Plant-Mediated Enantioselective Transformation of Indan-1-one and Indan-1-ol. Part 2
Abstract
1. Introduction
2. Results and Discussion
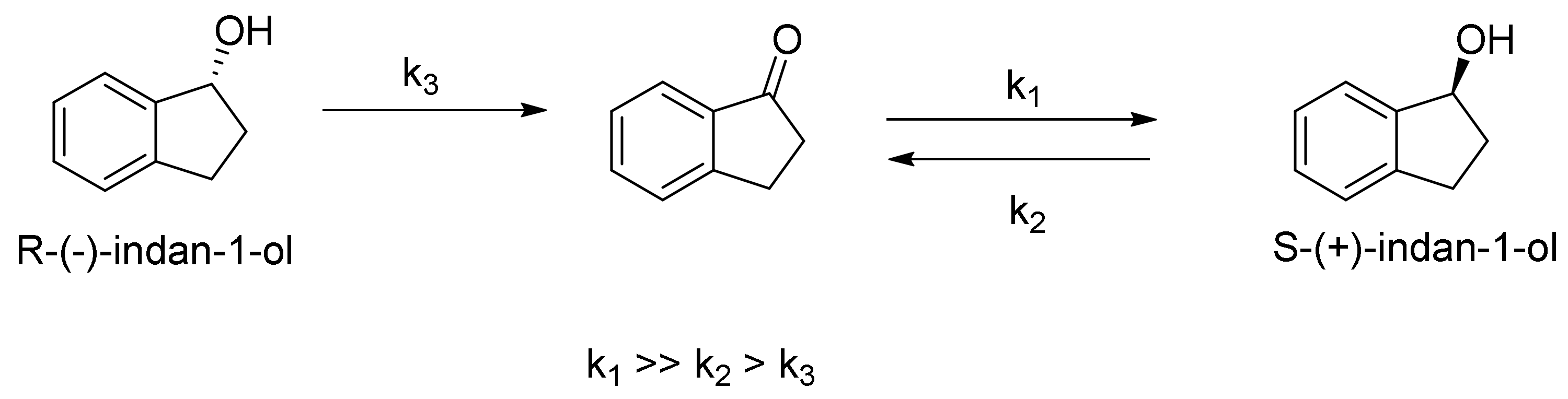
2.1. Reduction of Indan-1-one
2.2. Oxidation of Indan-1-ol
3. Materials and Methods
3.1. Initiation and Stabilization of Callus Culture for Biotransformation
3.2. Method of Conducting Biotransformation
3.3. Methods of Identification of the Biotransformation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Catallo, W.J.; Shupe, T.F.; Eberhardt, T.L. Hydrothermal processing of biomass from invasive aquatic plants. Biomass Bioenergy 2008, 32, 140–145. [Google Scholar] [CrossRef]
- Lawton, R.O.; Alexander, L.D.; Setzer, W.N.; Byler, K.G. Floral essential oil of Guettarda poasana inhibits yeast growth. Biotropica 1993, 25, 483–486. [Google Scholar] [CrossRef]
- Fushimi, K.; Horikawa, M.; Suzuki, K.; Sekiya, A.; Kanno, S.; Shimura, S.; Kawagishi, H. Applanatines A to E from the culture broth of Ganoderma applanatum. Tetrahedron 2010, 66, 9332–9335. [Google Scholar] [CrossRef]
- Gallou, I.; Senanayake, C.H. cis-1-Amino-2-indanol in drug design and applications to asymmetric processes. Chem. Rev. 2006, 106, 2843–2874. [Google Scholar] [CrossRef]
- Calitz, C.; Gouws, C.; Viljoen, J.; Steenekamp, J.; Wiesner, L.; Abay, E.; Hamman, J. Herb-Drug pharmacokinetic interactions: Transport and metabolism of Indinavir in the presence of selected herbal products. Molecules 2015, 20, 22113–22127. [Google Scholar] [CrossRef]
- Lourenco, N.M.T.; Barreiros, S.; Afonso, C.A.M. Enzymatic resolution of Indinavir precursor in ionic liquids with reuse of biocatalyst and media by product sublimation. Green Chem. 2007, 9, 734–736. [Google Scholar] [CrossRef]
- Kameyama, M.; Siqueira, F.A.; Garcia-Mijares, M.; Silva, L.F., Jr.; Silva, M.T.A. Indatraline: Synthesis and effect on the motor activity of Wistar rats. Molecules 2011, 16, 9421–9438. [Google Scholar] [CrossRef]
- Xu, R.; Wang, K.; Rizzi, J.P.; Huang, H.; Grina, J.A.; Schlachter, S.T.; Wang, B.; Wehn, P.M.; Yang, H.; Dixon, D.D.; et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell Renal cell carcinoma. J. Med. Chem. 2019, 62, 6876–6893. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Li, Z. Recent advances in enzymatic oxidation of alcohols. Curr. Opin. Chem. Biol. 2018, 43, 77–86. [Google Scholar] [CrossRef]
- Nasário, F.D.; Cazetta, T.; Moran, P.J.S.; Rodrigues, J.A.R. Deracemization of 1-phenylethanol via tandem biocatalytic oxidation and reduction. Tetrahedron Asymmetry 2016, 27, 404–409. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M.; Galek, R. Plant-Mediated enantioselective transformation of indan-1-one and indan-1-ol. Catalysts 2019, 9, 844. [Google Scholar] [CrossRef]
- Mączka, W.; Sołtysik, D.; Wińska, K.; Grabarczyk, M.; Szumny, A. Plant-Mediated biotransformations of S(+)- and R(-)-carvones. Appl. Sci. 2018, 8, 2605. [Google Scholar] [CrossRef]
- Mączka, W.K.; Mironowicz, A. Enantioselective hydrolysis of 1-aryl ethyl acetates and reduction of aryl methyl ketones using carrot, celeriac and horseradish enzyme systems. Tetrahedron Asymmetry 2002, 13, 2299–2302. [Google Scholar] [CrossRef]
- Mączka, W.K.; Mironowicz, A. Enantioselective reduction of bromo- and methoxy-acetophenone derivatives using carrot and celeriac enzymatic system. Tetrahedron Asymmetry 2004, 15, 1965–1967. [Google Scholar] [CrossRef]
- Mączka, W.K.; Mironowicz, A. Biotransformation of isoprenoids and shikimic acid derivatives by vegetable enzymatic system. Z. Naturforsch. 2004, 59, 309–316. [Google Scholar] [CrossRef]
- Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Anioł, M. Plant-Mediated stereoselective biotransformation of phenylglyoxylic acid esters. Z. Naturforsch. 2014, 69, 309–316. [Google Scholar]
- Cordell, G.A.; Lemos, T.L.G.; Monte, F.J.Q.; de Mattos, M.C. Vegetables as chemical reagents. J. Nat. Prod. 2007, 70, 478–492. [Google Scholar] [CrossRef]
- Meshram, S.H.; Ramesh, T.; Nanubolu, J.B.; Srivastava, A.K.; Adari, B.R.; Sahu, N. Green synthesis of enantiopure quinoxaline alcohols using Daucus carota. Chirality 2019, 31, 312–320. [Google Scholar] [CrossRef]
- Kazici, H.C.; Bayraktar, E.; Mehmetoglu, Ü. Production of precursors for anti-Alzheimer drugs: Asymmetric bioreduction in a packed-bed bioreactor using immobilized D. carota cells. Prep. Biochem. Biotechnol. 2017, 47, 67–73. [Google Scholar] [CrossRef]
- Omori, A.T.; Lobo, F.G.; Gonçalves do Amaral, A.C.; de Oliveira, C.S. Purple carrots: Better biocatalysts for the enantioselective reduction of acetophenones than common orange carrots (D. carota). J. Mol. Catal. B Enzym. 2016, 127, 93–97. [Google Scholar] [CrossRef]
- Yadav, J.S.; Nanda, S.; Thirupathi Reddy, P.; Bhaskar Rao, A. Efficient enantioselective reduction of ketones with Daucus carota root. J. Org. Chem. 2002, 67, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Utsukihara, T.; Horiuchi, A. Production of chiral aromatic alcohol by acetophenone and 1-arylethanol derivatives using vegetables. J. Chem. 2019, 58, 69–74. [Google Scholar]
- Mironowicz, A.; Kromer, K. Apple-Tree shoots and transformed carrot and apple roots used as biocatalysts in enantioselective acetate hydrolysis, alcohol oxidation and ketone reduction. Collect. Czech. Chem. Commun. 1998, 63, 1655–1662. [Google Scholar] [CrossRef]
- Naoshima, Y.; Akakabe, Y. Biotransformation of aromatic ketones with cell cultures of carrot, tobacco and Gardenia. Phytochemistry 1991, 30, 3595–3597. [Google Scholar] [CrossRef]
- Akakabe, Y.; Naoshima, Y. Biotransformation of acetophenone with immobilized cells of carrot, tobacco and Gardenia. Phytochemistry 1994, 35, 3–661. [Google Scholar] [CrossRef]
- Baskar, B.; Ganesh, S.; Lokeswari, T.S.; Chadha, A. Highly stereoselective reduction of 4-aryl-2-oxobut-3-enoic carboxylic esters by plant cell culture of Daucus carota. J. Mol. Catal. B: Enzym. 2004, 27, 13–17. [Google Scholar] [CrossRef]
- Caron, D.; Coughlan, A.P.; Simard, M.; Bernier, J.; Piché, Y.; Chênevert, R. Stereoselective reduction of ketones by Daucus carota hairy root cultures. Biotech. Lett. 2005, 27, 713–716. [Google Scholar] [CrossRef]
- Nagaki, M.; Soma, N.; Ono, K.; Yamanouchi, K.; Tsujiguchi, T.; Kawakami, J.; Chounan, Y. Biotransformation of indanol, fluorenol and their analogs using tissue-cultured cells and their antimicrobial activity. Trans. Mat. Res. Soc. 2019, 44, 29–33. [Google Scholar] [CrossRef]
- Bennamane, M.; Razi, S.; Zeror, S.; Aribi-Zouioueche, L. Preparation of chiral phenylethanols using various vegetables grown in Algeria. Biocatal. Agric. Biotechnol. 2018, 14, 52–56. [Google Scholar] [CrossRef]
- Uzura, A.; Katsuragi, T.; Tani, Y. Conversion of various aromatic compounds by resting cells of Fusarium moniliforme strain MS31. J. Biosci. Bioeng. 2001, 92, 381–384. [Google Scholar] [CrossRef]
- Stampfer, W.; Kosjek, B.; Faber, K.; Kroutil, W. Biocatalytic asymmetric hydrogen transfer employing Rhodococcus ruber DSM 44541. J. Org. Chem. 2003, 68, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fu, Q.; Gan, J. Metabolism of pharmaceutical and personal care products by carrot cell cultures. Environ. Pollut. 2016, 211, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Song, Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Pazmino, D.M.; Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Archilla-Ruiz, A.; Del Rio, L.A.; Sandalio, L.M. Differential response of young and adult leaves to herbicide 2,4-dichlorophenoxyacetic acid in pea plants: Role of reactive oxygen species. Plant Cell Environ. 2011, 34, 1874–1889. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.-H.; Matak-Vinković, D.; Coyne, A.G.; Abell, C. Insight into protein conformation and subcharging by DMSO from native ion mobility mass spectrometry. Chem. Select. 2016, 1, 5686–5690. [Google Scholar] [CrossRef]
- Könst, P.; Merkens, H.; Kara, S.; Kochius, S.; Vogel, A.; Zuhse, R.; Holtmann, D.; Arends, I.W.C.E.; Hollmann, F. Oxidation von Aldehyden mit Alkoholdehydrogenasen. Angew. Chem. Int. Ed. 2012, 51, 9914–9917. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Time [days] | Ketone [%] | Alcohol | |
|---|---|---|---|
| [%] | ee [%] | ||
| 5 | 46.7 | 53.3 | 99%S |
| 10 | 56.3 | 43.7 | 99%S |
| 15 | 55.6 | 44.4 | 99%S |
| Time [days] | Ketone [%] | Alcohol [%] | |
|---|---|---|---|
| [%] | ee [%] | ||
| 1 | 93.7 | 6.3 | 99% S(+) |
| 2 | 88.9 | 11.1 | 99% S(+) |
| 3 | 87.6 | 12.4 | 99% S(+) |
| 7 | 67.5 | 32.5 | 99% S(+) |
| 8 | 65.5 | 34.5 | 99% S(+) |
| 9 | 64 | 36.0 | 99% S(+) |
| 10 | 66.8 | 33.2 | 99% S(+) |
| Time [days] | Ketone [%] | Alcohol | |
|---|---|---|---|
| % | ee [%] | ||
| 5 | 43.7 | 56.3 | 27.1 R(-) |
| 10 | 61.1 | 38.9 | 34.4 S(+) |
| 15 | 63.3 | 36.7 | 57.4 S(+) |
| Time [days] | Ketone [%] | Alcohol | |
|---|---|---|---|
| % | ee [%] | ||
| 1 | 10.7 | 89.3 | 10.0 R(-) |
| 2 | 15.5 | 84.5 | 13.8 R(-) |
| 3 | 22.2 | 77.9 | 13.6 R(-) |
| 7 | 50.3 | 49.7 | 6.5 S(+) |
| 8 | 55.0 | 45.0 | 14.7 S(+) |
| 9 | 55.6 | 44.4 | 21.9 S(+) |
| 10 | 56.5 | 43.5 | 23.9 S (+) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mączka, W.; Wińska, K.; Grabarczyk, M.; Galek, R. Plant-Mediated Enantioselective Transformation of Indan-1-one and Indan-1-ol. Part 2. Molecules 2019, 24, 4342. https://doi.org/10.3390/molecules24234342
Mączka W, Wińska K, Grabarczyk M, Galek R. Plant-Mediated Enantioselective Transformation of Indan-1-one and Indan-1-ol. Part 2. Molecules. 2019; 24(23):4342. https://doi.org/10.3390/molecules24234342
Chicago/Turabian StyleMączka, Wanda, Katarzyna Wińska, Małgorzata Grabarczyk, and Renata Galek. 2019. "Plant-Mediated Enantioselective Transformation of Indan-1-one and Indan-1-ol. Part 2" Molecules 24, no. 23: 4342. https://doi.org/10.3390/molecules24234342
APA StyleMączka, W., Wińska, K., Grabarczyk, M., & Galek, R. (2019). Plant-Mediated Enantioselective Transformation of Indan-1-one and Indan-1-ol. Part 2. Molecules, 24(23), 4342. https://doi.org/10.3390/molecules24234342






