23), 4356. https://doi.org/10.3390/molecules24234356









