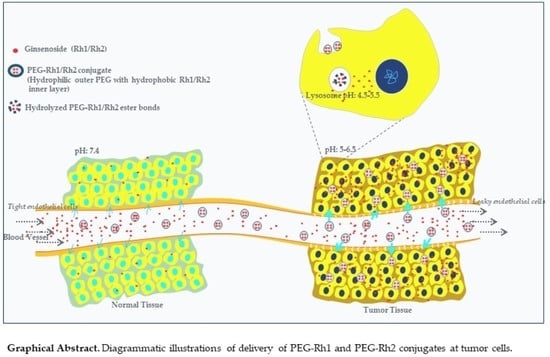
Preparation of Polyethylene Glycol-Ginsenoside Rh1 and Rh2 Conjugates and Their Efficacy against Lung Cancer and Inflammation
Abstract
1. Introduction
2. Results and Discussion
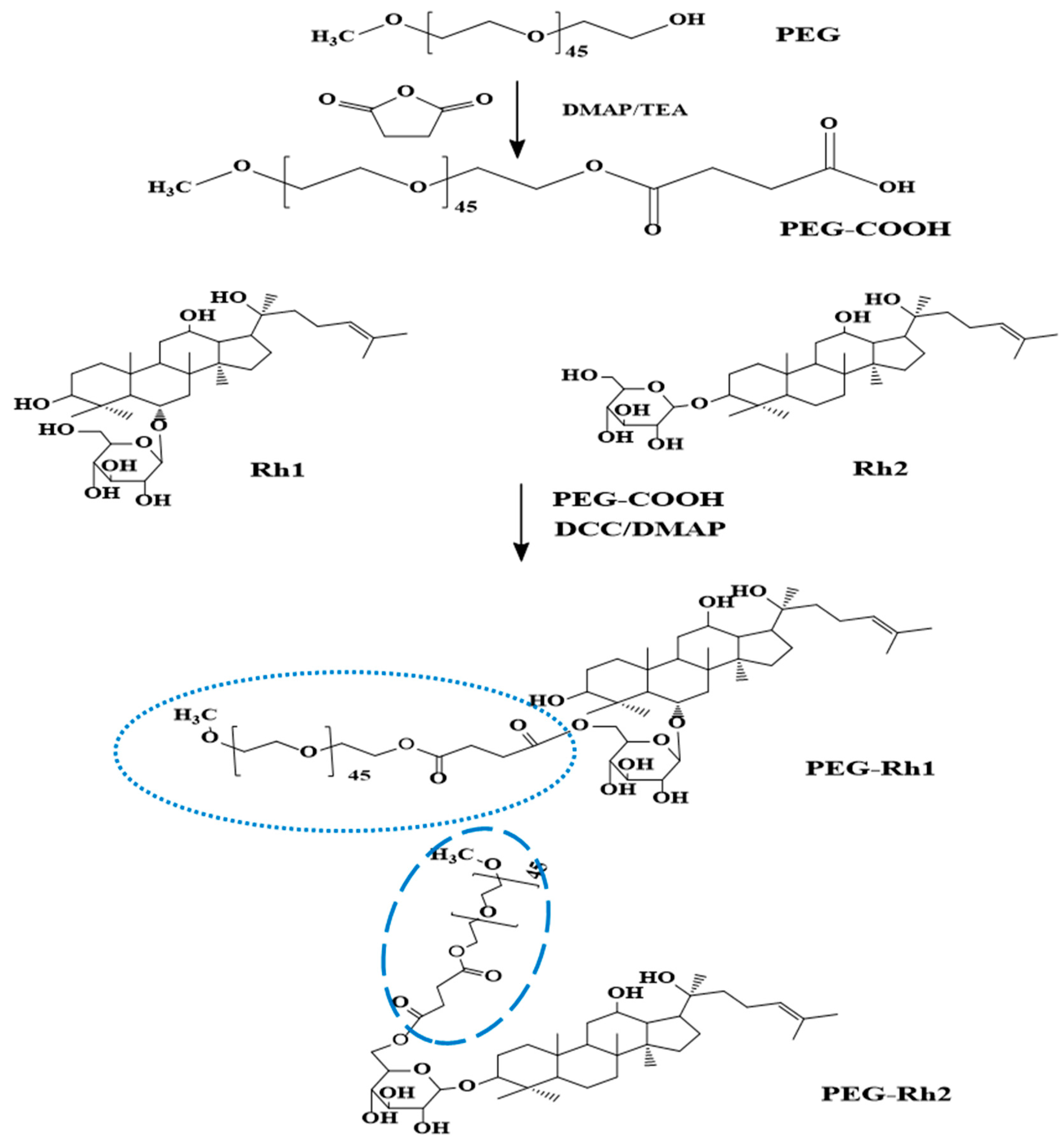
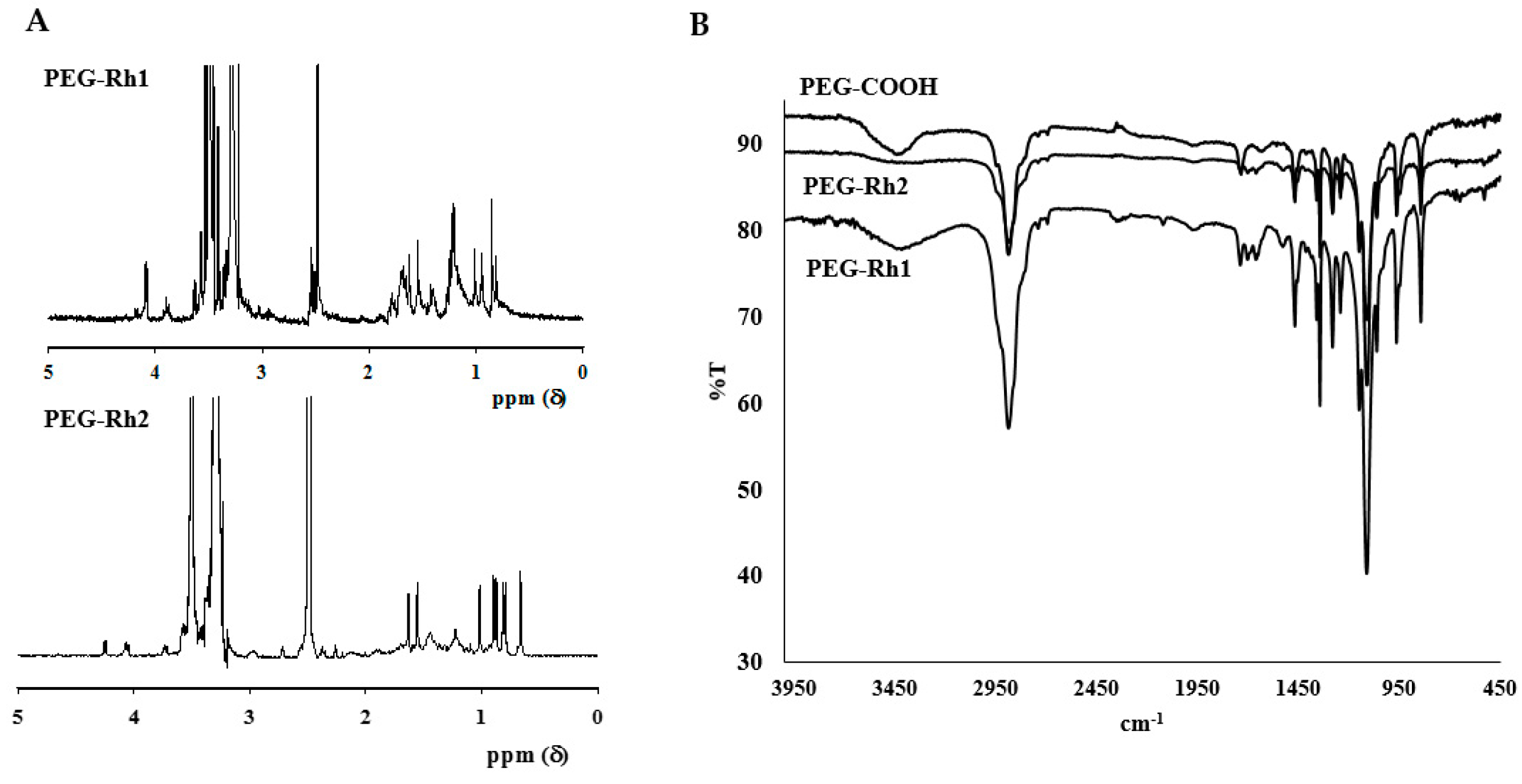
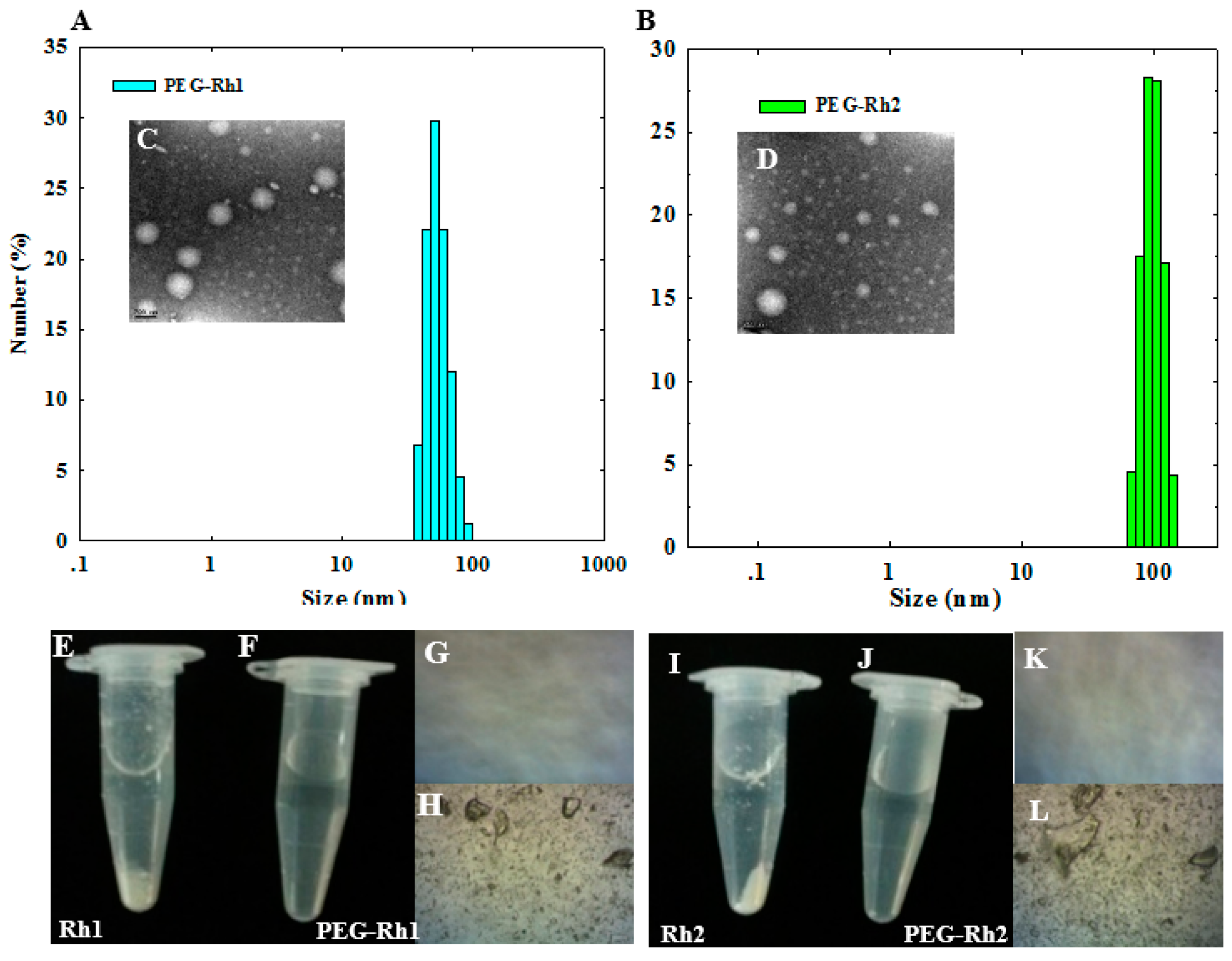
2.1. Synthesis and Physiochemical Characterization of PEG-Rh1 and PEG-Rh2 Conjugates
2.2. Solubility of the PEG-Rh1 and PEG-Rh2 Conjugates
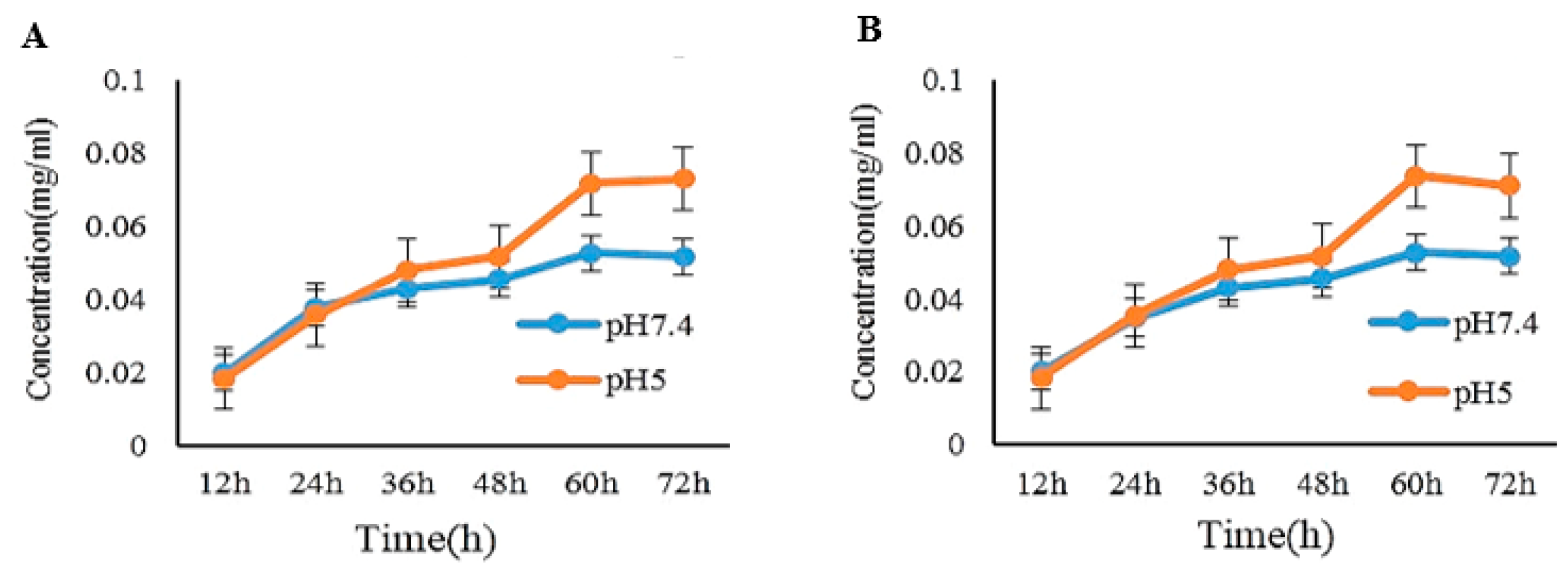
2.3. pH-Dependent Release of Rh1 and Rh2 from the PEG-Rh1 and PEG-Rh2 Conjugates
2.4. In Vitro Cytotoxicity Inhibition of lipopolysaccharide (LPS)-Induced nitric oxide (NO) Production by PEG-Rh1 and PEG-Rh2 Conjugates
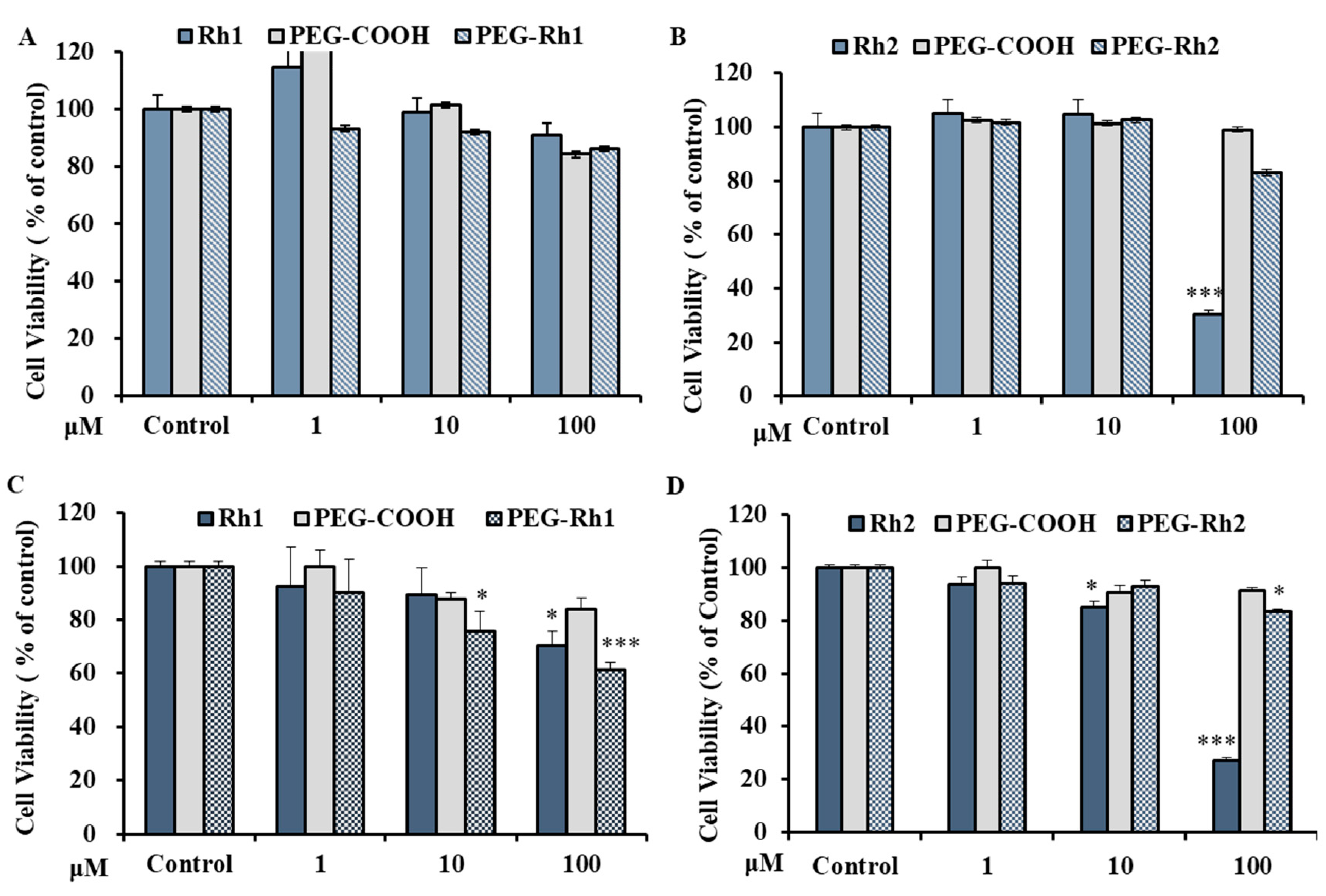
2.5. In Vitro Cytotoxicity of PEG-Rh1 and PEG-Rh2 in Lung Cancer A549 Cell Line
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PEG-Rh1and PEG-Rh2Conjugates
3.3. Characterizations of the Structure of the PEG-Rh1 and PEG-Rh2 Conjugates
3.4. In Vitro pH-Dependent Release of Rh1 and Rh2 from the PEG-Rh1 and PEG-Rh2 Conjugates
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Measurement of Nitrite Levels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [PubMed]
- Bonomi, P. Paclitaxel poliglumex (PPX, CT-2103): Macromolecular medicine for advanced non-small-cell lung cancer. Expert Rev. Anticancer Ther. 2007, 7, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, R.L.; Buckle, T.; Lambert, G.; Garrigue, J.S.; Beijnen, J.H.; Schellens, J.H.M.; van Tellingen, O. Paclitaxel in self-micro emulsifying formulations: Oral bioavailability study in mice. Investig. New Drugs 2010, 29, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.-H.; Son, S.; Jo, D.-G.; Ahn, C.-H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Thambi, T.; Park, J.H. Recent Advances in Shell-Sheddable Nanoparticles for Cancer Therapy. J. Biomed. Nanotechnol. 2014, 10, 1841–1862. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Thambi, T.; Deepagan, V.G.; Saravanakumar, G.; Ko, H.; Kang, Y.M.; Park, J.H. Carboxymethyl Dextran-Cyclodextrin Conjugate as the Carrier of Doxorubicin. J. Nanosci. Nanotechnol. 2013, 13, 7271–7278. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Thambi, T.; Park, J.H. Mineralized cyclodextrin nanoparticles for sustained protein delivery. Carbohydr. Polym. 2013, 97, 643–649. [Google Scholar] [CrossRef]
- Min, K.H.; Park, K.; Kim, Y.-S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.-W.; Kim, I.-S.; Jeong, S.Y.; Kim, K.; et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218. [Google Scholar] [CrossRef]
- Thambi, T.; You, D.G.; Han, H.S.; Deepagan, V.G.; Jeon, S.M.; Suh, Y.D.; Choi, K.Y.; Kim, K.; Kwon, I.C.; Yi, G.-R.; et al. Bioreducible Carboxymethyl Dextran Nanoparticles for Tumor-Targeted Drug Delivery. Adv. Healthc. Mater. 2014, 3, 1829–1838. [Google Scholar] [CrossRef]
- Thambi, T.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Yoo, C.K.; Park, J.H. Bioreducible Block Copolymers Based on Poly(Ethylene Glycol) and Poly(γ-Benzyl l-Glutamate) for Intracellular Delivery of Camptothecin. Bioconjug. Chem. 2011, 22, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Son, S.; Lee, D.S.; Park, J.H. Poly(ethylene glycol)-b-poly(lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomater. 2016, 29, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhan, P.; de Clercq, E.; Lou, H.; Liu, X. Current drug research on PEGylation with small molecular agents. Prog. Polym. Sci. 2013, 38, 421–444. [Google Scholar] [CrossRef]
- Cai, L.; Qiu, N.; Xiang, M.; Tong, R.; Yan, J.; He, L.; Shi, J.; Chen, T.; Wen, J.; Wang, W.; et al. Improving aqueous solubility and antitumor effects by nanosized gambogic acid-mPEG₂₀₀₀ micelles. Int. J. Nanomed. 2014, 9, 243–255. [Google Scholar]
- Liu, M.; Wang, L.; Hu, K.; Feng, J. Study on the stability of ginsenoside Rg1 before and after PEG modification. Med. Plant 2013, 4, 28–31. [Google Scholar]
- Li, J.; Wang, Y.; Yang, C.; Wang, P.; Oelschlager, D.K.; Zheng, Y.; Tian, D.-A.; Grizzle, W.E.; Buchsbaum, D.J.; Wan, M. Polyethylene Glycosylated Curcumin Conjugate Inhibits Pancreatic Cancer Cell Growth through Inactivation of Jab1. Mol. Pharmacol. 2009, 76, 81–90. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Kim, Y.J.; Wang, C.; Jin, Y.; Subramaniyam, S.; Singh, P.; Wang, D.; Yang, D.C. Protopanaxadiol aglycone ginsenoside-polyethylene glycol conjugates: Synthesis, physicochemical characterizations, and in vitro studies. Artif. Cellsnanomed. Biotechnol. 2016, 44, 1803–1809. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Subramaniyam, S.; Kim, Y.J.; Natarajan, S.; Min, J.W.; Kim, S.Y.; Yang, D.C. Synthesis and pharmacokinetic characterization of a pH-sensitive polyethylene glycol ginsenoside CK (PEG-CK) conjugate, Bioscience. Biotechnol. Biochem. 2014, 78, 466–468. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, X.; Liu, Y.; Bo, L.; Liu, X. Synthesis of poly(ethylene glycol)–metaxalone conjugates and study of its controlled release in vitro. Int. J. Pharm. 2007, 332, 125–131. [Google Scholar] [CrossRef]
- Toshiyama, R.; Konno, M.; Eguchi, H.; Takemoto, H.; Noda, T.; Asai, A.; Koseki, J.; Haraguchi, N.; Ueda, Y.; Matsushita, K.; et al. Poly(ethylene glycol)–poly(lysine) block copolymer–ubenimex conjugate targets aminopeptidase N and exerts an antitumor effect in hepatocellular carcinoma stem cells. Oncogene 2019, 38, 244–260. [Google Scholar] [CrossRef]
- Kang, J.-W.; Cho, H.-J.; Lee, H.J.; Jin, H.-E.; Maeng, H.-J. Polyethylene glycol-decorated doxorubicin/carboxymethyl chitosan/gold nanocomplex for reducing drug efflux in cancer cells and extending circulation in blood stream. Int. J. Biol. Macromol. 2019, 125, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Jain, A. In Vitro and In Vivo Study of Poly(ethylene glycol) Conjugated Ibuprofen to Extend the Duration of Action. Sci. Pharm. 2011, 79, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.C.; Yao, J.C.; Benson, A.B.; Thomas, A.L.; Falk, S.; Mena, R.R.; Picus, J.; Wright, J.; Mulcahy, M.F.; Ajani, J.A.; et al. A phase II study of pegylated-camptothecin (pegamotecan) in the treatment of locally advanced and metastatic gastric and gastro-oesophageal junction adenocarcinoma. Cancer Chemother. Pharmacol. 2009, 63, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.-C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, V.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.-C. Till 2018: A survey of biomolecular sequences in genus Panax. J. Ginseng Res. 2019. [Google Scholar] [CrossRef]
- Wang, D.-D.; Kim, Y.-J.; Baek, N.I.; Mathiyalagan, R.; Wang, C.; Jin, Y.; Xu, X.Y.; Yang, D.-C. Glycosyltransformation of ginsenoside Rh2 to two novel ginsenosides by recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications. J. Ginseng Res. 2019. [Google Scholar] [CrossRef]
- Yang, J.-L.; Hu, Z.-F.; Zhang, T.-T.; Gu, A.-D.; Gong, T.; Zhu, P. Progress on the Studies of the Key Enzymes of Ginsenoside Biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef]
- Yang, W.-Z.; Hu, Y.; Wu, W.-Y.; Ye, M.; Guo, D.-A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Subramaniyam, S.; Kim, Y.J.; Kim, Y.-C.; Yang, D.C. Ginsenoside compound K-bearing glycol chitosan conjugates: Synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr. Polym. 2014, 112, 359–366. [Google Scholar] [CrossRef]
- Markus, J.; Mathiyalagan, R.; Kim, Y.J.; Han, Y.; Perez, Z.E.J.; Veronika, S.; Yang, D.-C. Synthesis of Hyaluronic Acid and O-Carboxymethyl Chitosan-Stabilized ZnO-Ginsenoside Rh2 Nanocomposites Incorporated with Aqueous Leaf Extract of Dendropanax morbifera Léveille: In Vitro Studies as Potential Sunscreen Agents. New J. Chem. 2019, 43, 9188–9200. [Google Scholar] [CrossRef]
- Moon, S.; Lee, H.; Mathiyalagan, R.; Kim, Y.; Yang, D.; Lee, D.; Min, J.; Jimenez, Z.; Yang, D. Synthesis of a Novel α-Glucosyl Ginsenoside F1 by Cyclodextrin Glucanotransferase and Its In Vitro Cosmetic Applications. Biomolecules 2018, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Yang, D.C. Ginseng nanoparticles: A budding tool for cancer treatment. Nanomedicine 2017, 12, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, J.; Hu, X.; Yu, S.K.; Liu, C.; Pan, R.; Chang, Q.; Liu, X.; Liao, Y. Preparation and evaluation of self-microemulsions for improved bioavailability of ginsenoside-Rh1 and Rh2. Drug Deliv. Transl. Res. 2017, 7, 731–737. [Google Scholar] [CrossRef]
- Han, M.; Hou, J.-G.; Dong, C.-M.; Li, W.; Yu, H.-L.; Zheng, Y.-N.; Chen, L. Isolation, synthesis and structures of ginsenoside derivatives and their anti-tumor bioactivity. Molecules 2010, 15, 399–406. [Google Scholar] [CrossRef]
- Gai, Y.; Ma, Z.; Yu, X.; Qu, S.; Sui, D. Effect of ginsenoside Rh1 on myocardial injury and heart function in isoproterenol-induced cardiotoxicity in rats. Toxicol. Mech. Methods 2012, 22, 584–591. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Kim, B.; Kim, D.-H. Ginsenoside Rh1 Eliminates the Cytoprotective Phenotype of Human Immunodeficiency Virus Type 1-Transduced Human Macrophages by Inhibiting the Phosphorylation of Pyruvate Dehydrogenase Lipoamide Kinase Isozyme 1. Biol. Pharm. Bull. 2013, 36, 1088–1094. [Google Scholar] [CrossRef]
- Jung, J.-S.; Shin, J.A.; Park, E.-M.; Lee, J.-E.; Kang, Y.-S.; Min, S.-W.; Kim, D.-H.; Hyun, J.-W.; Shin, C.-Y.; Kim, H.-S. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: Critical role of the protein kinase a pathway and hemeoxygenase-1 expression. J. Neurochem. 2010, 115, 1668–1680. [Google Scholar] [CrossRef]
- Park, E.K.; Choo, M.K.; Han, M.J.; Kim, D.H. Ginsenoside Rh1 Possesses Antiallergic and Anti-Inflammatory Activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yoon, J.-H.; Cha, S.-W.; Lee, S.-G. Ginsenoside Rh1 inhibits the invasion and migration of THP-1 acute monocytic leukemia cells via inactivation of the MAPK signaling pathway. Fitoterapia 2011, 82, 911–919. [Google Scholar] [CrossRef]
- Lee, Y.; Jin, Y.; Lim, W.; Ji, S.; Choi, S.; Jang, S.; Lee, S. A ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2003, 84, 463–468. [Google Scholar] [CrossRef]
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.e.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta. Med. 2018, 84, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Yang, S.-M.; Huang, C.-Y.; Chen, J.-C.; Chang, W.-M.; Hsu, S.-L. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother. Pharmacol. 2005, 55, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Musende, A.G.; Eberding, A.; Wood, C.; Adomat, H.; Fazli, L.; Hurtado-Coll, A.; Jia, W.; Bally, M.B.; Guns, E.T. Pre-clinical evaluation of Rh2 in PC-3 human xenograft model for prostate cancer in vivo: Formulation, pharmacokinetics, biodistribution and efficacy. Cancer Chemother. Pharmacol. 2009, 64, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xue, J.; Lee, M.; Liu, L.; Zhang, D.; Sun, M.; Zheng, Y.; Sung, C. Ginsenoside Rh2 improves learning and memory in mice. J. Med. Food 2013, 16, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Lee, S.; Choi, W.-Y.; Lim, C.-J. Skin anti-photoaging properties of ginsenoside Rh2 epimers in UV-B-irradiated human keratinocyte cells. J. Biosci. 2014, 39, 673–682. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Kim, S.-H.; Lee, M.-S.; Kim, S.H.; Yang, H.-J.; Kim, M.-J.; Kim, H.-S.; Ha, J.; Kim, M.S.; Kwon, D.Y. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2007, 364, 1002–1008. [Google Scholar] [CrossRef]
- Park, E.K.; Lee, E.J.; Lee, S.H.; Koo, K.H.; Sung, J.Y.; Hwang, E.H.; Park, J.H.; Kim, C.W.; Jeong, K.C.; Park, B.K.; et al. Induction of apoptosis by the ginsenoside Rh2 by internalization of lipid rafts and caveolae and inactivation of Akt. Br. J. Pharm. 2010, 160, 1212–1223. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, S.-J.; Kim, J.-S.; Kang, H.-S. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem. Toxicol. 2012, 50, 2736–2741. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Noh, H.-Y.; Kim, Y.-J.; Kim, Y.-J.; Jin, C.-G.; Yang, D.-C. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells. Sci. Bull. 2015, 60, 773–784. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathiyalagan, R.; Wang, C.; Kim, Y.J.; Castro-Aceituno, V.; Ahn, S.; Subramaniyam, S.; Simu, S.Y.; Jiménez-Pérez, Z.E.; Yang, D.C.; Jung, S.-K. Preparation of Polyethylene Glycol-Ginsenoside Rh1 and Rh2 Conjugates and Their Efficacy against Lung Cancer and Inflammation. Molecules 2019, 24, 4367. https://doi.org/10.3390/molecules24234367
Mathiyalagan R, Wang C, Kim YJ, Castro-Aceituno V, Ahn S, Subramaniyam S, Simu SY, Jiménez-Pérez ZE, Yang DC, Jung S-K. Preparation of Polyethylene Glycol-Ginsenoside Rh1 and Rh2 Conjugates and Their Efficacy against Lung Cancer and Inflammation. Molecules. 2019; 24(23):4367. https://doi.org/10.3390/molecules24234367
Chicago/Turabian StyleMathiyalagan, Ramya, Chao Wang, Yeon Ju Kim, Verónica Castro-Aceituno, Sungeun Ahn, Sathiyamoorthy Subramaniyam, Shakina Yesmin Simu, Zuly Elizabeth Jiménez-Pérez, Deok Chun Yang, and Seok-Kyu Jung. 2019. "Preparation of Polyethylene Glycol-Ginsenoside Rh1 and Rh2 Conjugates and Their Efficacy against Lung Cancer and Inflammation" Molecules 24, no. 23: 4367. https://doi.org/10.3390/molecules24234367
APA StyleMathiyalagan, R., Wang, C., Kim, Y. J., Castro-Aceituno, V., Ahn, S., Subramaniyam, S., Simu, S. Y., Jiménez-Pérez, Z. E., Yang, D. C., & Jung, S.-K. (2019). Preparation of Polyethylene Glycol-Ginsenoside Rh1 and Rh2 Conjugates and Their Efficacy against Lung Cancer and Inflammation. Molecules, 24(23), 4367. https://doi.org/10.3390/molecules24234367