Tetramethoxystilbene-Loaded Liposomes Restore Reactive-Oxygen-Species-Mediated Attenuation of Dilator Responses in Rat Aortic Vessels Ex vivo
Abstract
:1. Introduction
2. Results
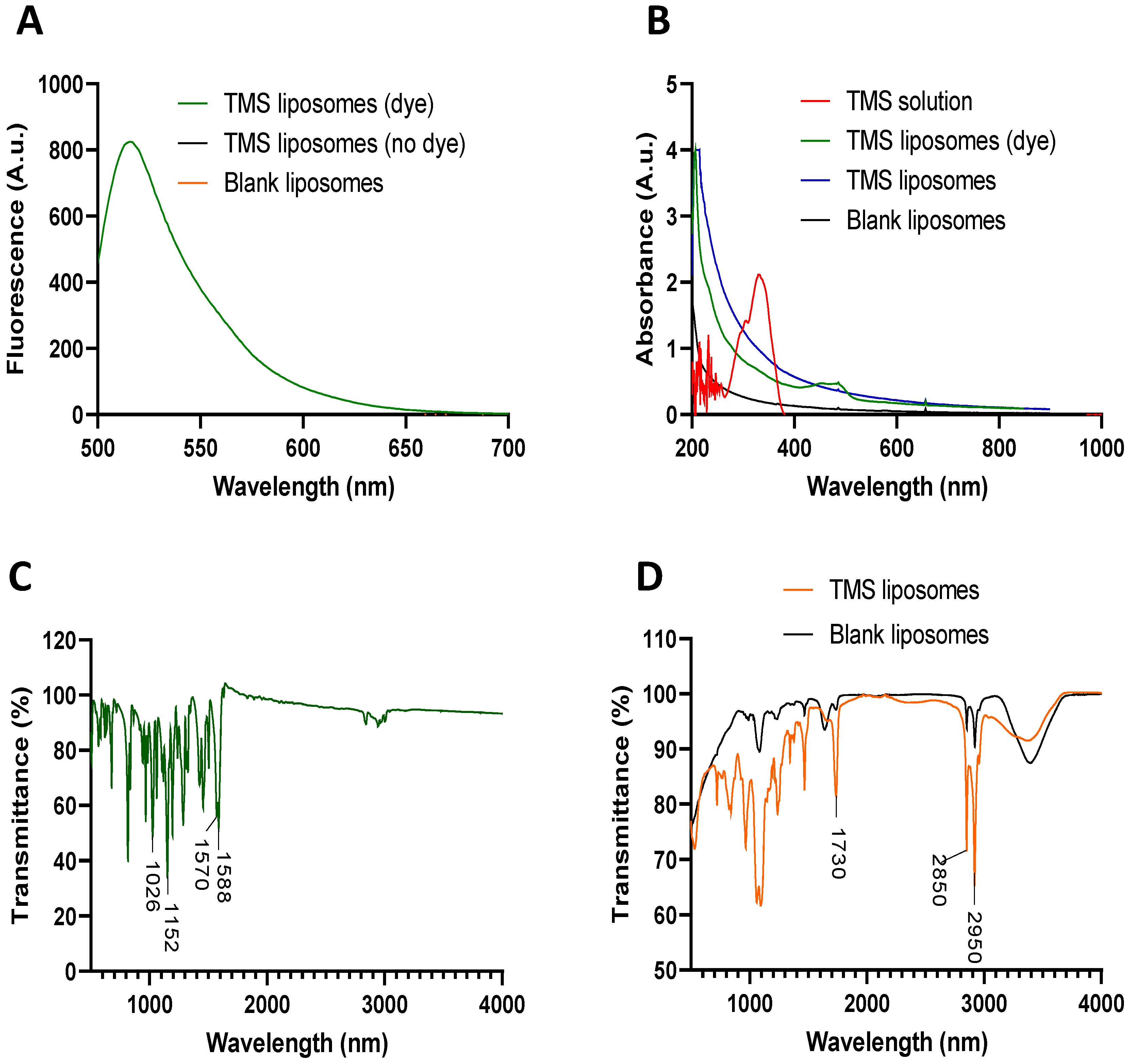
2.1. Production and Characterisation of TMS-Loaded Liposomes
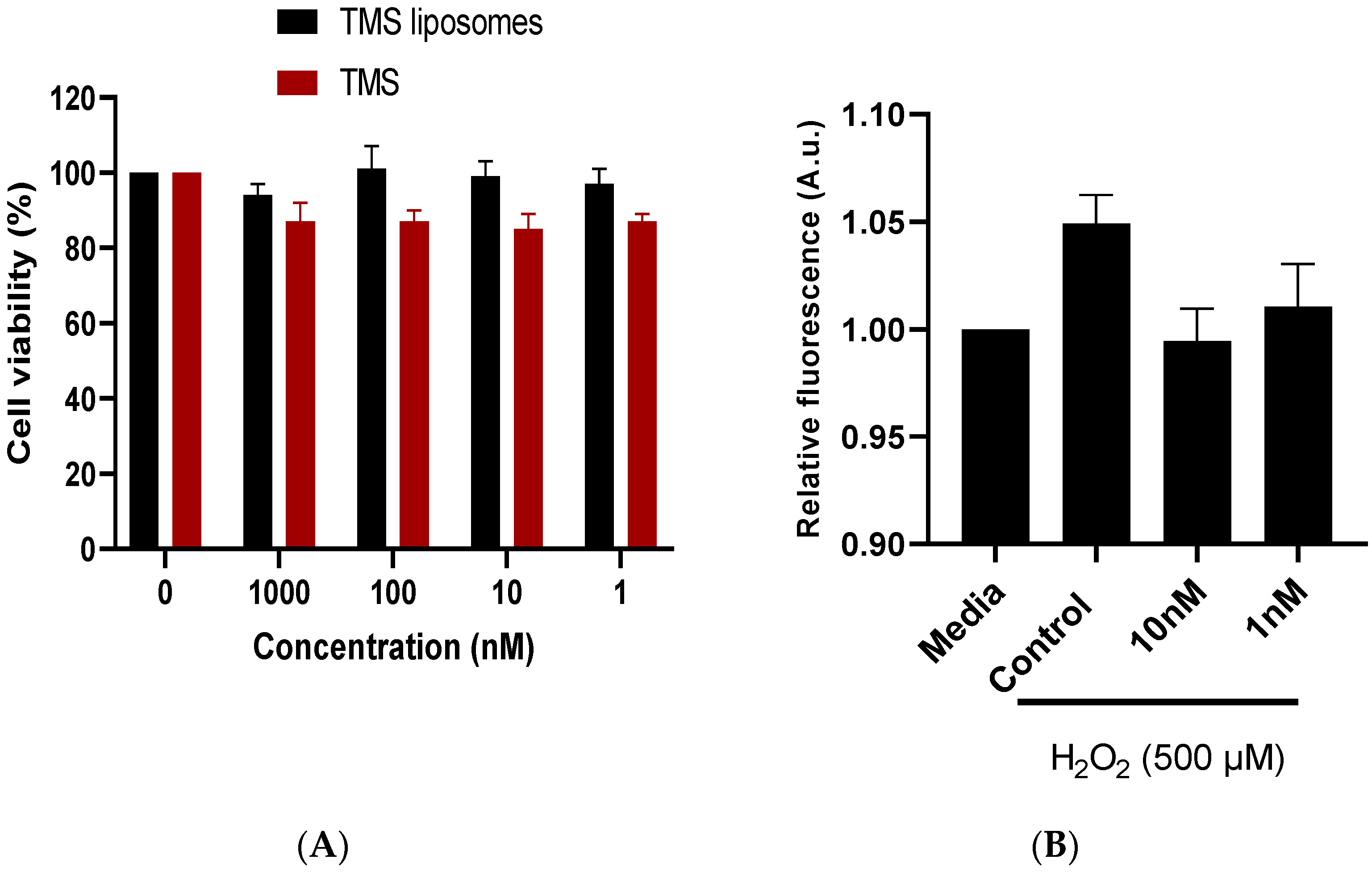
2.2. TMS-Loaded Liposomes Maintained Cell Viability In Vitro
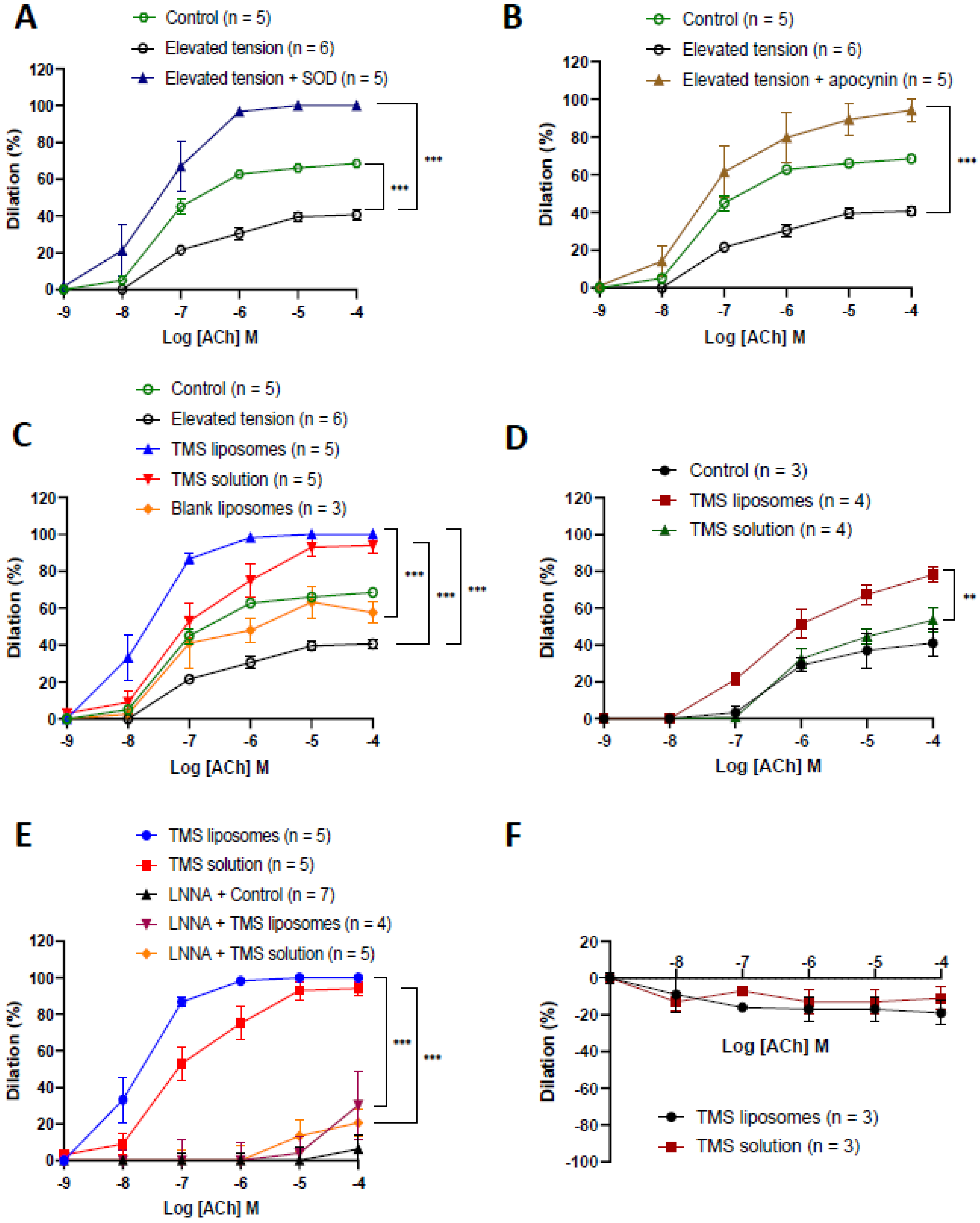
2.3. TMS-Loaded Liposomes Restored Endothelium-Dependent Dilation via Nitric Oxide
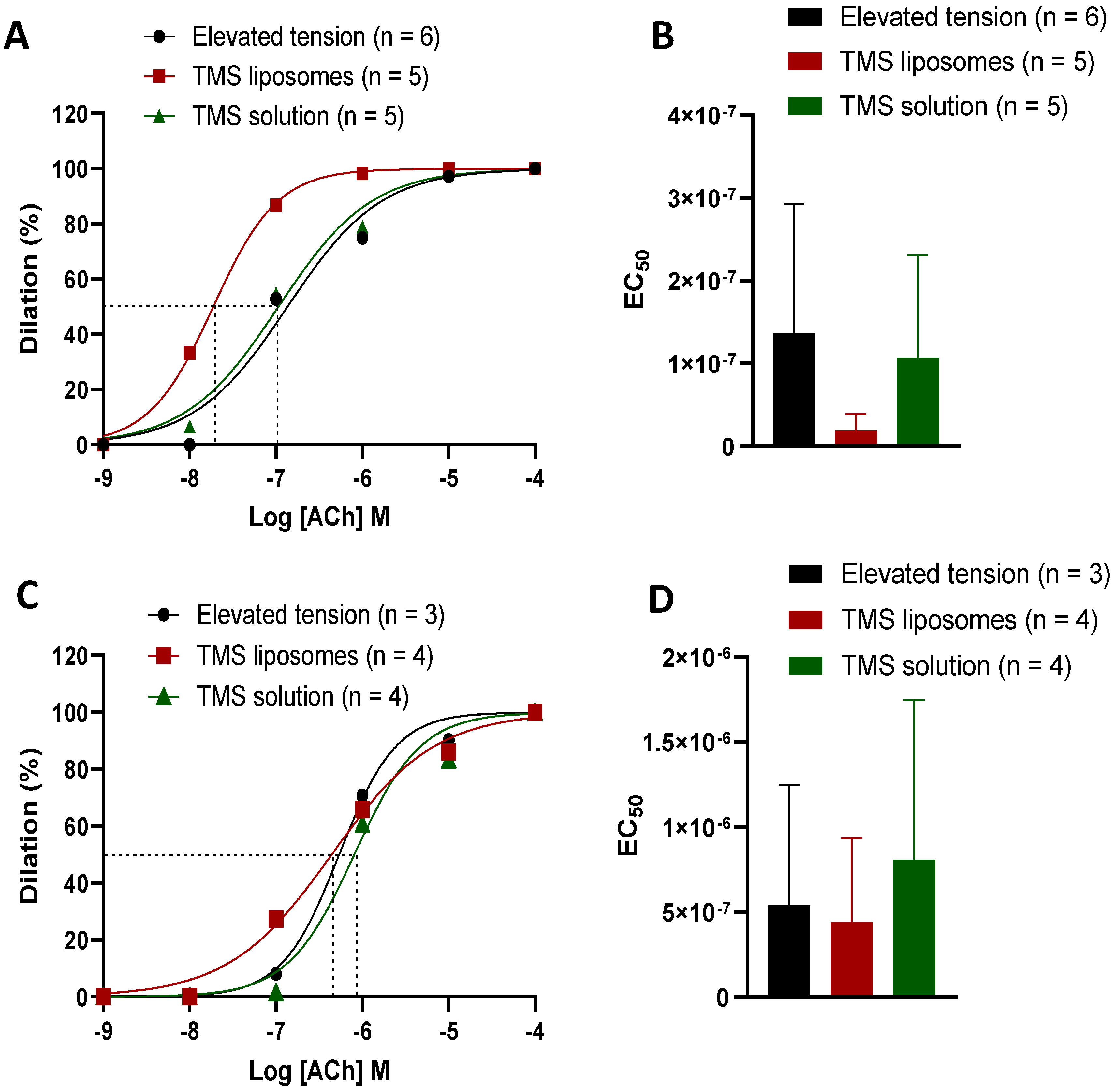
2.4. TMS-Loaded Liposomes Demonstrated Better Efficacy of ACh-Induced Dilation than TMS Solution
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Materials
4.2. Liposome Synthesis and Characterisation
4.3. Cell Culture Studies
4.4. Vascular Function Studies
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, W.; Druhan, L.; Chen, C.; Hemann, C.; Chen, Y.; Berka, V.; Tsai, A.; Zweier, J. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: Transition from reversible to irreversible enzyme inhibition. Biochemistry 2010, 49, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Csiszar, A.; Tucsek, Z.; Sosnowska, D.; Gautam, T.; Koller, A.; Schwartzman, M.; Sonntag, W.; Ungvari, Z. Role Of 20-HETE, TRPC channels, and Bkca in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1698–H1708. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Resveratrol and cardiovascular diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.; Robertson, I.; Dyck, J. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Jennings, B.; Montanez, D.; May, M.; Estes, A.; Fang, X.; Yaghini, F.; Kanu, A.; Malik, K. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc. Drugs Ther. 2014, 28, 145–161. [Google Scholar] [CrossRef]
- Sahan-Firat, S.; Jennings, B.; Yaghini, F.; Song, C.; Estes, A.; Fang, X.; Farjana, N.; Khan, A.; Malik, K. 2,3′,4,5′-tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: Contribution of cytochrome P-450 1B1. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1891–H1901. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, W.; Go, M.; Tringali, C.; Spatafora, C.; Ho, P. Quantification of trans-3, 4, 5, 4′-tetramethoxystilbene in rat plasma by HPLC: Application to pharmacokinetic study. J. Agric. Food Chem. 2011, 59, 1072–1077. [Google Scholar] [CrossRef]
- Lin, H.; Choo, Q.; Ho, P. Quantification of oxyresveratrol analog trans-2, 4, 3′, 5′-tetramethoxystilbene in rat plasma by a rapid HPLC method: Application in a pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2010, 24, 1373–1378. [Google Scholar] [CrossRef]
- Hu, Y.; Gaillard, P.; Rip, J.; de Lange, E.; Hammarlund-Udenaes, M. In vivo quantitative understanding of pegylated liposome’s influence on brain delivery of diphenhydramine. Mol. Pharm. 2018, 15, 5493–5500. [Google Scholar] [CrossRef] [PubMed]
- Janeczek, A.; Scarpa, E.; Horrocks, M.; Tare, R.; Rowland, C.; Jenner, D.; Newman, T.; Oreffo, R.; Lee, S.; Evans, N. PEGylated liposomes associate with Wnt3A protein and expand putative stem cells in human bone marrow populations. Nanomedicine 2017, 12, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Han, H. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2017, 48, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Arta, A.; Eriksen, A.; Melander, F.; Kempen, P.; Larsen, M.; Andresen, T.; Urquhart, A. Endothelial protein C–targeting liposomes show enhanced uptake and improved therapeutic efficacy in human retinal endothelial cells. Investig. Opthalmol. Vis. Sci. 2018, 59, 2119. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Kader, K. Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. ASAIO J. 2007, 53, 17–22. [Google Scholar] [CrossRef]
- Gupta, U.; Singh, V.; Kumar, V.; Khajuria, Y. Spectroscopic studies of cholesterol: Fourier transform infra-red and vibrational frequency analysis. Mater. Focus 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Pople, P.; Singh, K. Development and evaluation of colloidal modified nanolipid carrier: Application to topical delivery of tacrolimus. Eur. J. Pharm. Biopharm. 2011, 79, 82–94. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef]
- Schmitt, C.; Heiss, E.; Dirsch, V. Effect of resveratrol on endothelial cell function: Molecular mechanisms. BioFactors 2010, 36, 342–349. [Google Scholar] [CrossRef]
- Drummond, G.; Selemidis, S.; Griendling, K.; Sobey, C. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef]
- Virdis, A.; Gesi, M.; Taddei, S. Impact of apocynin on vascular disease in hypertension. Vasc. Pharmacol. 2016, 87, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Jennings, B.; Yaghini, F.; Sahan-Firat, S.; Song, C.; Estes, A.; Fang, X. Contribution of cytochrome P450 1B1 to hypertension and associated pathophysiology: A novel target for antihypertensive agents. Prostaglandins Other Lipid Mediat. 2012, 98, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kang, K. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol. Res. 2014, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kessner, S.; Krause, A.; Rothe, U.; Bendas, G. Investigation of the cellular uptake of E-Selectin-targeted immunoliposomes by activated human endothelial cells. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1514, 177–190. [Google Scholar] [CrossRef]
- Voigt, J.; Christensen, J.; Shastri, V. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc. Natl. Acad. Sci. USA 2014, 111, 2942–2947. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.; Bilia, A.; Bergonzi, M. Stealth and cationic nanoliposomes as drug delivery systems to increase andrographolide BBB permeability. Pharmaceutics 2018, 10, 128. [Google Scholar] [CrossRef]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective targeting of a novel vasodilator to the uterine vasculature to treat impaired uteroplacental perfusion in pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.; Agemy, L.; Teesalu, T.; Glazier, J.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef]
- Farooq, A.; Shukur, A.; Astley, C.; Tosheva, L.; Kelly, P.; Whitehead, D.; Azzawi, M. Titania coating of mesoporous silica nanoparticles for improved biocompatibility and drug release within blood vessels. Acta Biomater. 2018, 76, 208–216. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaabalawi, A.; Astley, C.; Renshall, L.; Beards, F.; Lightfoot, A.P.; Degens, H.; Whitehead, D.; Alexander, Y.; Harris, L.K.; Azzawi, M. Tetramethoxystilbene-Loaded Liposomes Restore Reactive-Oxygen-Species-Mediated Attenuation of Dilator Responses in Rat Aortic Vessels Ex vivo. Molecules 2019, 24, 4360. https://doi.org/10.3390/molecules24234360
Zaabalawi A, Astley C, Renshall L, Beards F, Lightfoot AP, Degens H, Whitehead D, Alexander Y, Harris LK, Azzawi M. Tetramethoxystilbene-Loaded Liposomes Restore Reactive-Oxygen-Species-Mediated Attenuation of Dilator Responses in Rat Aortic Vessels Ex vivo. Molecules. 2019; 24(23):4360. https://doi.org/10.3390/molecules24234360
Chicago/Turabian StyleZaabalawi, Azziza, Cai Astley, Lewis Renshall, Frances Beards, Adam P. Lightfoot, Hans Degens, Debra Whitehead, Yvonne Alexander, Lynda K Harris, and May Azzawi. 2019. "Tetramethoxystilbene-Loaded Liposomes Restore Reactive-Oxygen-Species-Mediated Attenuation of Dilator Responses in Rat Aortic Vessels Ex vivo" Molecules 24, no. 23: 4360. https://doi.org/10.3390/molecules24234360
APA StyleZaabalawi, A., Astley, C., Renshall, L., Beards, F., Lightfoot, A. P., Degens, H., Whitehead, D., Alexander, Y., Harris, L. K., & Azzawi, M. (2019). Tetramethoxystilbene-Loaded Liposomes Restore Reactive-Oxygen-Species-Mediated Attenuation of Dilator Responses in Rat Aortic Vessels Ex vivo. Molecules, 24(23), 4360. https://doi.org/10.3390/molecules24234360





