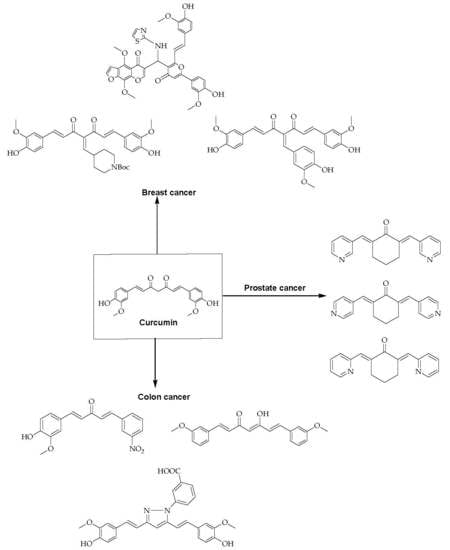
Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers
Abstract
1. Introduction
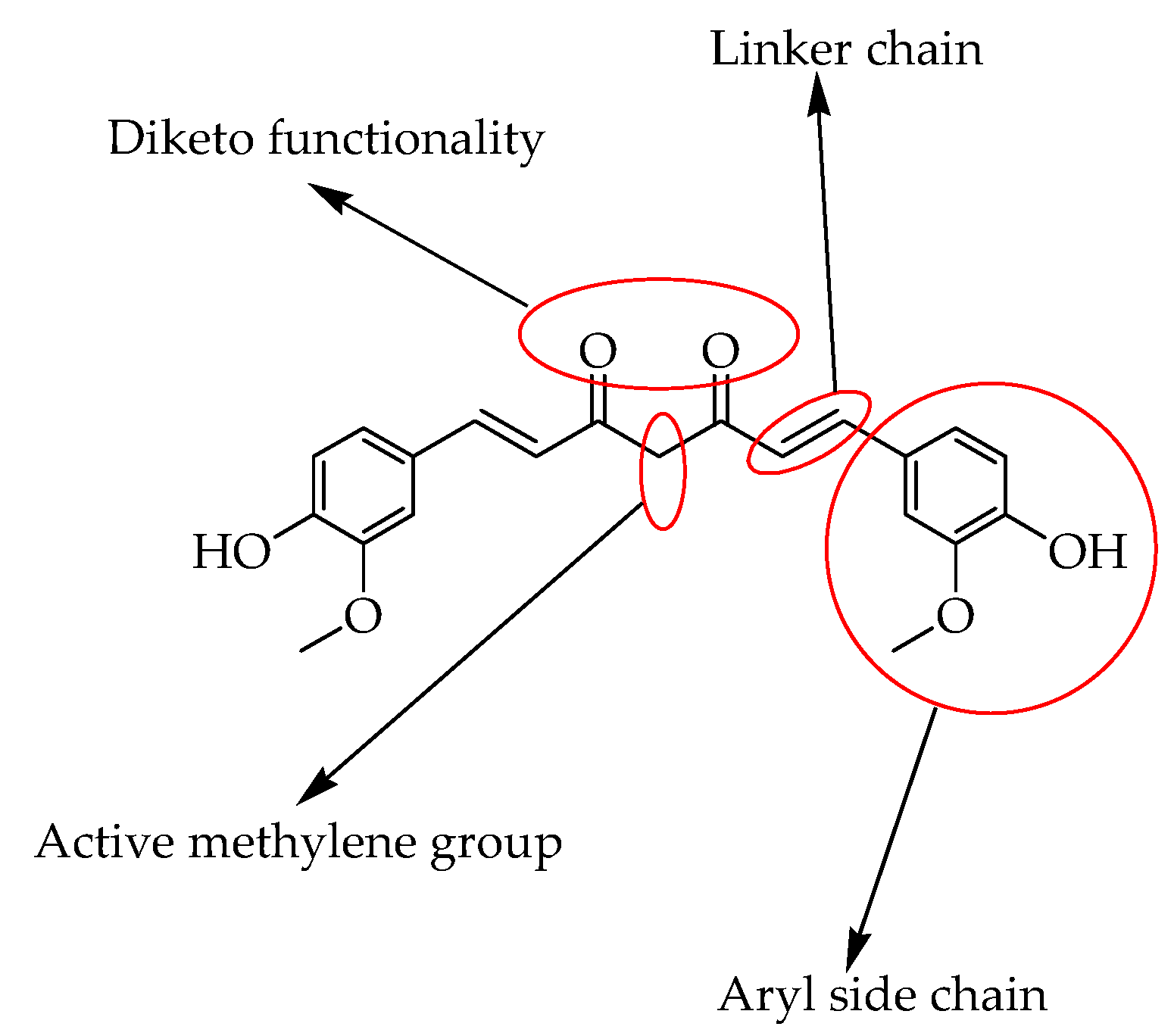
2. Anticancer Activity of Curcumin and Its Derivatives
- Low aqueous solubility;
- Instability in aqueous condition;
- Poor bioavailability;
- Poor cellular uptake.
2.1. Advantages of Using Curcumin Derivatives
2.2. Resistance to the Currently Used Medicine
2.3. Mode of Action of Curcumin Derivatives
3. Three Different Types of Cancers
3.1. Breast Cancer
3.1.1. Curcumin Derivatives as Breast Cancer Inhibitors
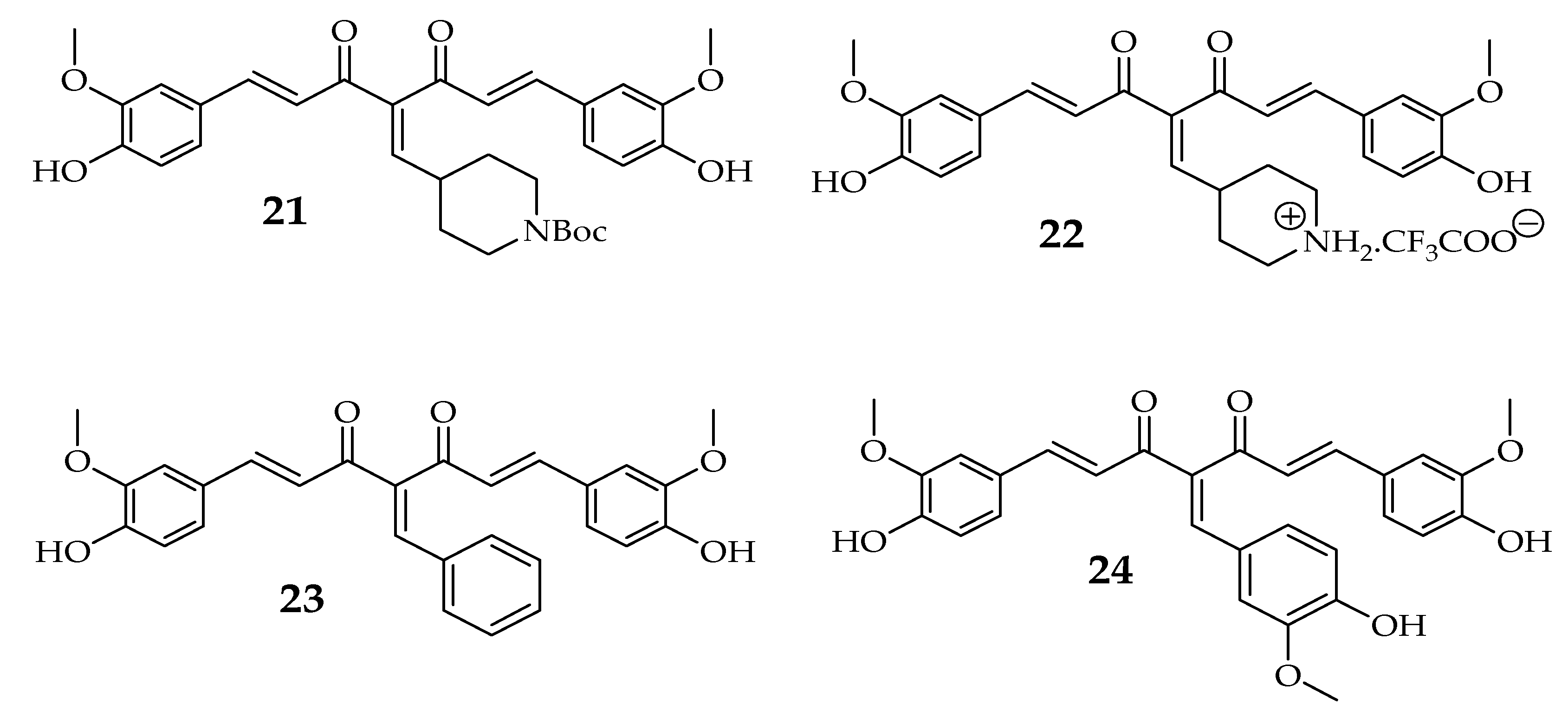
3.1.2. Clinical Studies of Curcumin Derivatives in Breast Cancer
3.2. Prostate Cancer
3.2.1. Curcumin Derivatives as Prostate Cancer Inhibitors
3.2.2. Clinical Studies of Curcumin Derivatives on Prostate Cancer
3.3. Colon Cancer
3.3.1. Curcumin Derivatives as Colon Cancer Inhibitors
3.3.2. Clinical Studies of Curcumin Derivatives
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Borik, R.M.; Fawzy, N.M.; Abu-bakr, S.M.; Aly, M.S. Docking Studies of Novel Heterocyclic Derivatives Obtained via Reactions Involving Curcumin. Molecules 2018, 23, 1398. [Google Scholar] [CrossRef]
- Damalas, C.A. Potential Uses of Turmeric (‘Curcuma longa’) Products as Alternative Means of Pest Management in Crop Production. Plant. Omi. 2011, 4, 136–141. [Google Scholar]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Nawaz, A.; Khan, G.M.; Hussain, A.; Ahmad, A.A.; Khan, A. Curcumin: A Natural Product of Biological Importance. Gomal. Univ. J. Res. 2011, 27, 7–14. [Google Scholar]
- Ding, L.; Ma, S.; Lou, H.; Sun, L.; Ji, M. Synthesis and biological evaluation of curcumin derivatives with water-soluble groups as potential antitumor agents: An in vitro investigation using tumor cell lines. Molecules 2015, 20, 21501–21514. [Google Scholar] [CrossRef]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharm. 2012, 69, 65–70. [Google Scholar] [CrossRef]
- Mahal, A.; Wu, P.; Jiang, Z.H.; Wei, X. Synthesis and Cytotoxic Activity of Novel Tetrahydrocurcumin Derivatives Bearing Pyrazole Moiety. Nat. Prod. Bioprospect. 2017, 7, 461–469. [Google Scholar] [CrossRef]
- Tripathi, A.; Misra, K. Designing and development of novel curcumin analogues/congeners as inhibitors of breast cancer stem cells growth. Chem. Eng. Trans. 2016, 49, 79–84. [Google Scholar]
- Vallianou, N.C.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential Anticancer Properties and Mechanisms of Action of Curcumin. Anticancer. Res. 2015, 35, 645–651. [Google Scholar]
- Beevers, C.S.; Huang, S. Pharmacological and clinical properties of curcumin. Bot. Targets. Ther. 2011, 1, 5–18. [Google Scholar]
- Grynkiewicz, G.; Ślifirski, P. Curcumin and curcuminoids in quest for medicinal status. Acta. Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hackler, L., Jr.; Ózsvári, B.; Gyuris, M.; Sipos, P.; Fábián, G.; Molnár, E.; Marton, A.; Faragó, N.; Mihály, J.; Nagy, L.I.; et al. The curcumin analog C-150, influencing NF-κB, UPR and Akt/Notch pathways has potent anticancer activity in vitro and in vivo. PLoS ONE 2016, 11, e0149832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Z.; Wang, C.; Zhou, H.; Liu, W.; Kanchana, K.; Dai, X.; Zou, P.; Gu, J.; Cai, L.; et al. Curcumin derivative WZ35 efficiently suppresses colon cancer progression through inducing ROS production and ER stress-dependent apoptosis. Am. J. Cancer. Res. 2017, 7, 275–288. [Google Scholar] [PubMed]
- Liu, Y.; Zhou, J.; Hu, Y.; Wang, J.; Yuan, C. Curcumin inhibits growth of human breast cancer cells through demethylation of DLC1 promoter. Mol. Cell. Biochem. 2017, 425, 47–58. [Google Scholar] [CrossRef]
- Pröhl, M.; Schubert, U.S.; Weigand, W.; Gottschaldt, M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Coord. Chem. Rev. 2016, 307, 32–41. [Google Scholar] [CrossRef]
- Kumar, A.P.; Garcia, G.E.; Ghosh, R.; Rajnarayanan, R.V.; Alworth, W.L.; Slaga, T.J. 4-Hydroxy-3-methoxybenzoic acid methyl ester: A curcumin derivative targets Akt/NFκB cell survival signaling pathway: Potential for prostate cancer management. Neoplasia 2003, 5, 255–266. [Google Scholar] [CrossRef]
- Somers-Edgar, T.J.; Taurin, S.; Larsen, L.; Chandramouli, A.; Nelson, M.A.; Rosengren, R.J. Mechanisms for the activity of heterocyclic cyclohexanone curcumin derivatives in estrogen receptor negative human breast cancer cell lines. Invest. New. Drugs 2011, 29, 87–97. [Google Scholar] [CrossRef]
- Shehzad, A.; Khan, S.; Shehzad, O.; Lee, Y.S. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drugs Today 2010, 46, 523–532. [Google Scholar] [CrossRef]
- Mahal, A.; Wu, P.; Jiang, Z.H.; Wei, X. Schiff Bases of Tetrahydrocurcumin as Potential Anticancer Agents. Chem. Select. 2019, 4, 366–369. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Das, M.; Manna, K. Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight. J. Toxicol. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food. Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ju, Y.; Wang, J.; Zhou, R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef]
- Chen, D.; Dai, F.; Chen, Z.; Wang, S.; Cheng, X.; Sheng, Q.; Lin, J.; Chen, W. Dimethoxy curcumin induces apoptosis by suppressing survivin and inhibits invasion by enhancing E-cadherin in colon cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clinical. Res. 2016, 22, 3215. [Google Scholar] [CrossRef]
- Howells, L.M.; Mitra, A.; Manson, M.M. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int. J. Cancer. 2007, 121, 175–183. [Google Scholar] [CrossRef]
- Zhao, Z.; Pi, C.; Ye, Y.; Zhao, L.; Wei, Y. Recent advances of analogues of curcumin for treatment of cancer. Eur. J. Med. Chem. 2019, 180, 524–535. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS. J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Sa, G.; Das, T.; Banerjee, S.; Chakraborty, J. Curcumin: From exotic spice to modern anticancer drug. Al. Ameen. J. Med. Sci. 2010, 3, 21–37. [Google Scholar]
- Zhang, Q.; Li, D.; Liu, Y.; Wang, H.; Zhang, C.; Huang, H.; He, Y.; Chen, X.; Du, Z.; Zheng, X. Potential anticancer activity of curcumin analogs containing sulfone on human cancer cells. Arch. Biol. Sci. 2016, 68, 125–133. [Google Scholar] [CrossRef]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Kuroda, K.; Ramamoorthy, A.; Yasuhara, K. Modulation of raft domains in a lipid bilayer by boundary-active curcumin. Chem. Commun. 2014, 50, 3427–3430. [Google Scholar] [CrossRef] [PubMed]
- Teymouri, M.; Barati, N.; Pirro, M.; Sahebkar, A. Biological and pharmacological evaluation of dimethoxycurcumin: A metabolically stable curcumin analogue with a promising therapeutic potential. J. Cell. Physiol. 2018, 233, 124–140. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front. Oncol. 2018, 8, 614. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Ohori, H.; Yamakoshi, H.; Tomizawa, M.; Shibuya, M.; Kakudo, Y.; Takahashi, A.; Takahashi, S.; Kato, S.; Suzuki, T.; Ishioka, C.; et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer. Ther. 2006, 5, 2563–2571. [Google Scholar] [CrossRef]
- Kumar Dikkala, P.; Shirisha, S.D.S.N. Curcumin and Its Biological Importance: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1100–1105. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Cui, R.; Lin, J.; Ding, X. Curcumin in Treating Breast Cancer: A Review. J. Lab. Autom. 2016, 21, 723–731. [Google Scholar] [CrossRef]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer. Res. 2017, 36, 1–16. [Google Scholar] [CrossRef]
- Liu, H.T.; Ho, Y.S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness. 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lim, J.E.; Hong, J.H. Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate. Cancer Prostatic. Dis. 2010, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Bueso-Ramos, C.; Chatterjee, D.; Pantazis, P.; Aggarwal, B.B. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 2001, 20, 7597–7609. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.; Meng, B.; Suo, J.; Wang, H.; Xie, H.; Jin, Q.; Yao, L.; Wang, R.; Zhang, L. The effect of curcumin on proliferation and apoptosis in LNCaP prostate cancer cells. Chinese J. Clin. Oncol. 2006, 3, 55–60. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, N.Y.; Suh, J.; Kim, D.H.; Kim, W.; Ann, J.; Lee, J.; Baek, J.H.; Na, H.K.; Surh, Y.J. Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer. Lett. 2018, 431, 219–229. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Ives, K.L.; Evers, B.M. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin. Cancer. Res. 2006, 12, 5346–5355. [Google Scholar] [CrossRef]
- Villegas, I.; Sánchez-Fidalgo, S.; Alarcon de la Lastra, C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol. Nutr. Food. Res. 2008, 52, 1040–1061. [Google Scholar] [CrossRef]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin Nanoformulations for Colorectal Cancer: A Review. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Ramasamy, T.S.; Ayob, A.Z.; Myint, H.H.L.; Thiagarajah, S.; Amini, F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: Insights into the mechanism of the therapeutic efficacy. Cancer. Cell. Int. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Naqvi, S.T.Q.; Muhammad, S.A. Curcumin: A polyphenol with molecular targets for cancer control. Asian. Pacific. J. Cancer. Prev. 2016, 17, 2735–2739. [Google Scholar]
- Houssami, N.; Macaskill, P.; Marinovich, M.L.; Dixon, J.M.; Irwig, L.; Brennan, M.E.; Solin, L.J. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur. J. Cancer. 2010, 46, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, P.; Balci, F.L.; Crowe, J.P. Optimizing Surgical Margins in Breast Conservation. Int. J. Surg. 2012. [Google Scholar] [CrossRef] [PubMed]
- Khazaei Koohpar, Z.; Entezari, M.; Movafagh, A.; Hashemi, M. Anticancer Activity of Curcumin on Human Breast Adenocarcinoma: Role of Mcl-1 Gene. Iran. J. Cancer. Prev. 2015, 8. [Google Scholar] [CrossRef]
- Agrawal, D.K.; Kumar Mishra, P. Curcumin and ItsAnalogues: Potential AnticancerAgents Dinesh. Med. Res. Rev. 2010, 35, 818–860. [Google Scholar]
- Cridge, B.J.; Larsen, L.; Rosengren, R.J. Curcumin and its derivatives in breast cancer: Current developments and potential for the treatment of drug-resistant cancers. Oncol. Discov. 2013, 1, 6. [Google Scholar] [CrossRef]
- Razak, N.A.; Akhtar, M.N.; Abu, N.; Ho, W.Y.; Tan, S.W.; Zareen, S.; Nizam bin Taj-ud-din, S.; Long, K.; Alitheen, N.B.; Yeap, S.K. The in vivo anti-tumor effect of curcumin derivative (2E,6E)-2, 6-bis (4-hydroxy-3-methoxybenzylidene) cyclohexanone (BHMC) on 4T1 breast cancer cells. RSC. Adv. 2017, 7, 36185–36192. [Google Scholar] [CrossRef]
- Reddy, C.A.; Somepalli, V.; Golakoti, T.; Kanugula, A.K.; Karnewar, S.; Rajendiran, K.; Vasagiri, N.; Prabhakar, S.; Kuppusamy, P.; Kotamraju, S.; et al. Mitochondrial-targeted curcuminoids: A strategy to enhance bioavailability and anticancer efficacy of curcumin. PLoS ONE 2014, 9, e89351. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, S.; Surolia, A.; Panda, D. C1, a highly potent novel curcumin derivative, binds to tubulin, disrupts microtubule network and induces apoptosis. Biosci. Rep. 2016, 36, e00323. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer. Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Zhao, S.Q.; Du, Z.Y.; Zheng, X.; Zhang, K. Pyridine analogues of curcumin exhibit high activity for inhibiting CWR-22Rv1 human prostate cancer cell growth and androgen receptor activation. Oncol. Lett. 2016, 11, 4160–4166. [Google Scholar] [CrossRef] [PubMed]
- Parsai, S.; Keck, R.; Skrzypczak-Jankun, E.; Jankun, J. Analysis of the anticancer activity of curcuminoids, thiotryptophan and 4-phenoxyphenol derivatives. Oncol. Lett. 2014, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.H.; Shih, C.C.; Lee, K.H. Novel anti-prostate cancer curcumin analogues that enhance androgen receptor degradation activity. Anti-Cancer. Agents. Med. Chem. (Formerly. Curr. Med. Chem. Agents.) 2019, 9, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.A.; Chou, F.J.; Wang, K.; Yang, R.; Ding, J.; Zhang, Q.; Li, G.; Yeh, S.; Xu, D.; Chang, C. Androgen receptor (AR) degradation enhancer ASC-J9® in an FDA-approved formulated solution suppresses castration resistant prostate cancer cell growth. Cancer Lett. 2018, 417, 182–191. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The Curcumin Analog EF24 Targets NF-κB and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef]
- Lin, T.H.; Izumi, K.; Lee, S.O.; Lin, W.J.; Yeh, S.; Chang, C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 2013, 4, 764–769. [Google Scholar] [CrossRef]
- Ohtsu, H.; Xiao, Z.; Ishida, J.; Nagai, M.; Wang, H.K.; Itokawa, H.; Su, C.Y.; Shih, C.; Chiang, T.; Chang, E.; et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem. 2002, 45, 5037–5042. [Google Scholar] [CrossRef]
- Ahsan, M.J. Evaluation of anticancer activity of curcumin analogues bearing a heterocyclic nucleus. Asian Pac. J. Cancer Prev. 2016, 17, 1739–1744. [Google Scholar] [CrossRef]
- Elias, G.; Jacob, P.J.; Hareeshbabu, E.; Mathew, V.B.; Krishnan, B.; Krishnakumar, K. Curcumin: Transforming the spice to a wonder drug. Int. J. Pharm. Sci. Res. 2015, 6, 2671–2680. [Google Scholar]
- Zhou, D.; Ding, N.; Zhao, S.; Li, D.; Van Doren, J.; Qian, Y.; Wei, X.; Zheng, X. Synthesis and evaluation of curcumin-related compounds containing inden-2-one for their effects on human cancer cells. Biol. Pharm. Bull. 2014, 37, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: A double blinded, randomized, placebo-controlled study. Nutri. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Chaithongyot, S.; Asgar, A.; Senawong, G.; Yowapuy, A.; Lattmann, E.; Sattayasai, N.; Senawong, T. Anticancer effects of curcuma C20-dialdehyde against colon and cervical cancer cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 6513–6519. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, J.; Banerjee, S.; Kanwar, S.S.; Yu, Y.; Patel, B.B.; Sarkar, F.H.; Majumdar, A.P. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer 2011, 128, 951–961. [Google Scholar] [CrossRef]
- Butterworth, A.S.; Higgins, J.P.T.; Pharoah, P. Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur. J. Cancer 2006, 42, 216–227. [Google Scholar] [CrossRef]
- Lynch, H.T.; de la Chapelle, A. Hereditary Colorectal Cancer Colorectal Cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and Familial Colon Cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Andre, W. Cancer risk in patients with inflammatory bowel disease: A Population-Based Study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, A.; Li, W.; Cai, P.; Yang, B.; Zhang, M.; Gu, Y.; Shu, Y.; Sun, Y.; Shen, Y.; et al. Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed. Pharm. 2014, 68, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- LaValle, C.R.; George, K.M.; Sharlow, E.R.; Lazo, J.S.; Wipf, P.; Wang, Q.J. Protein kinase D as a potential new target for cancer therapy. Biochim. Biophys. Acta - Rev. Cancer 2010, 1806, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Prev. Biomark. 2005, 14, 120–125. [Google Scholar]
- Zheng, A.; Li, H.; Wang, X.; Feng, Z.; Xu, J.; Cao, K.; Zhou, B.; Wu, J.; Liu, J. Anticancer effect of a curcumin derivative B63: ROS production and mitochondrial dysfunction. Curr. Cancer Drug Targets 2014, 14, 156–166. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Q.; Wang, S.; Dai, F.; Cheng, X.; Cheng, X.; Chen, W.; Zhang, M.; Chen, D. In vitro additive antitumor effects of Dimethoxycurcumin and 5-fluorouracil in colon cancer cells. Cancer Med. 2017, 6, 1698–1706. [Google Scholar] [CrossRef]
- Tamvakopoulos, C.; Dimas, K.; Sofianos, Z.D.; Hatziantoniou, S.; Han, Z.; Liu, Z.L.; Wyche, J.H.; Pantazis, P. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin. Cancer Res. 2007, 13, 1269–1277. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer. 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Kim, J.M.; Araki, S.; Kim, D.J.; Park, C.B.; Takasuka, N.; Baba-Toriyama, H.; Ota, T.; Nir, Z.; Khachik, F.; Shimidzu, N.; et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1, 2-dimethylhydrazine initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Z.; Cao, Y.; Zhu, C.; Zuo, Y.; Huang, L.; Wen, G.; Shang, N.; Chen, Y.; Yue, X.; et al. MC37, a new mono-carbonyl curcumin analog, induces G2/M cell cycle arrest and mitochondria-mediated apoptosis in human colorectal cancer cells. Eur. J. Pharmacol. 2017, 796, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules. 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.M.; Yeap, S.K.; Abu, N.; Lim, K.L.; Ky, H.; Pauzi, A.Z.M.; Ho, W.Y.; Tan, S.W.; Alan-Ong, H.K.; Zareen, S.; et al. Synthetic curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell. Int. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.T.; Figg, W.D. The potential role of curcumin in prostate cancer: The importance of optimizing pharmacokinetics in clinical studies. Transl. Cancer Res. 2016, 5, S1107–S1110. [Google Scholar] [CrossRef]
- Teiten, M.H.; Gaascht, F.; Eifes, S.; Dicato, M.; Diederich, M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010, 5, 61. [Google Scholar] [CrossRef]
- Basile, V.; Belluti, S.; Ferrari, E.; Gozzoli, C.; Ganassi, S.; Quaglino, D.; Saladini, M.; Imbriano, C. bis-Dehydroxy-Curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy. PLoS ONE 2013, 8, e53664. [Google Scholar] [CrossRef]
- Sarika, P.R.; James, N.R.; Nishna, N.; Kumar, P.A.; Raj, D.K. Galactosylated pullulan–curcumin conjugate micelles for site specific anticancer activity to hepatocarcinoma cells. Colloids. Surf. B Biointerfaces 2015, 133, 347–355. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M. Combinations of the antioxidants sulforaphane or curcumin and the conventional antineoplastics cisplatin or doxorubicin as prospects for anticancer chemotherapy. Eur. J. Pharmacol. 2019, 859, 172513. [Google Scholar] [CrossRef]

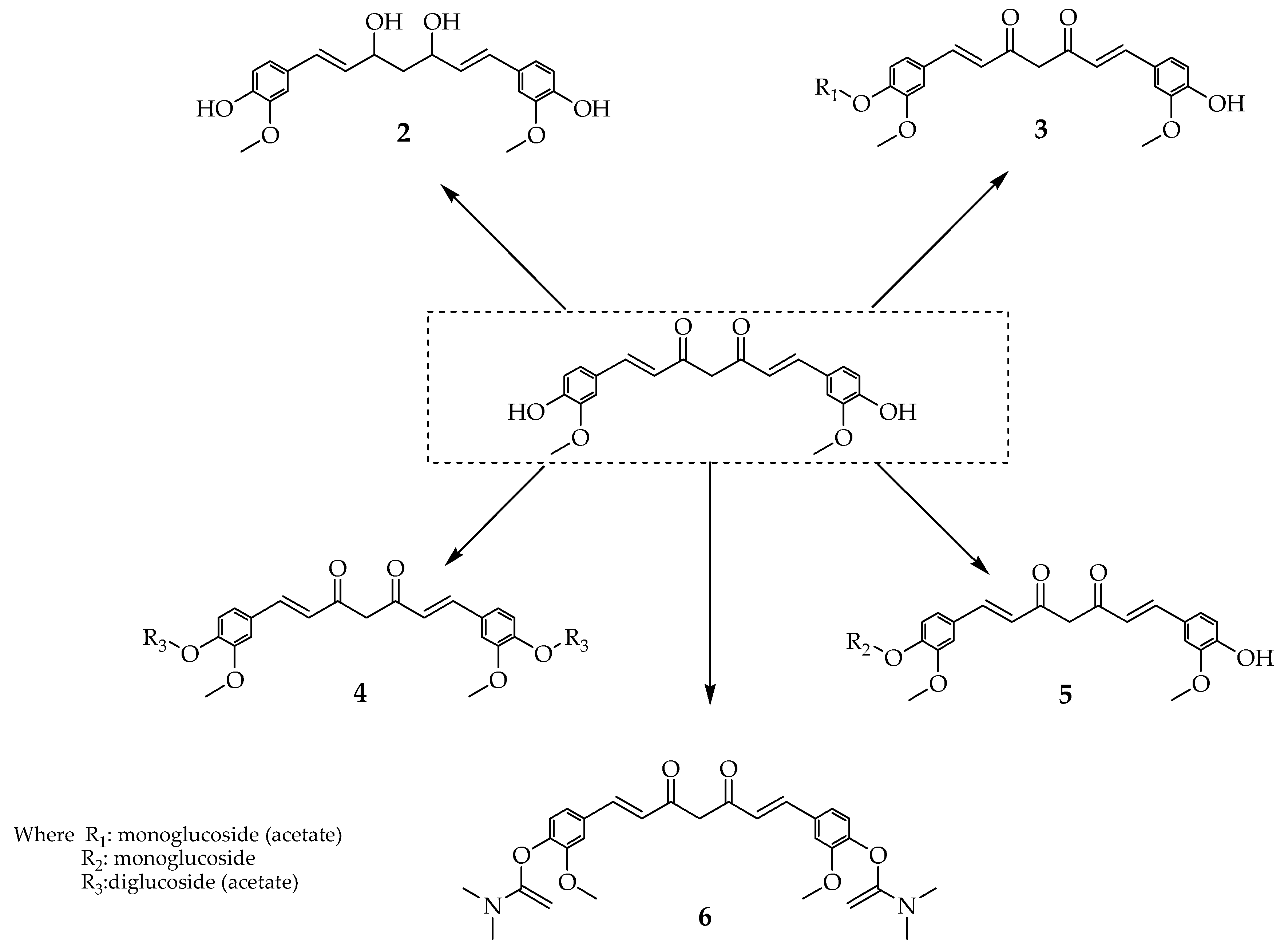
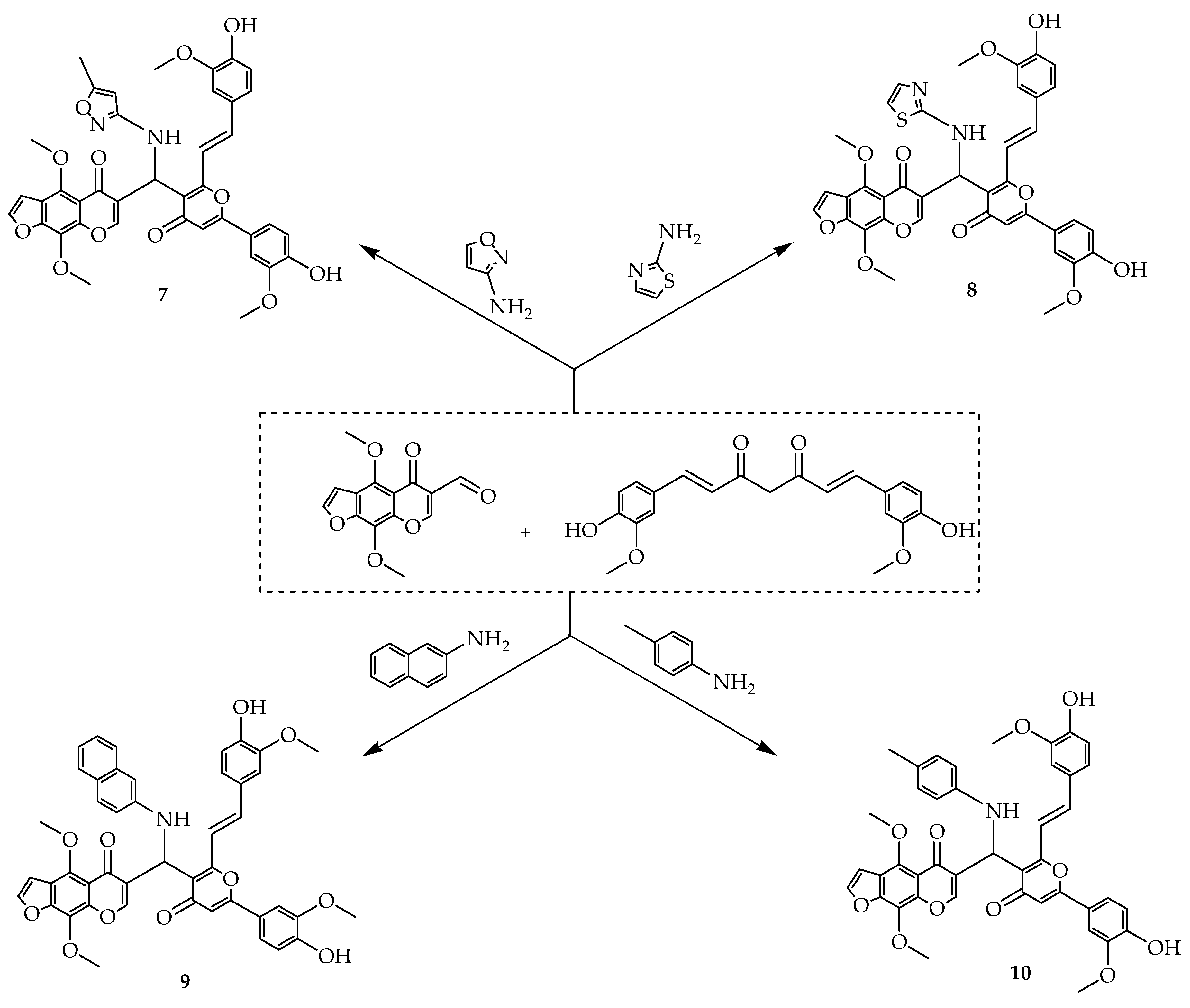


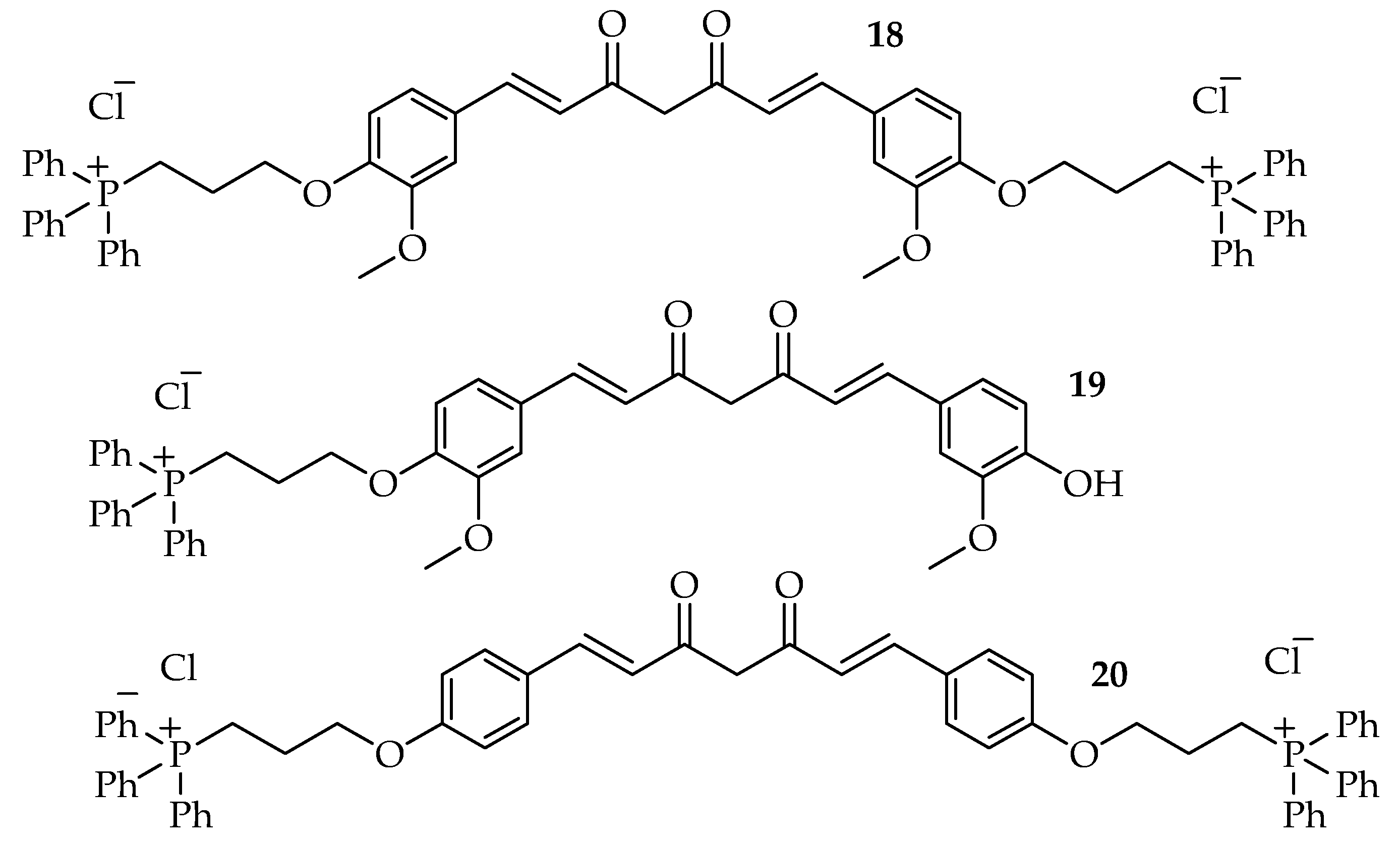
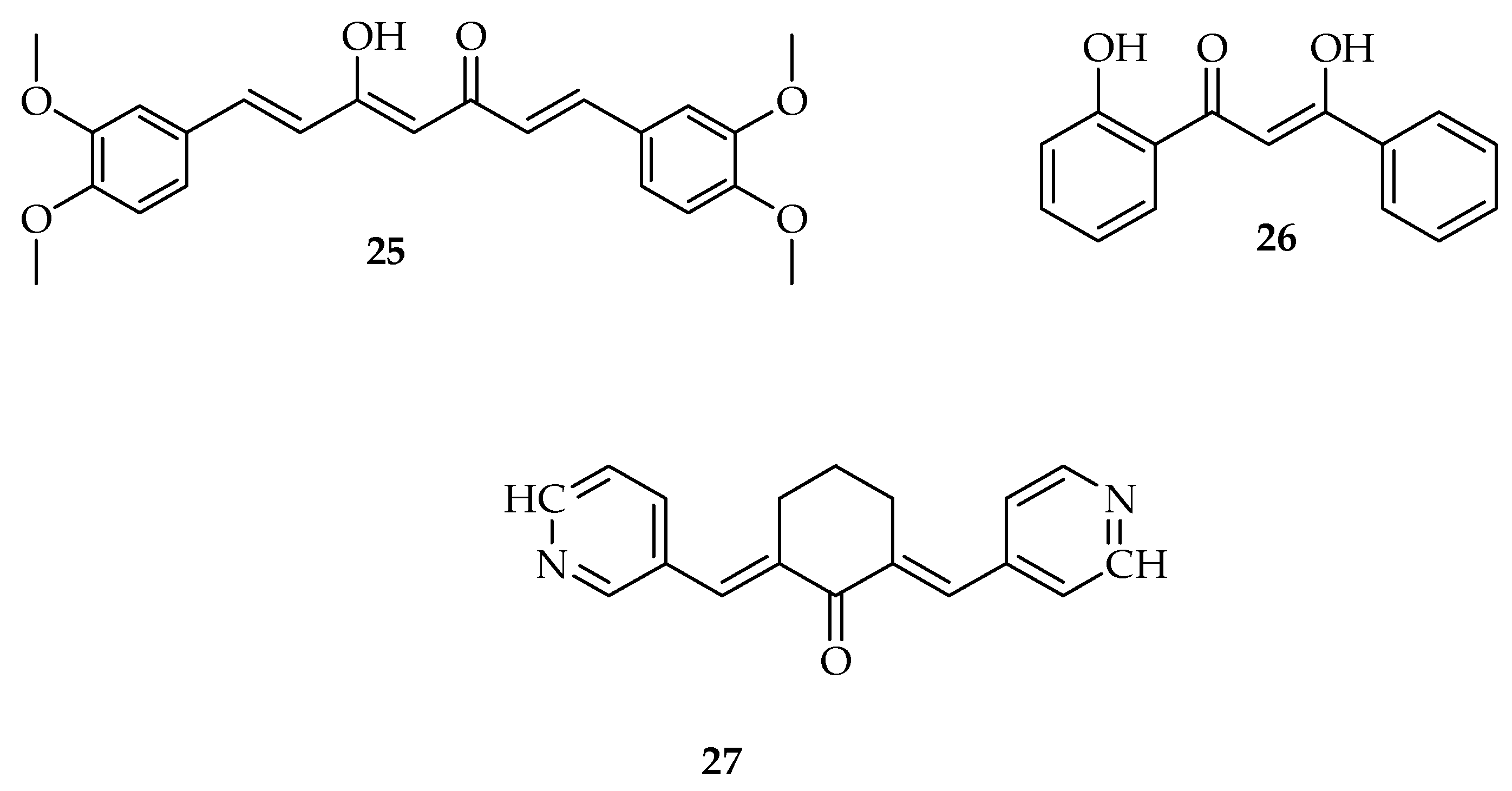
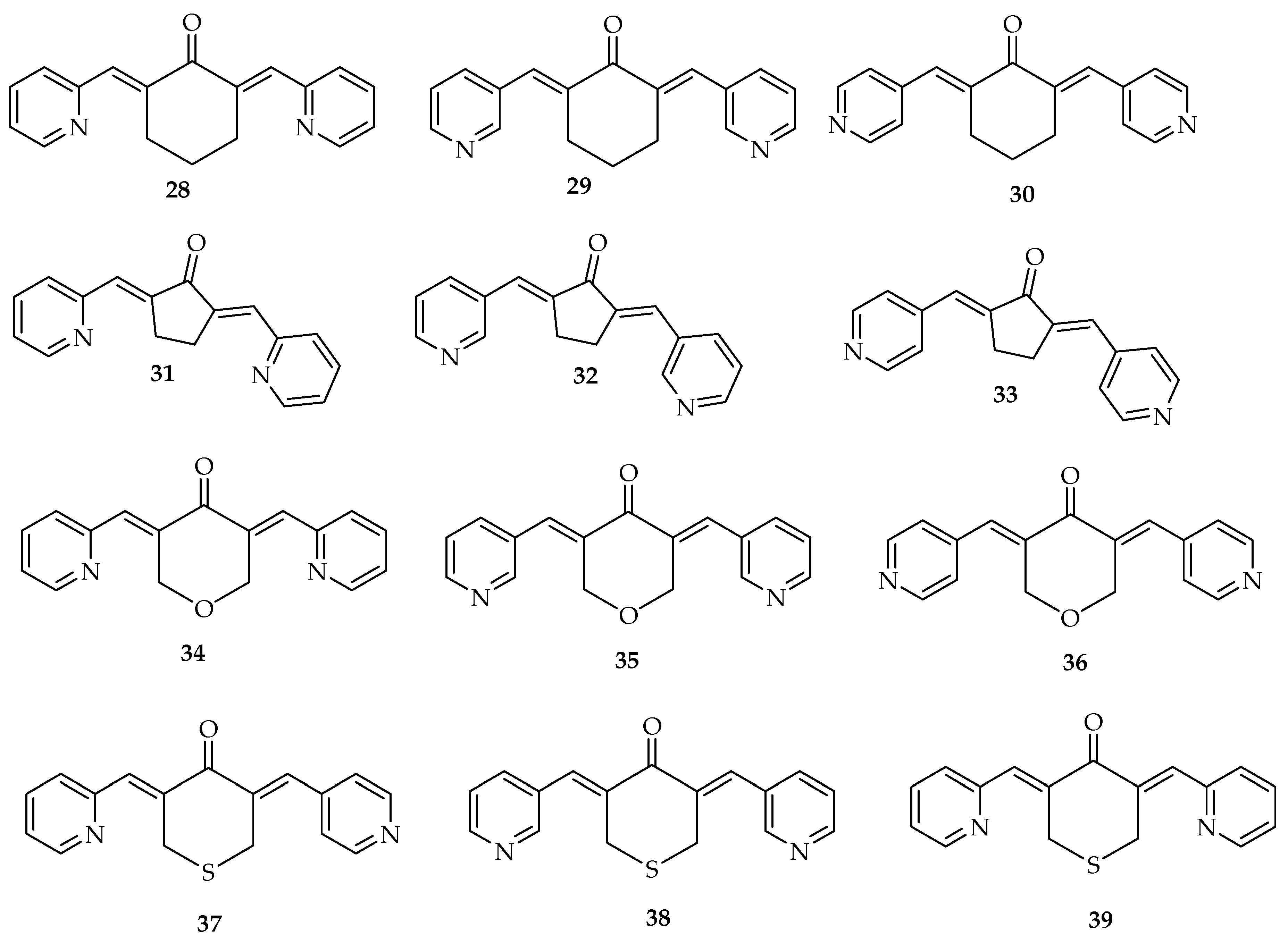
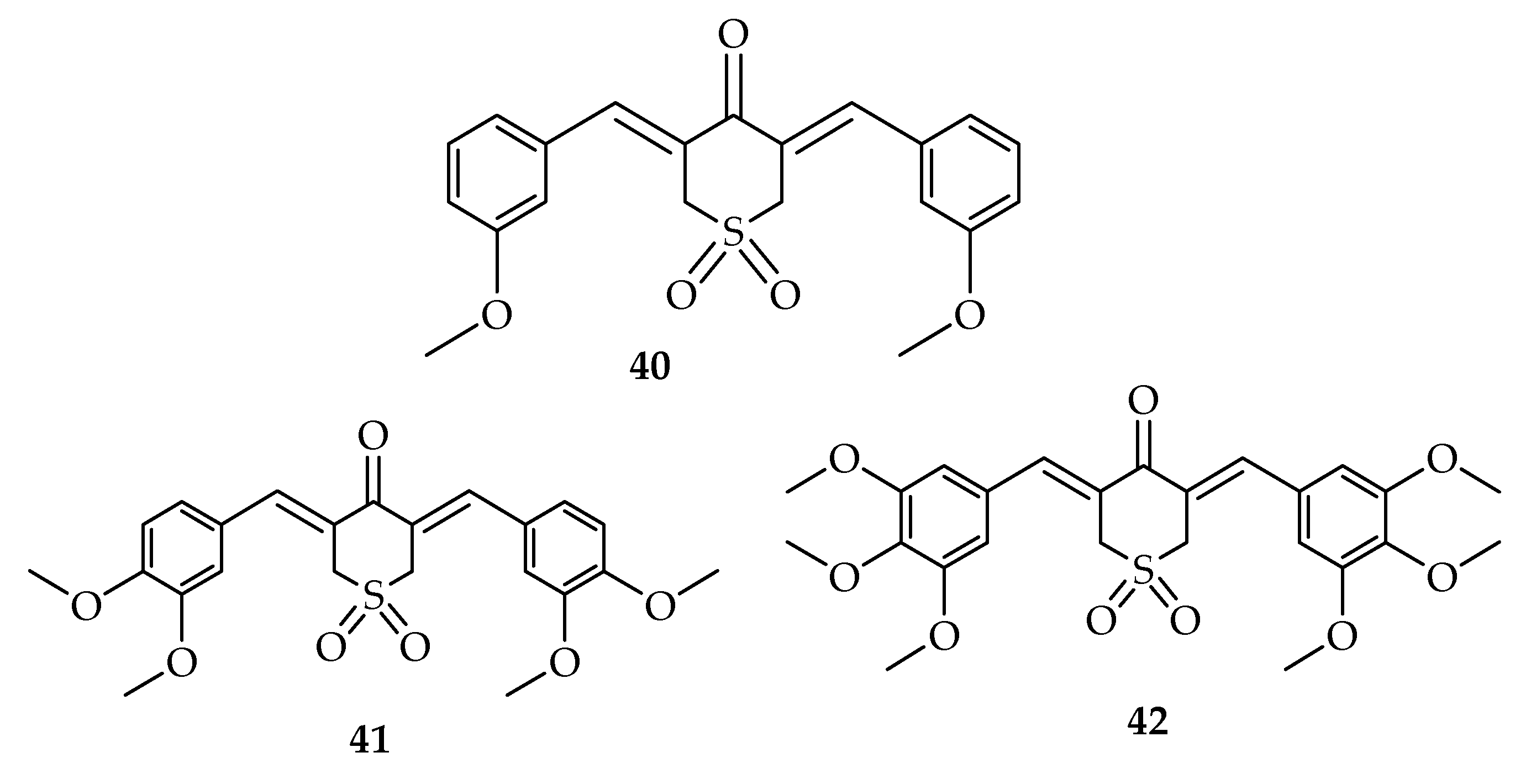
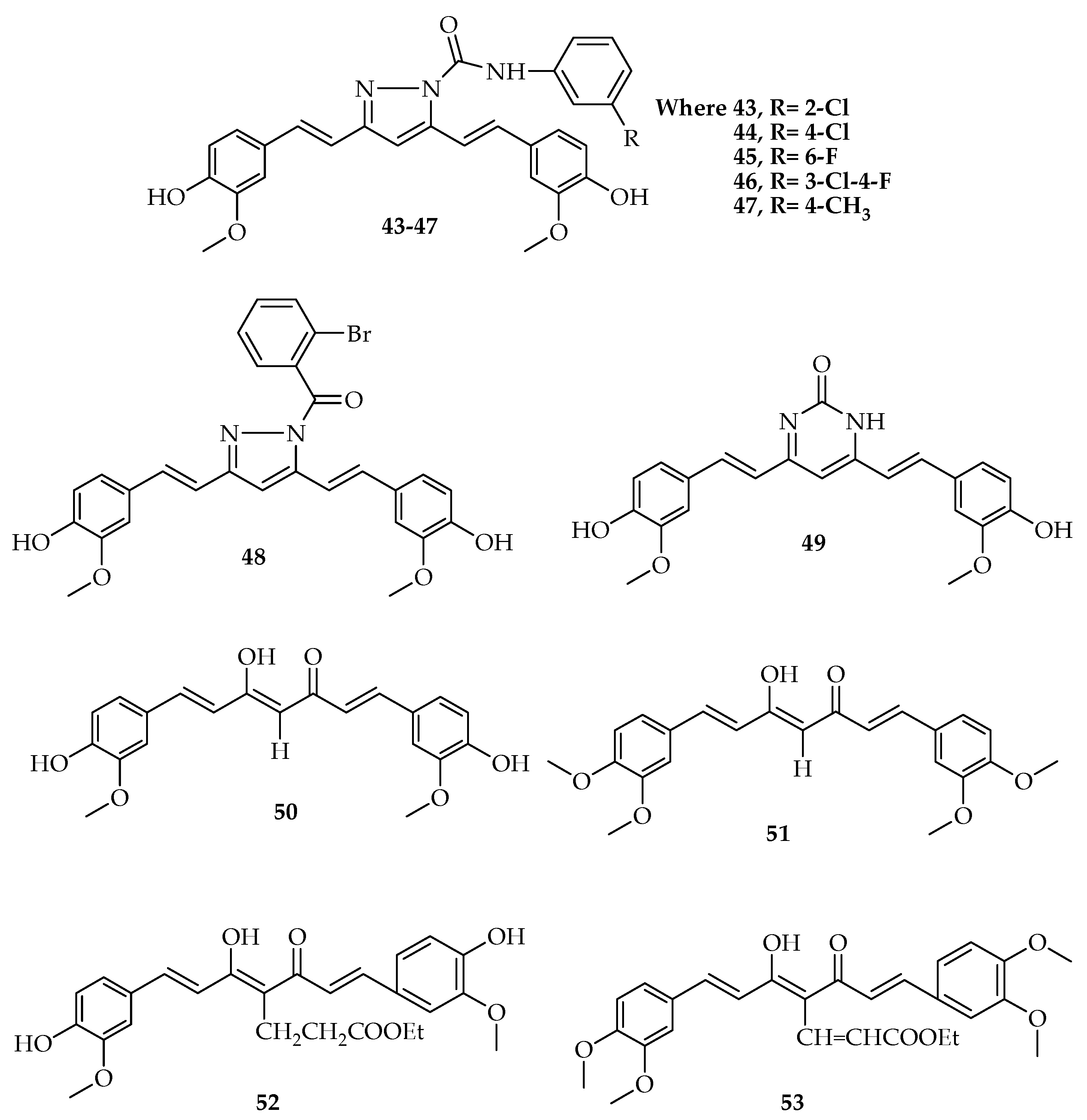
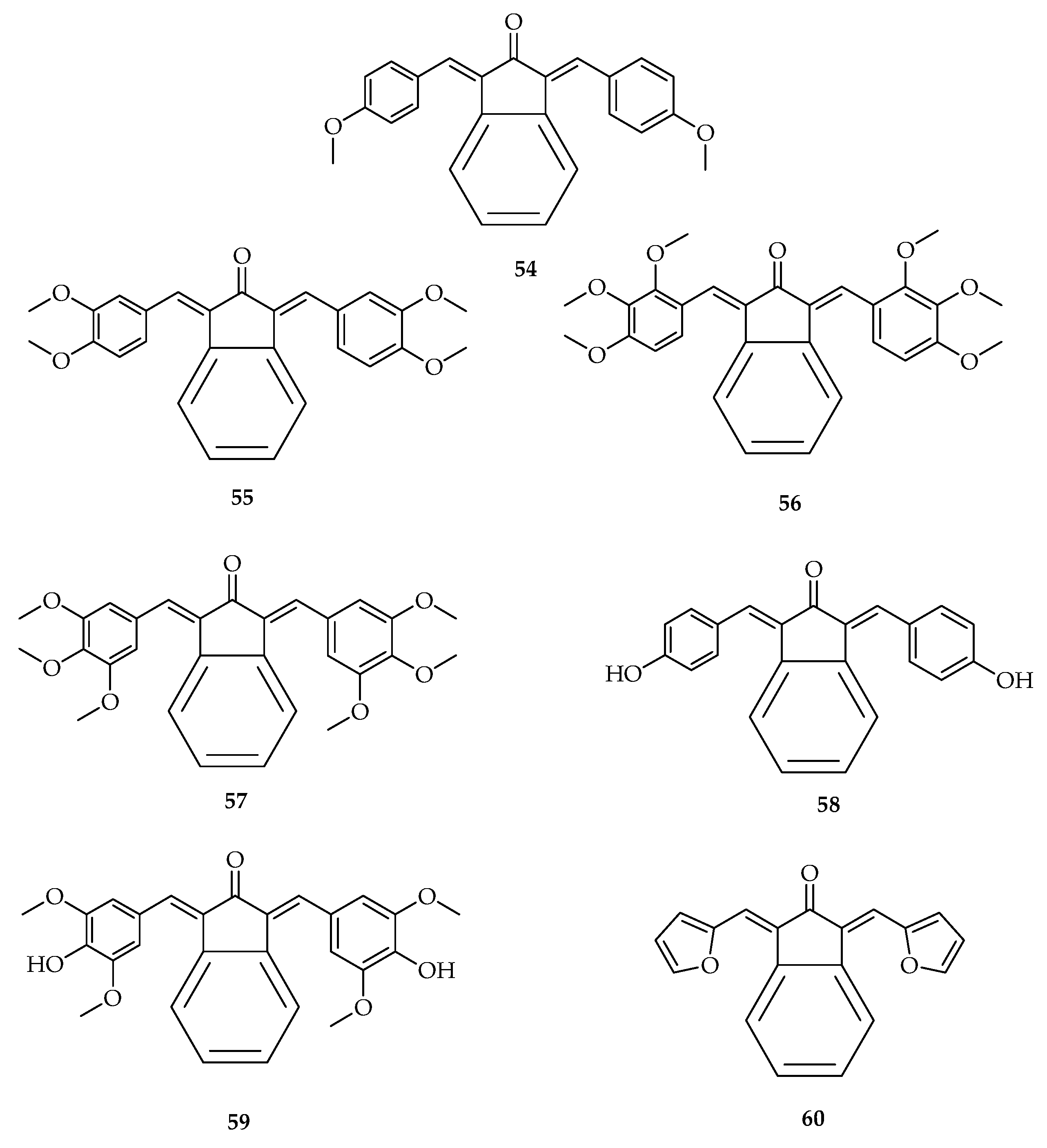
| Types of Cancer | Molecular Targets of Curcumin | References |
|---|---|---|
| Breast cancer | Regulates the apoptosis, cell phase-related genes, and micro-RNA in breast cancer cells. | [38] |
| Modulates carcinogenesis of the breast. | [39] | |
| Inhibits the proliferation of BCSC (breast cancer stem cells). | [40] | |
| Inhibit the stem-like properties and regulates the EMT (epithelial-mesenchymal transition) process. | [41] | |
| Prostate cancer | Inhibits proliferation and the ability of colony formation of prostate cancer cells. | [42] |
| Inhibits phosphorylation of downstream targets of the LNCaP cells. | [43] | |
| Inhibits the NF-κb-regulate gene products in the DU-145 cells. | [44] | |
| Inhibits the expression of the androgen receptor of the LNCaP cells. | [45] | |
| Colon cancer | Suppresses the oncogenicity of human-colon cancer cells in human colon-cancer cells. | [46] |
| Inhibits AP-1 and NF-κB signaling pathways, suppress JNK activation induced by carcinogens. | [47] | |
| Inhibits PKC activation by inhibiting the release of Ca2+ from the endoplasmic reticulum. | [48] | |
| Inhibits carcinogenesis in various types of cancer including colorectal cancer and curcumin is able to inhibit the inflammatory response and the oxidative stress and to induce apoptosis in cancer cells | [49] | |
| Suppresses the expression of EGFR, mediated by the reduction of Egr-1 activity in Caco2, and HT29 colon cancer cells, inhibiting colon cancer cell growth. | [50] | |
| Reduce the size of tumor mass and growth in both in vivo and in vitro studies by affecting many intracellular events that are associated with cancer progression and cancer stem cells formation. | [51] | |
| Suppresses LPS-induced cyclooxygenase-2 gene expression by inhibiting AP-1 DNA binding in BV2 microglial cells. | [52] | |
| Inhibits the cell proliferation followed by suppression of EGFR gene and cyclin D1 gene expression. | [53] |
| Compounds | IC50 µM |
|---|---|
| MCF-7 | |
| 7 | 33 |
| 8 | 20 |
| 9 | 33 |
| 10 | 37 |
| Compounds | IC50 µM | ||||
|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | MDA-MB-468 | MDA-MB-453 | SKBr3 | |
| 1 | - | 7.6 | 1 | - | - |
| 15 | 2.4 | 2.8 | 0.3 | 4.7 | 5.7 |
| 16 | 1.7 | 2.7 | 0.3 | 1.3 | 3.8 |
| 17 | - | 5 | - | - | - |
| Compounds | IC50 µM |
|---|---|
| MCF-7 | |
| 1 | 40.32 |
| 18 | 2.31 |
| 19 | 5.31 |
| 20 | 3.84 |
| Compound | IC50 µM |
|---|---|
| MCF-7 | |
| 1 | 17.1 ± 0.7 |
| 21 | 1.5 ± 0.7 |
| 22 | 26.2 ± 3 |
| 23 | 2.9 ± 0.4 |
| 24 | 6.3 ± 0.2 |
| Compounds | IC50 µM |
|---|---|
| PC-3 | |
| 1 | 16.99 ± 2.1 |
| 28 | 0.53.± 0.1 |
| 29 | 0.92 ± 0.1 |
| 30 | 0.95 ± 0.2 |
| 31 | 4.75 ± 0.5 |
| 32 | 4.99 ± 0.5 |
| 33 | 3.03 ± 0.4 |
| 34 | 2.18 ± 0.2 |
| 35 | 1.07 ± 0.1 |
| 36 | 1.80 ± 0.2 |
| 37 | 0.66 ± 0.1 |
| 38 | 0.55 ± 0.1 |
| 39 | 0.49 ± 0.1 |
| Compounds | IC50 µM | |||
|---|---|---|---|---|
| PC-3 | H1299 | HT-29 | BxPC-3 | |
| 1 | 21.64 ± 1.83 | 19.87 ± 0.94 | 18.39 ± 0.35 | 18.25 ± 1.27 |
| 40 | 1.73 ± 0.26 | 1.24 ± 0.08 | 0.19 ± 0.14 | 1.01 ± 0.11 |
| 41 | 0.85 ± 0.10 | 0.58 ± 0.04 | 0.38 ± 0.15 | 0.32 ± 0.08 |
| 42 | 0.72 ± 0.17 | 0.46 ± 0.01 | 0.29 ± 0.09 | 0.29 ± 0.09 |
| Compounds | IC50 µM | |
|---|---|---|
| PC-3 | LNCaP | |
| 50 | 7.7 | 3.8 |
| 51 | 1.1 | 1.3 |
| 52 | 5.1 | 1.5 |
| 53 | 1.0 | 0.2 |
| Compounds | IC50 µM | |
|---|---|---|
| PC-3 | RWPE-1 | |
| 1 | 19.98 ± 2.4 | 15.62 ± 1.5 |
| 54 | 8.30 ± 0.9 | 39.26 ± 5.1 |
| 55 | 3.05 ± 0.4 | 18.13 ± 5.4 |
| 56 | 10.06 ± 1.3 | 18.44 ± 1.1 |
| 57 | 0.64 ± 0.1 | 9.12 ± 0.4 |
| 58 | 9.6 ± 1.1 | 29.23 ± 3.9 |
| 59 | 2.46 ± 0.3 | 4.2 ± 0.5 |
| 60 | 8.12 ± 0.9 | 27.66 ± 2.3 |
| Compounds | R1 | R2 | R3 | IC50 (μM) ± SD) |
|---|---|---|---|---|
| 68 | OMe | OMe | Me | 3.84 ± 0.19 |
| 69 | OMe | OMe | Et | 1.84 ± 0.11 |
| 70 | H | H | Me | 3.78 ± 0.31 |
| 71 | H | H | Et | 5.97 ± 0.28 |
| 72 | OMe | H | Me | 4.40 ± 0.15 |
| 73 | OMe | H | Et | 9.60 ± 0.31 |
| Type of Cancers | Curcumin Derivatives | Molecular Targets | References |
|---|---|---|---|
| Breast cancer | 28 | Inhibits many different types of steroid receptors in breast cancer cells | [26] |
| 29 | On MCF-7, reduce the number of cells and induced shrinkage of cells. It significantly downregulated the expression of PLK1, whereas improved the appearance of p21 and WEE-1 | [95] | |
| 30 | Induces G2/M-phase cell cycle arrest and apoptosis significantly. It modulates the expression of main cell signaling proteins, precisely, in AKt, SKBr3 cells, and protein levels of Her-2. | [17] | |
| 15 and 16 | Inhibits AKt, STAT3, and HER2/Neu pathways and also | [58] | |
| induced apoptosis at IC50 value of 10 µM. | |||
| 2–6 | Prevents the development of breast cancer stem cell growth by decreasing P-gp mediated efflux process | [8] | |
| 18 | Inhibits Akt and STAT3 phosphorylation and significantly increased ERK phosphorylation. | [60] | |
| 21 | Induces p53 mediated apoptosis against MCF-7 cells. In MCF-7 cells, it disturbs microtubules and induces p53 dependent apoptotic cell death | [61] | |
| Prostate cancer | 28 | Increases androgen receptor degradation in androgen-dependent prostate cancer cells. | [20] |
| Reduces ARs with the F876L mutation in DU-145 and C4-2 cells, and destroys prostate cancer stem/progenitor (S/P) cell invasion through the alteration of EZH2/STAT3 signaling in mice with CWR-22Rv1 CD133+ S/P xenografts. | [96] | ||
| 40–42 | Reduces ARs with the F876L mutation in DU-145 and C4–2 cells, and destroy prostate cancer stem/progenitor (S/P) cell invasion through the alteration of EZH2/STAT3 signaling in mice with CWR-22Rv1 CD133+ S/P xenografts. | [96] | |
| Reduces the level of phosphorylated signal transducer and activator of transcription-3 (p-STAT3) (Tyr705). | [30] | ||
| 68 and 69 | Prevents the growth of androgen-dependent and -independent prostate cancer cells with a sub micromolar range of IC50 values. | [97] | |
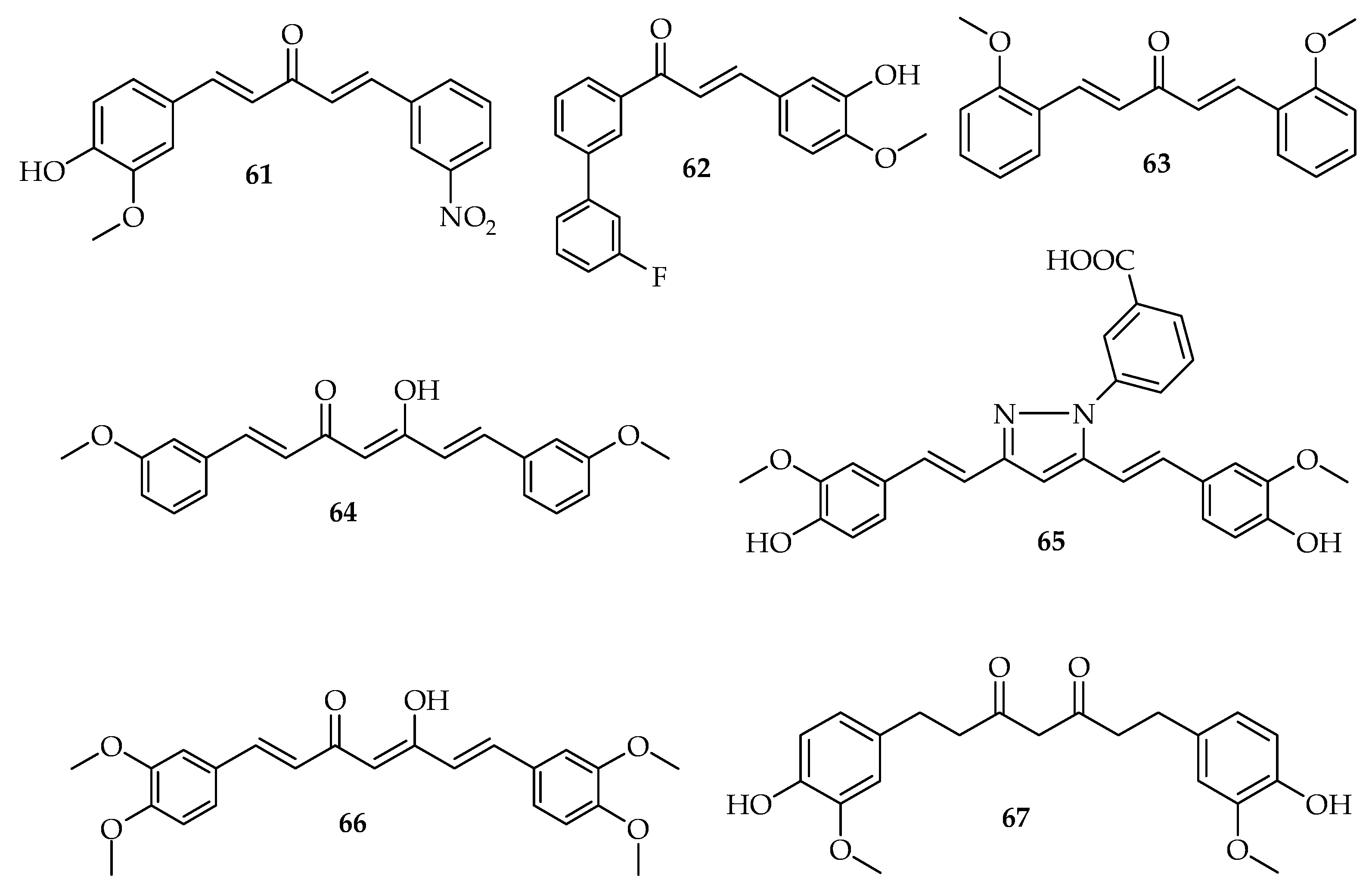
| Colon cancer | 61 | Inhibits cell the proliferation of colon cells. | [13] |
| 63 | Shows antiproliferative effects. Induces the cell cycle arrest, the necrosis, and the apoptosis in human colon cancer. | [86] | |
| 64 | Induces the autophagy and enhances the antiproliferative activity on colon cancer cells. | [98] | |
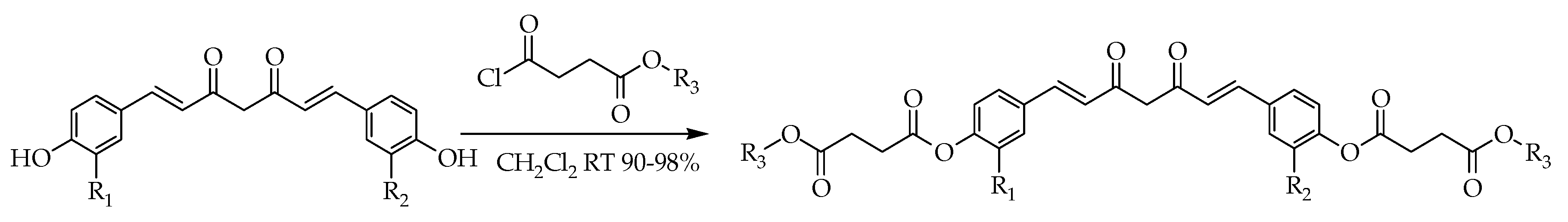
| 66 | Inhibits the proliferation and inducing apoptosis. | [25,87,88,89] | |
| 62 | Inhibits the growth of colon cancer cells and blocks cell cycle progression. | [91] | |
| 67 | Inhibits the aberrant crypt foci (ACF) development and cell proliferation. | [90] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. https://doi.org/10.3390/molecules24234386
Mbese Z, Khwaza V, Aderibigbe BA. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules. 2019; 24(23):4386. https://doi.org/10.3390/molecules24234386
Chicago/Turabian StyleMbese, Zintle, Vuyolwethu Khwaza, and Blessing Atim Aderibigbe. 2019. "Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers" Molecules 24, no. 23: 4386. https://doi.org/10.3390/molecules24234386
APA StyleMbese, Z., Khwaza, V., & Aderibigbe, B. A. (2019). Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules, 24(23), 4386. https://doi.org/10.3390/molecules24234386