Matrix Metalloproteinase Triple-Helical Peptide Inhibitors: Potential Cross-Reactivity with Caspase-11
Abstract
1. Introduction
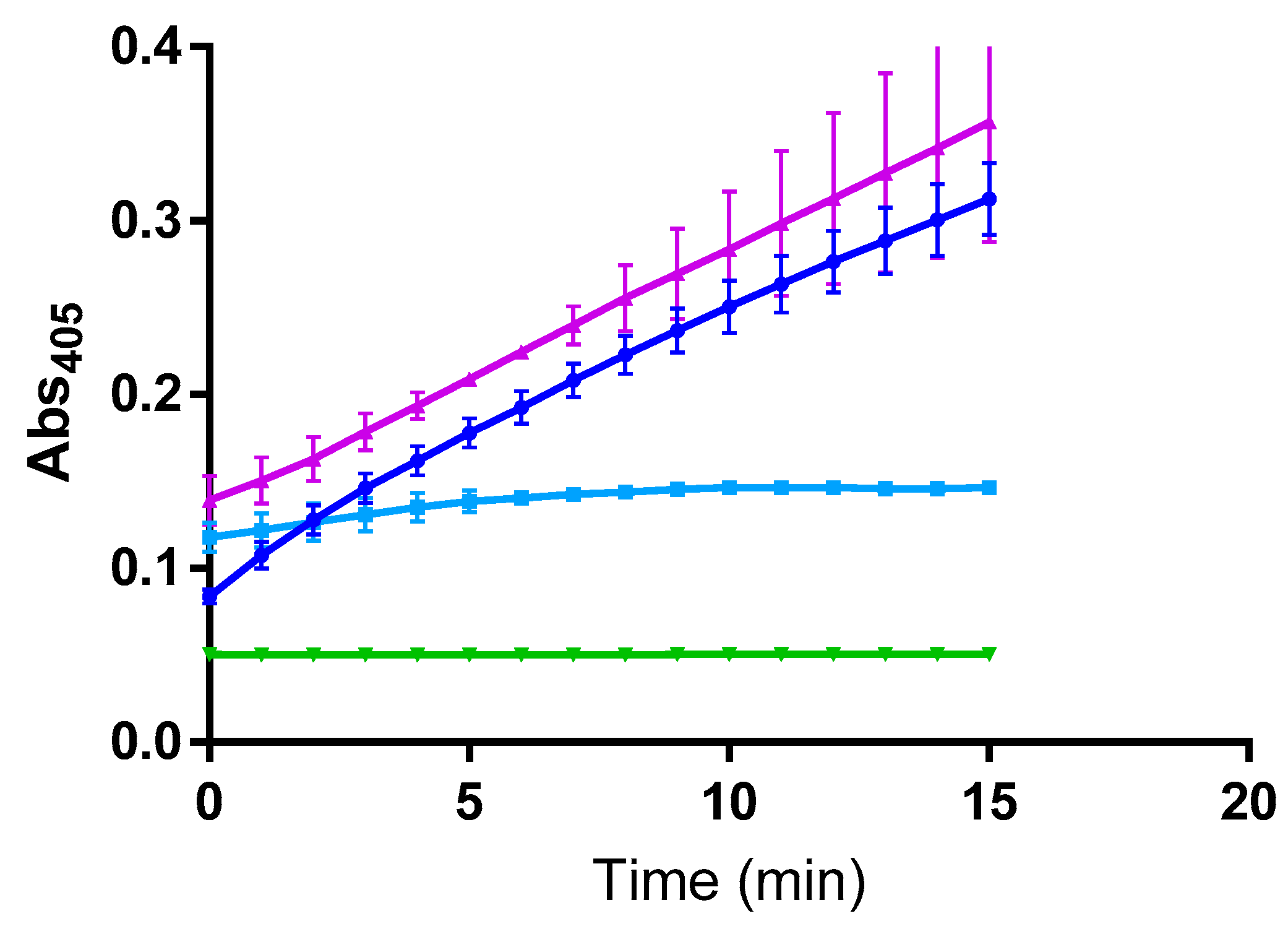
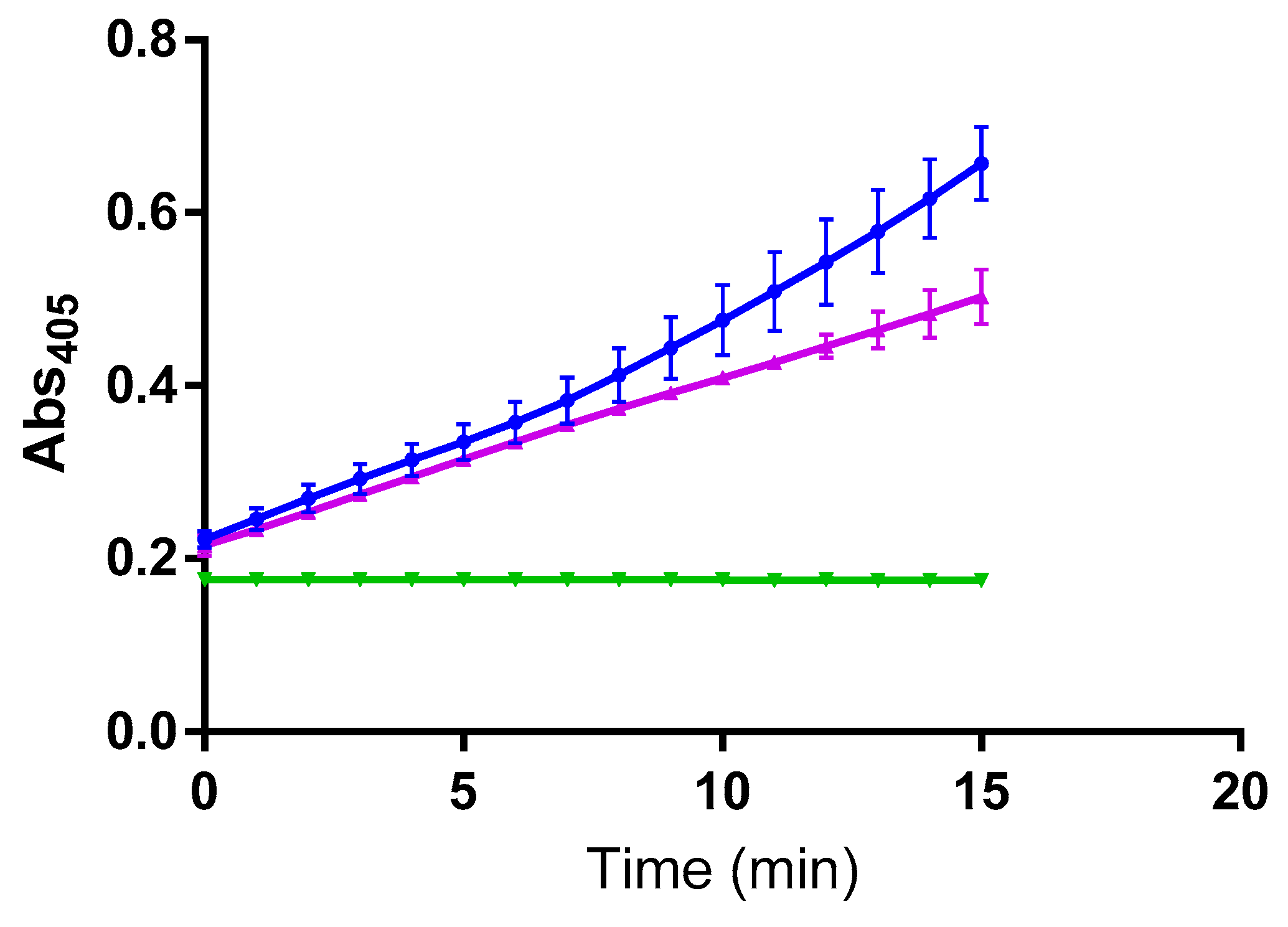
2. Results
3. Discussion
4. Materials and Methods
4.1. Caspase Enzyme Assay
4.2. Matrix Metalloproteinases (MMPs)
4.3. Inhibition Kinetic Studies
Author Contributions
Funding
Conflicts of Interest
References
- Fingleton, B. Matrix metalloproteinases as valid clinical targets. Curr. Pharm. Design 2007, 13, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Van den Steen, P.E.; Sang, Q.-X.A.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Yang, Y.; Rosenberg, G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009, 158, 983–994. [Google Scholar] [CrossRef]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef]
- Muri, L.; Leppert, D.; Grandgirard, D.; Leib, S.L. MMPs and ADAMs in neurological infectious diseases and multiple sclerosis. Cell Mol. Life Sci. 2019, 76, 3097–3116. [Google Scholar] [CrossRef]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J.H. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef]
- Vandenbroucke, R.C.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Murphy, G. Riding the metalloproteinase roller coaster. J. Biol. Chem. 2017, 292, 7708–7718. [Google Scholar] [CrossRef]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors - Novel strategies bring new prospects. Biochim. Biophys. Acta 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Xie, X.-W.; Wan, R.-Z.; Liu, Z.-P. Recent research advances in selective matrix metalloproteinase-13 inhibitors as anti-osteoarthritis agents. ChemMedChem 2017, 12, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; de Groot, R. Monoclonal antibodies against metzincin targets. Br. J. Pharmacol. 2019, 176, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Cuniasse, P.; Devel, L.; Makaritis, A.; Beau, F.; Georgiadis, D.; Matziari, M.; Yiotakis, A.; Dive, V. Future challenges facing the development of specific active-site-directed synthetic inhibitors of MMPs. Biochimie 2005, 87, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, D.; Dive, V. Phosphinic Peptides as Potent Inhibitors of Zinc-Metalloproteases. Top. Curr. Chem. 2015, 360, 1–38. [Google Scholar]
- Lauer-Fields, J.L.; Brew, K.; Whitehead, J.K.; Li, S.; Hammer, R.P.; Fields, G.B. Triple-helical transition-state analogs: A new class of selective matrix metalloproteinase inhibitors. J. Am. Chem. Soc. 2007, 129, 10408–10417. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Whitehead, J.K.; Li, S.; Hammer, R.P.; Brew, K.; Fields, G.B. Selective modulation of matrix metalloproteinase 9 (MMP-9) functions via exosite inhibition. J. Biol. Chem. 2008, 283, 20087–20095. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Chalmers, M.J.; Busby, S.A.; Minond, D.; Griffin, P.R.; Fields, G.B. Identification of Specific Hemopexin-like Domain Residues That Facilitate Matrix Metalloproteinase Collagenolytic Activity. J. Biol. Chem. 2009, 284, 24017–24024. [Google Scholar] [CrossRef]
- Bhowmick, M.; Stawikowska, R.; Tokmina-Roszyk, D.; Fields, G.B. Matrix Metalloproteinase Inhibition by Heterotrimeric Triple-Helical Peptide Transition State Analogs. ChemBioChem 2015, 16, 1084–1092. [Google Scholar] [CrossRef]
- Bhowmick, M.; Tokmina-Roszyk, D.; Onwuha-Ekpete, L.; Harmon, K.; Robichaud, T.; Fuerst, R.; Stawikowska, R.; Steffensen, B.; Roush, W.R.; Wong, H.; et al. Second Generation Triple-Helical Peptide Transition State Analog Matrix Metalloproteinase Inhibitors. J. Med. Chem. 2017, 60, 3814–3827. [Google Scholar] [CrossRef]
- Solan, P.D.; Dunsmore, K.E.; Denenberg, A.G.; Odoms, K.; Zingarelli, B.; Wong, H.R. A novel role for matrix metalloproteinase-8 in sepsis. Crit. Care Med. 2012, 40, 379–387. [Google Scholar] [CrossRef]
- Aguirre, A.; Blázquez-Prieto, J.; Amado-Rodriguez, L.; López-Alonso, I.; Batalla-Solís, E.; González-López, A.; Sánchez-Pérez, M.; Mayoral-Garcia, C.; Gutiérrez-Fernández, A.; Albaiceta, G.M. Matrix metalloproteinase-14 triggers an anti-inflammatory proteolytic cascade in endotoxemia. J. Mol. Med. 2017, 95, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Hulpiau, P.; Martens, L.; Vandenbroucke, R.E.; Van Wonterghem, E.; Perry, S.W.; Bruggeman, I.; Divert, T.; Choi, S.M.; Vuylsteke, M.; et al. Passenger Mutations Confound Interpretation of All Genetically Modified Congenic Mice. Immunity 2015, 43, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Dejonckheere, E.; Van Lint, P.; Demeestere, D.; Van Wonterghem, E.; Vanlaere, I.; Puimège, L.; Van Hauwermeiren, F.; De Rycke, R.; Mc Guire, C.; et al. Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. J. Neurosci. 2012, 32, 9805–9816. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Dejonckheere, E.; Van Hauwermeiren, F.; Lodens, S.; De Rycke, R.; Van Wonterghem, E.; Staes, A.; Gevaert, K.; López-Otin, C.; Libert, C. Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO Mol. Med. 2013, 5, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Vanlaere, I.; Van Hauwermeiren, F.; Van Wonterghem, E.; Wilson, C.; Libert, C. Pro-inflammatory effects of matrix metalloproteinase 7 in acute inflammation. Mucosal Immunol. 2014, 7, 579–588. [Google Scholar] [CrossRef]
- Wang, S.; Miura, M.; Jung, Y.K.; Zhu, H.; Li, E.; Yuan, J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 1998, 92, 501–509. [Google Scholar] [CrossRef]
- Arai, H.; Furuya, T.; Yasuda, T.; Miura, M.; Mizuno, Y.; Mochizuki, H. Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J. Biol. Chem. 2004, 279, 51647–51653. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Goethals, A.; Demon, D.; Bogaert, P.; Mak, T.W.; Cauwels, A.; Vandenabeele, P. An inactivating caspase-11 passenger mutation muddles sepsis research. Am. J. Respir. Crit. Care Med. 2013, 188, 120–121. [Google Scholar] [CrossRef]
- Ratnikov, B.I.; Cieplak, P.; Gramatikoff, K.; Pierce, J.; Eroshkin, A.; Igarashi, Y.; Kazanov, M.; Sun, Q.; Godzik, A.; Osterman, A.; et al. Basis for substrate recognition and distinction by matrix metalloproteinases. Proc. Natl. Acad. Sci. USA 2014, 111, E4148–E4155. [Google Scholar] [CrossRef]
- Kukreja, M.; Shiryaev, S.A.; Cieplak, P.; Muranaka, N.; Routenberg, D.A.; Chernov, A.V.; Kumar, S.; Remacle, A.G.; Smith, J.W.; Kozlov, I.A.; et al. High-Throughput Multiplexed Peptide-Centric Profiling Illustrates Both Substrate Cleavage Redundancy and Specificity in the MMP Family. Chem. Biol. 2015, 22, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, U.; Huesgen, P.F.; Schilling, O.; Bellac, C.L.; Butler, G.S.; Cox, J.H.; Dufour, A.; Goebeler, V.; Kappelhoff, R.; Keller, U.A.; et al. Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 2016, 49, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Stawikowski, M.; Knapinska, A.M.; Fields, G.B. Determining the Substrate Specificity of Matrix Metalloproteases using Fluorogenic Peptide Substrates. In Methods in Molecular Biology 1579: Matrix Metalloproteinases Methods and Protocols; Galea, C.A., Ed.; Humana Press/Springer Science + Business Media LLC: New York, NY, USA, 2017; pp. 137–183. [Google Scholar]
- Gonzalez Ramirez, M.L.; Poreba, M.; Snipas, S.J.; Groborz, K.; Drag, M.; Salvesen, G.S. Extensive peptide and natural protein substrate screens reveal that mouse caspase-11 has much narrower substrate specificity than caspase-1. J. Biol. Chem. 2018, 293, 7058–7067. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Katz, Y.; Bhusare, S.; Reich, R.; Röschenthaler, G.V.; Königsmann, M.; Breuer, E. Carbamoylphosphonate-based matrix metalloproteinase inhibitor metal complexes: solution studies and stability constants. Towards a zinc-selective binding group. J. Biol. Inorg. Chem. 2004, 9, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Saghatelian, A.; Jessani, N.; Joseph, A.; Humphrey, M.; Cravatt, B.F. Activity-based probes for the proteomic profiling of metalloproteases. Proc. Natl. Acad. Sci. USA 2004, 101, 10000–10005. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Tight Binding Inhibition. In Evaluation of Enzyme Inhibitors in Drug Discovery; Copeland, R.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 178–213. [Google Scholar]
- Yiallouros, I.; Vassiliou, S.; Yiotakis, A.; Zwilling, R.; Stöcker, W. Phosphinic peptides, the first potent inhibitors of astacin, behave as extremely slow-binding inhibitors. Biochem. J. 1998, 331, 375–379. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Marí, F.; Wei, S.; Fields, G.B.; Brew, K. Engineered Sarafotoxins as TIMP-like MMP Inhibitors. J. Biol. Chem. 2007, 282, 26948–26955. [Google Scholar] [CrossRef]
- Hyde, S.R.; Stith, R.D.; McCallum, R.E. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect. Immun. 1990, 58, 619–624. [Google Scholar]
- Wen, H. Sepsis induced by cecal ligation and puncture. Methods Mol. Biol. 2013, 1031, 117–124. [Google Scholar]
- Ross, C.; Chan, A.H.; Von Pein, J.; Boucher, D.; Schroder, K. Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome. Life Sci. Alliance 2018, 1, e201800237. [Google Scholar] [CrossRef]
- Wei, Y.; Fox, T.; Chambers, S.P.; Sintchak, J.; Coll, J.T.; Golec, J.M.; Swenson, L.; Wilson, K.P.; Charifson, P.S. The structures of caspases-1, -3, -7 and -8 reveal the basis for substrate and inhibitor selectivity. Chem. Biol. 2000, 7, 423–432. [Google Scholar] [CrossRef]
- Lavrik, I.N.; Golks, A.; Krammer, P.H. Caspases: Pharmacological manipulation of cell death. J. Clin. Invest. 2005, 115, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.M.; Choppin, G.R. Thermodynamics of Mg and Ca complexation with AMP, ADP, ATP. Inorg. Chim. Acta 1987, 138, 187–192. [Google Scholar] [CrossRef]
- Wilson, J.E.; Chin, A. Chelation of divalent cations by ATP, studied by titration calorimetry. Anal. Biochem. 1991, 193, 16–19. [Google Scholar] [CrossRef]
- Minond, D.; Lauer-Fields, J.L.; Cudic, M.; Overall, C.M.; Pei, D.; Brew, K.; Visse, R.; Nagase, H.; Fields, G.B. The roles of substrate thermal stability and P2 and P1’ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J. Biol. Chem. 2006, 281, 38302–38313. [Google Scholar] [CrossRef]
| Enzyme | Ki (nM) |
|---|---|
| MT1-MMP | 4704 ± 708.4 1 |
| MMP-8 | 124.6 ± 6.9 1 |
| MMP-8 | 378.1 ± 40.6 |
| MMP-1 | 169.2 ± 28.4 1 |
| MMP-3 | NI 1,2 |
| MMP-2 | 2.2 ± 0.20 |
| MMP-9 | 3.7 ± 0.40 |
| MMP-13 | 31.7 ± 7.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapinska, A.M.; Hart, M.; Drotleff, G.; Fields, G.B. Matrix Metalloproteinase Triple-Helical Peptide Inhibitors: Potential Cross-Reactivity with Caspase-11. Molecules 2019, 24, 4355. https://doi.org/10.3390/molecules24234355
Knapinska AM, Hart M, Drotleff G, Fields GB. Matrix Metalloproteinase Triple-Helical Peptide Inhibitors: Potential Cross-Reactivity with Caspase-11. Molecules. 2019; 24(23):4355. https://doi.org/10.3390/molecules24234355
Chicago/Turabian StyleKnapinska, Anna M., Melissa Hart, Gary Drotleff, and Gregg B. Fields. 2019. "Matrix Metalloproteinase Triple-Helical Peptide Inhibitors: Potential Cross-Reactivity with Caspase-11" Molecules 24, no. 23: 4355. https://doi.org/10.3390/molecules24234355
APA StyleKnapinska, A. M., Hart, M., Drotleff, G., & Fields, G. B. (2019). Matrix Metalloproteinase Triple-Helical Peptide Inhibitors: Potential Cross-Reactivity with Caspase-11. Molecules, 24(23), 4355. https://doi.org/10.3390/molecules24234355





