Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review
Abstract
1. Introduction
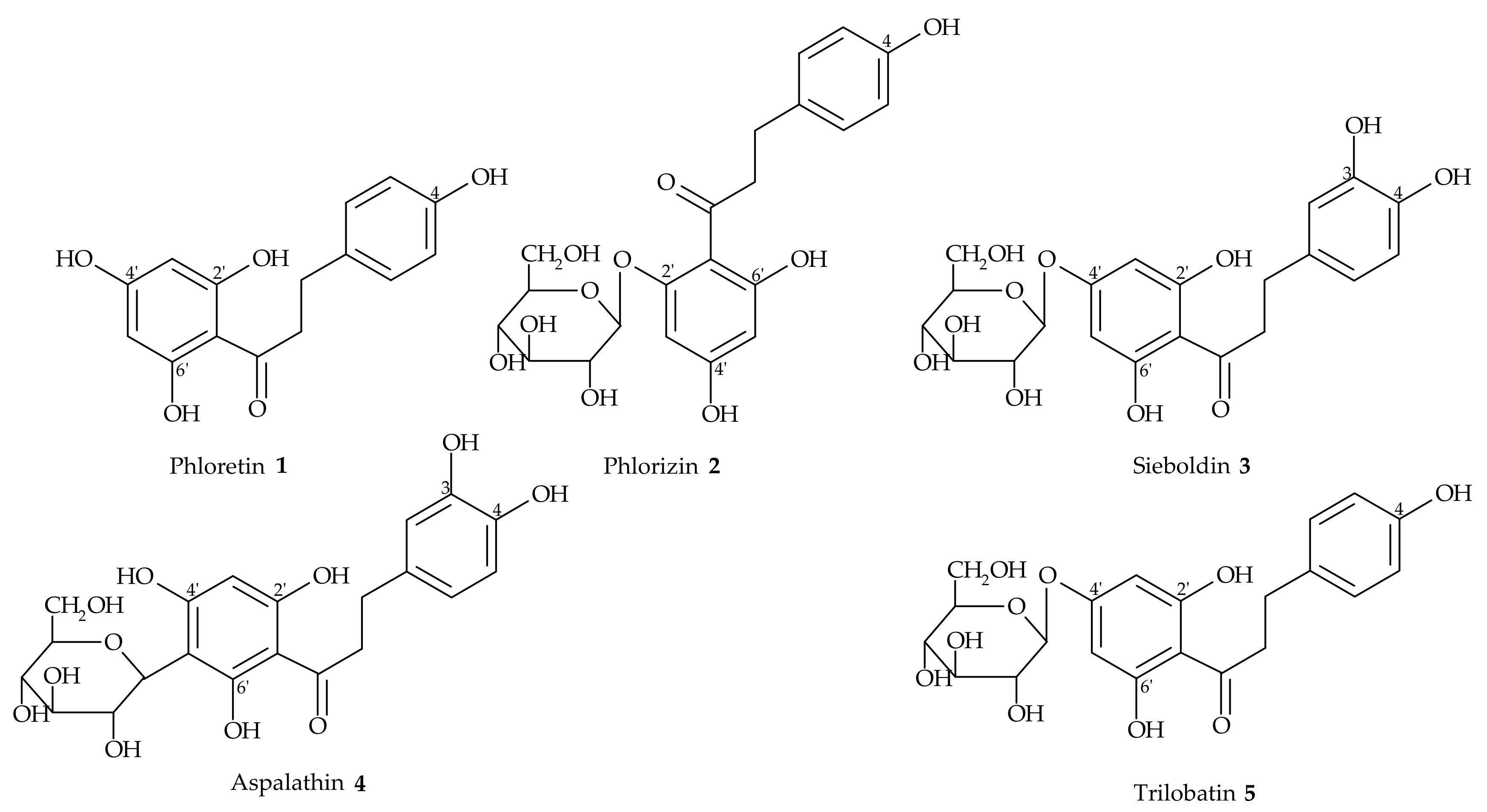
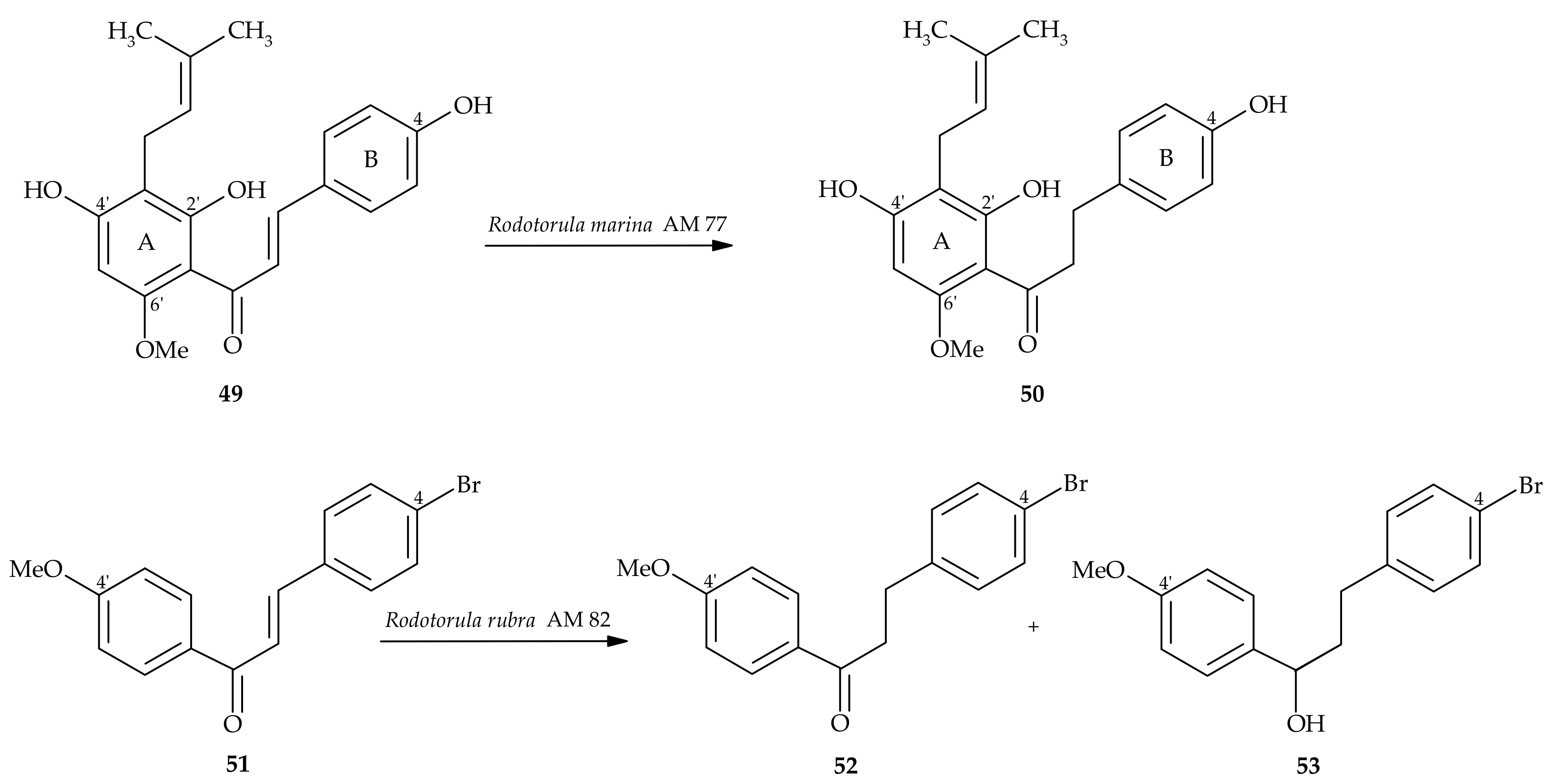
2. Dihydrochalcones from Natural Sources
3. Chemical Synthesis of Dihydrochalcones
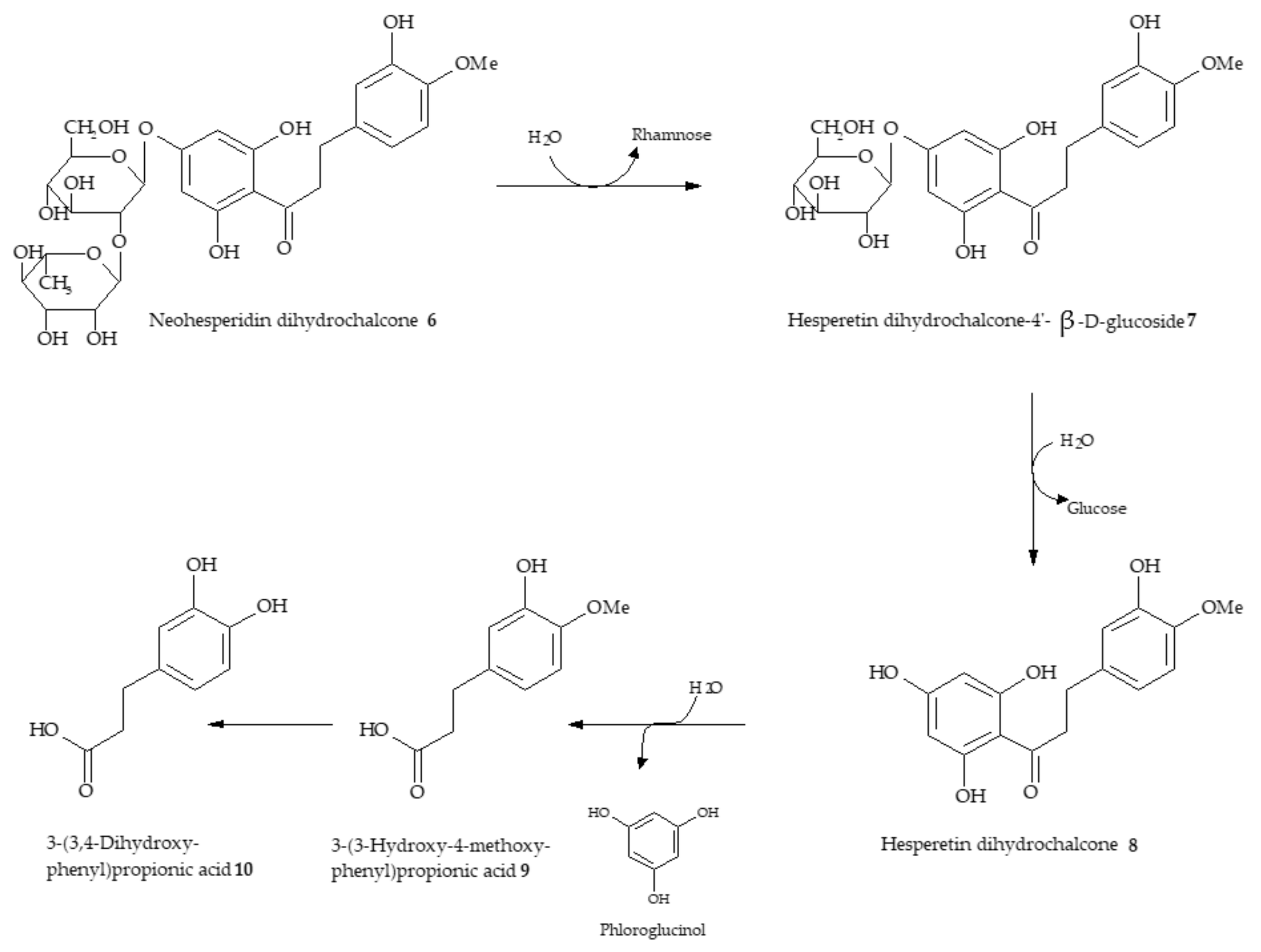
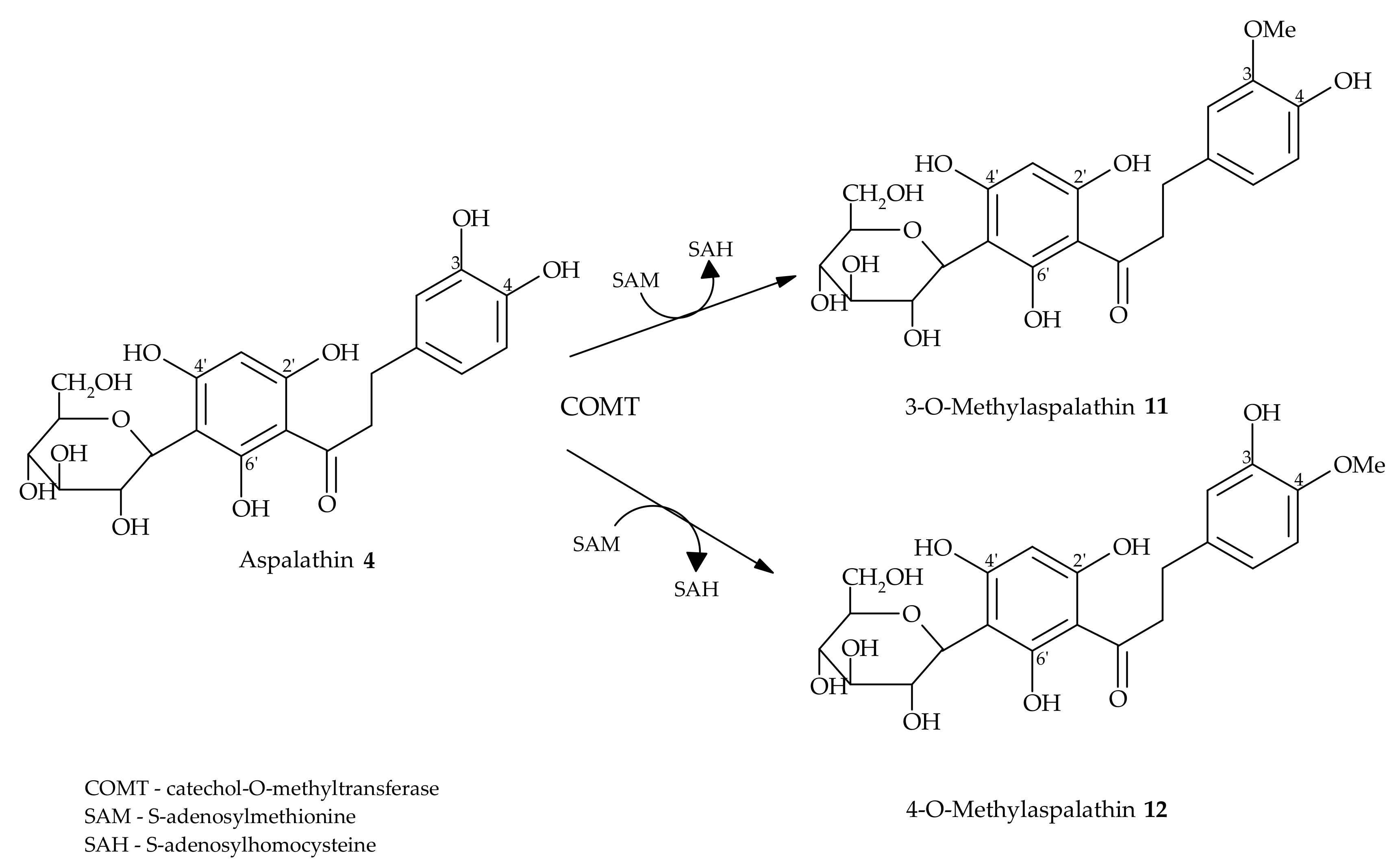
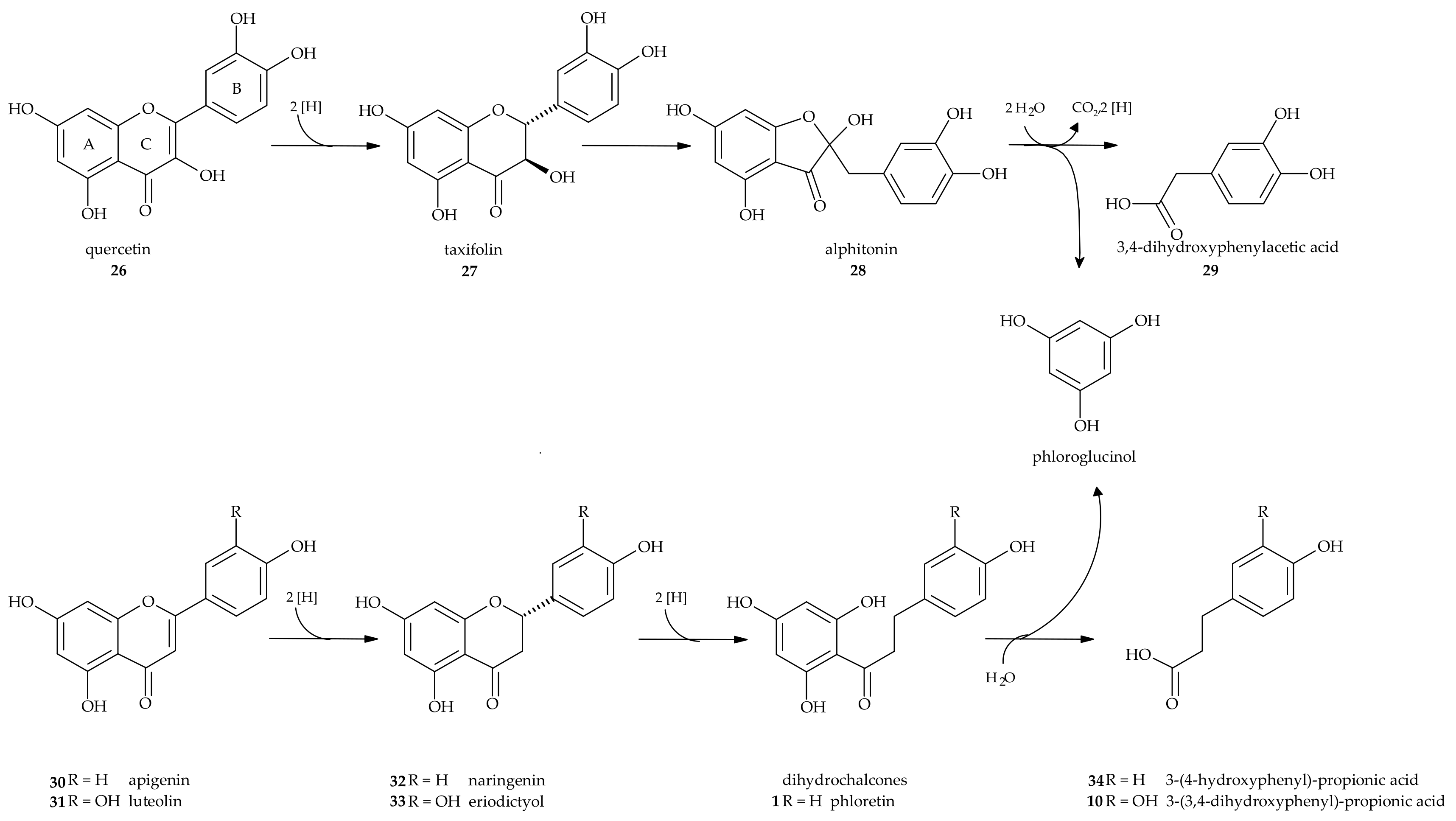
4. Metabolism
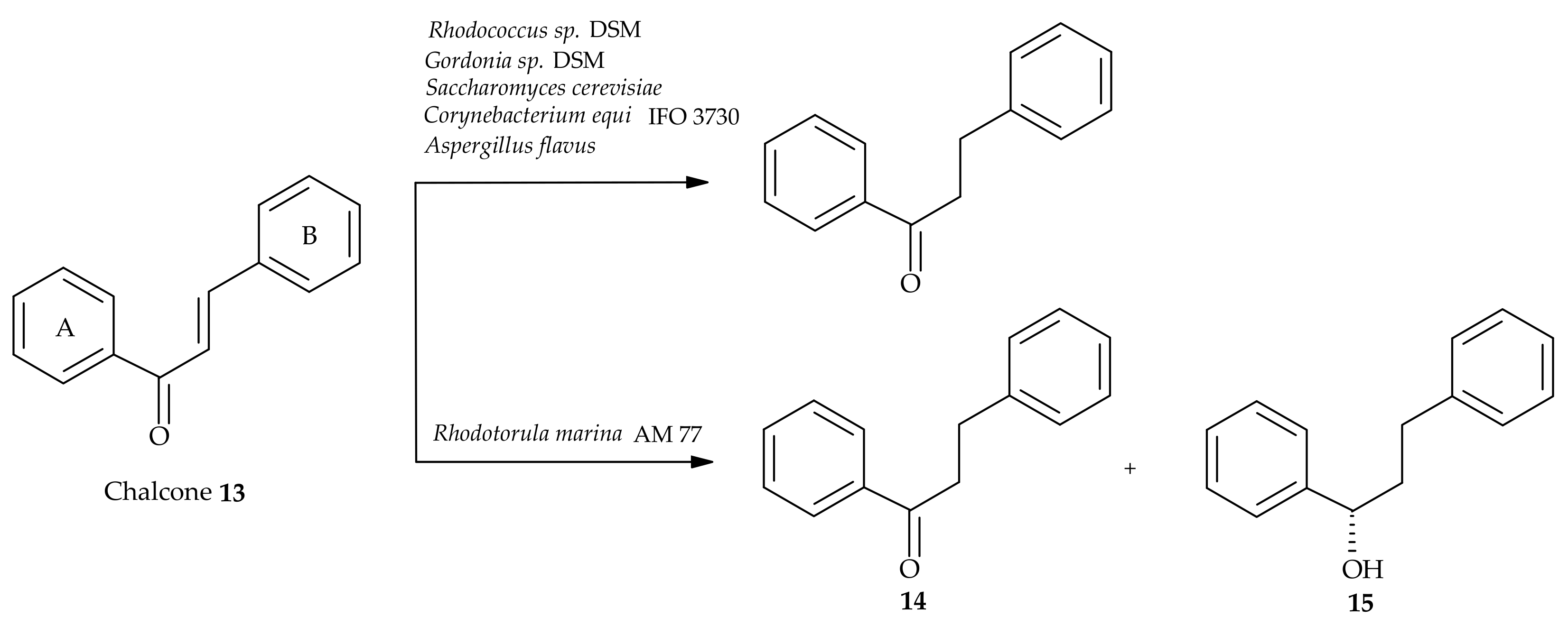
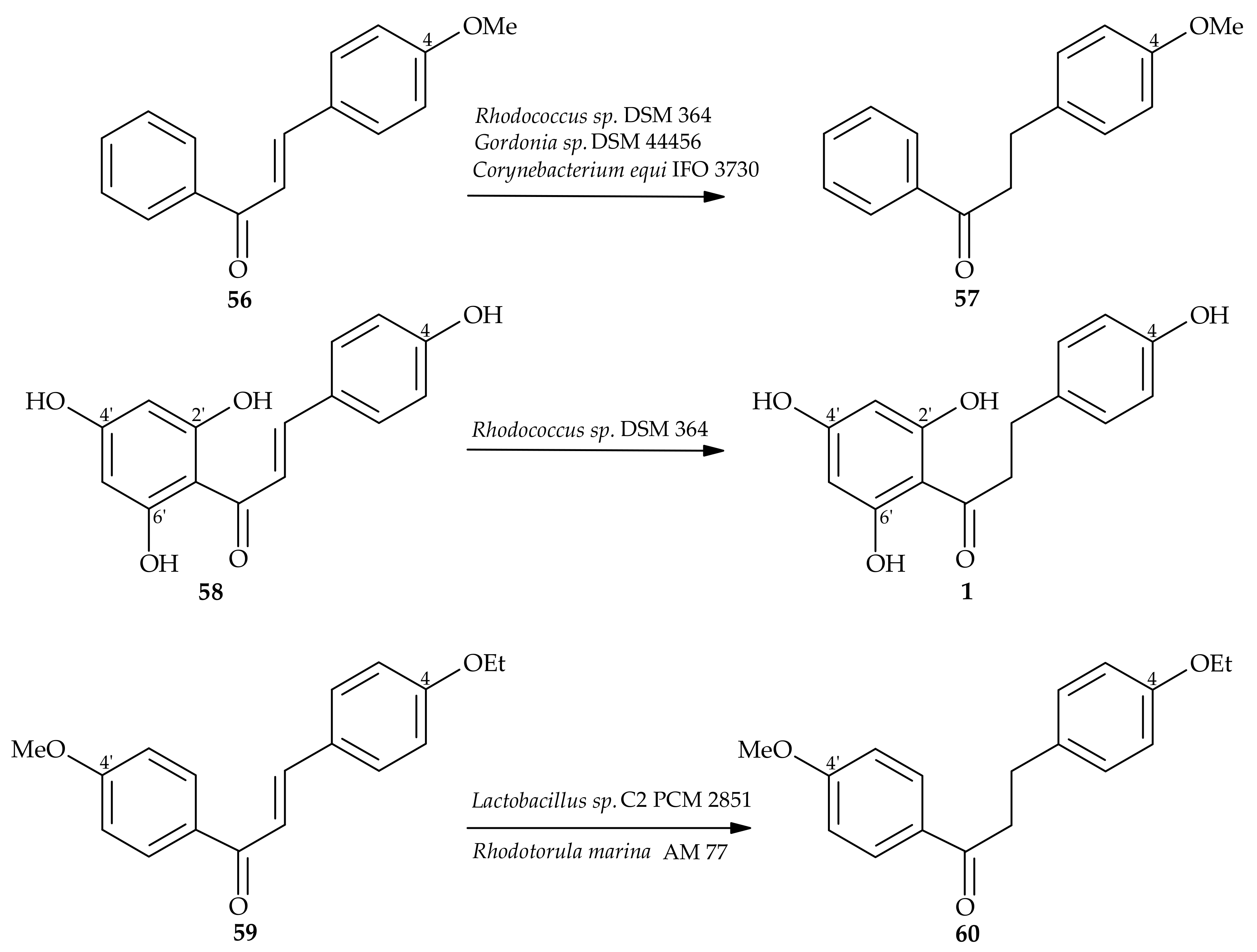
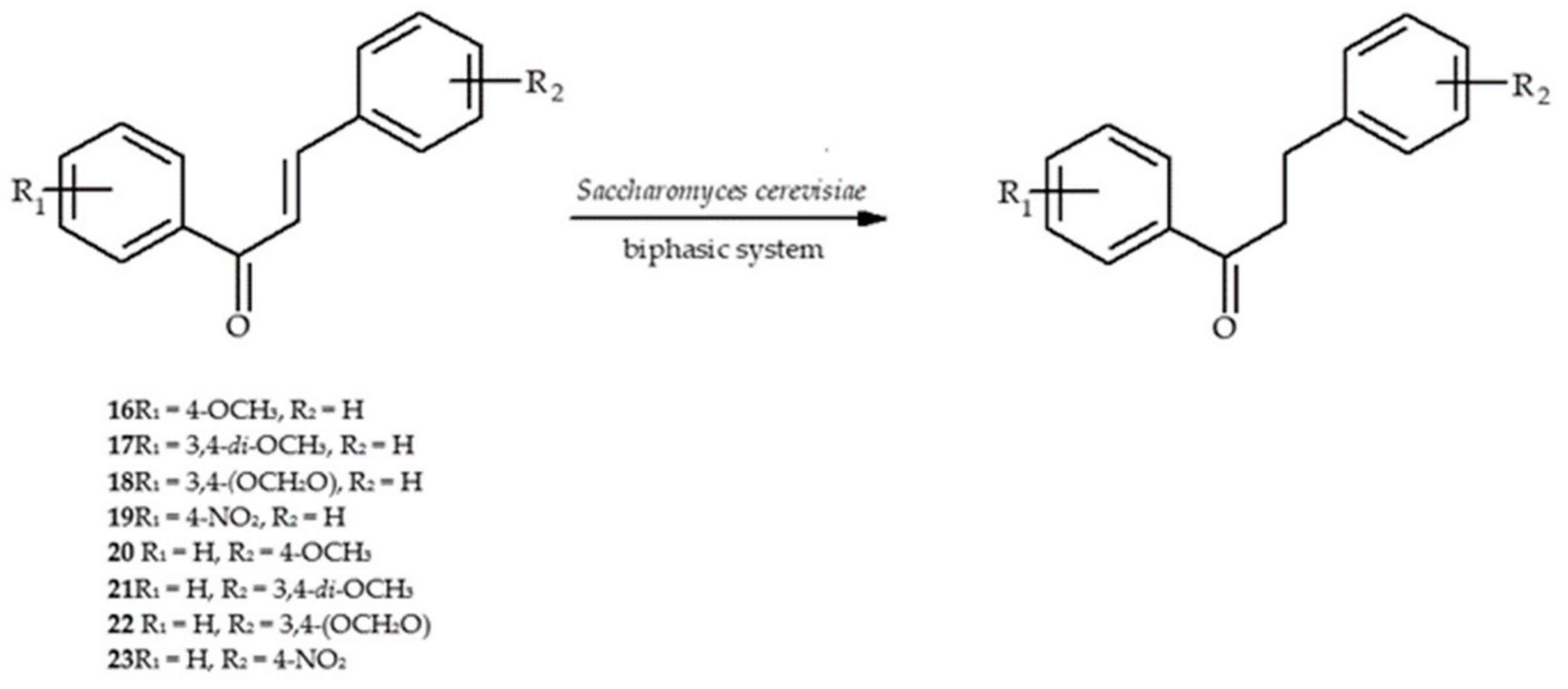
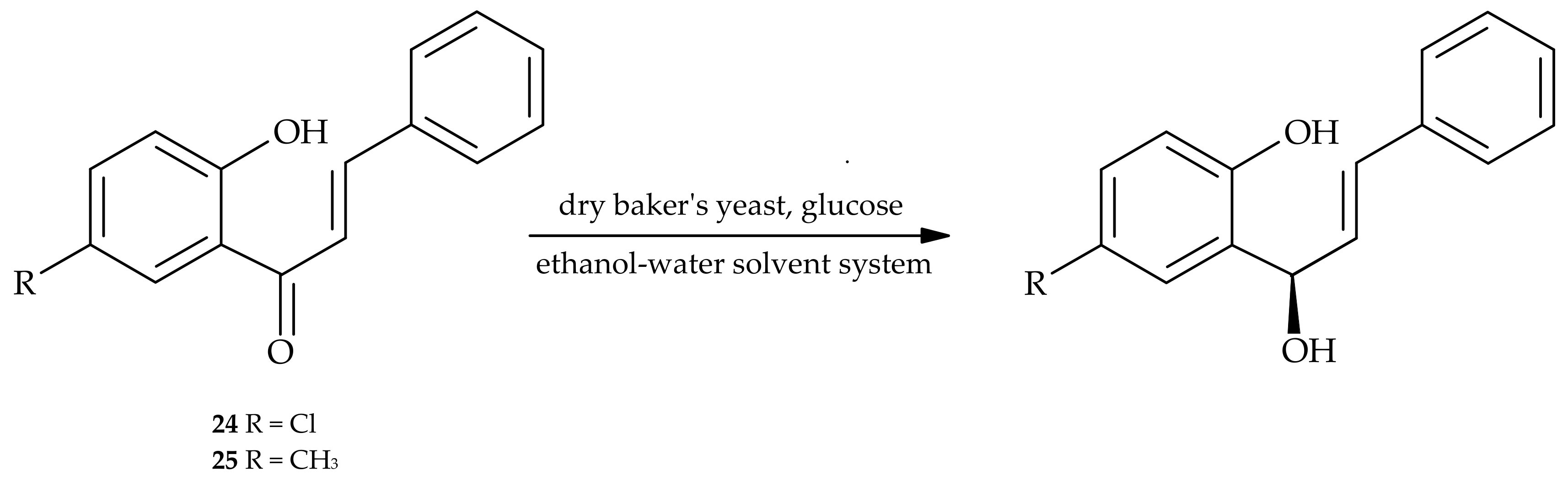
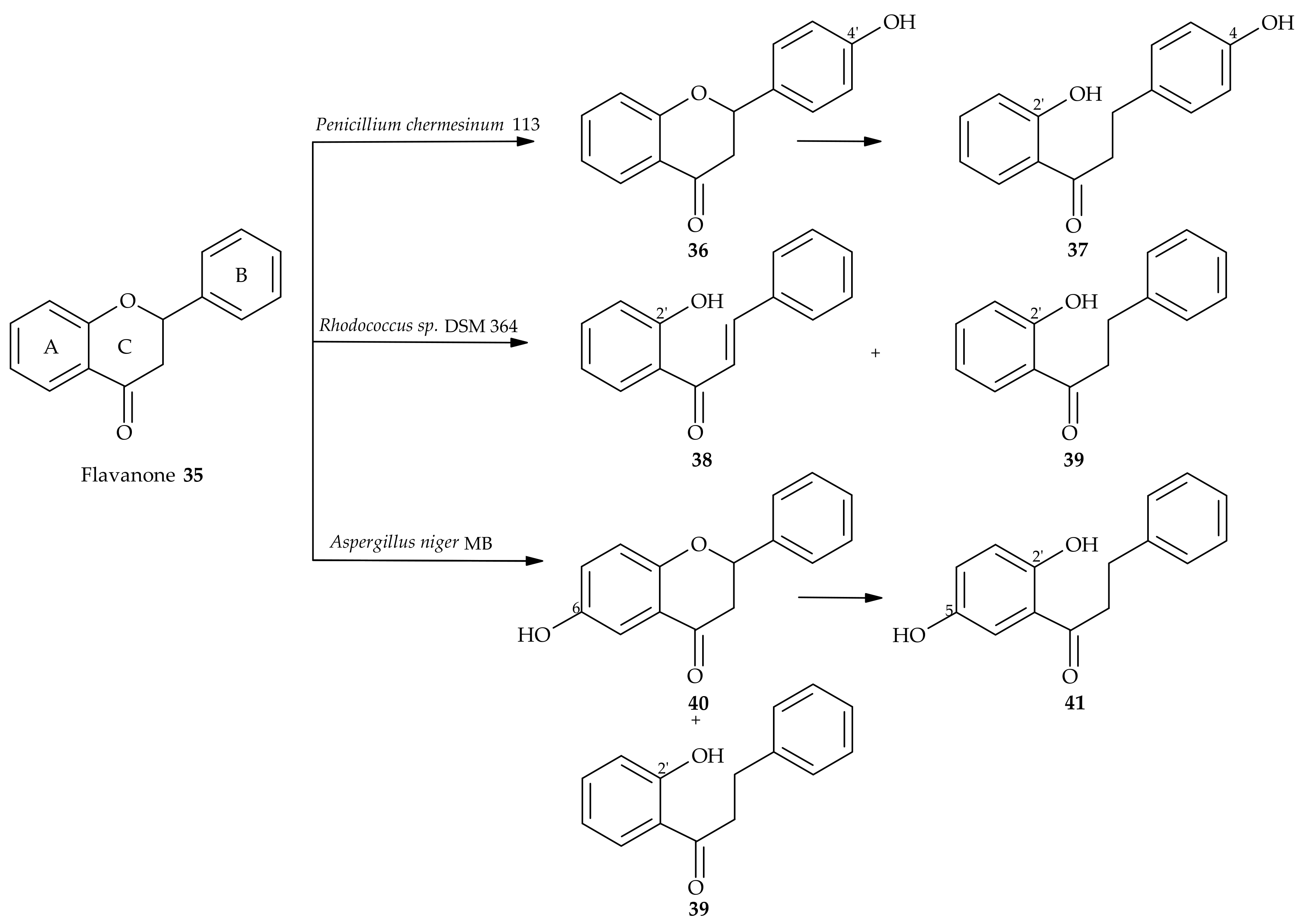
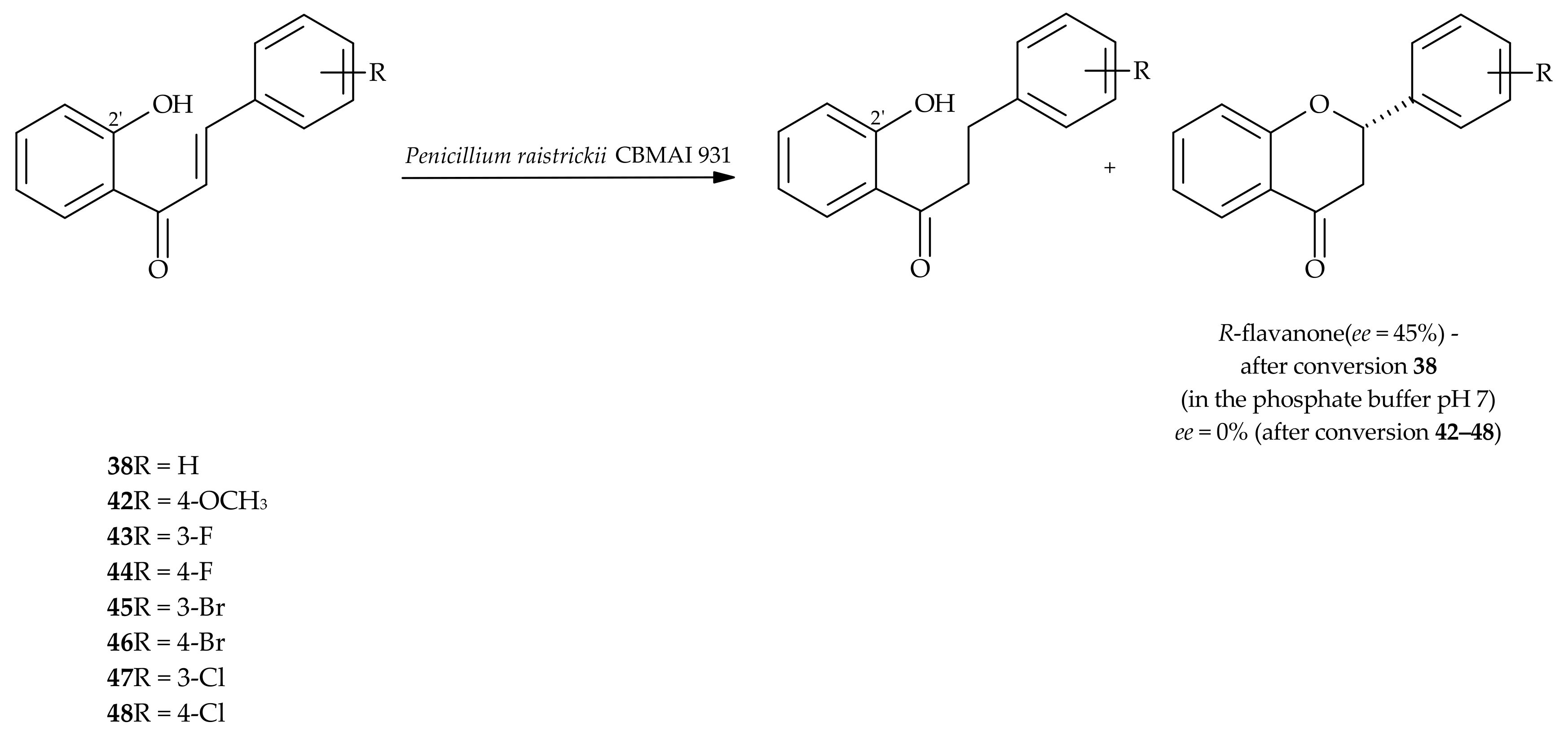
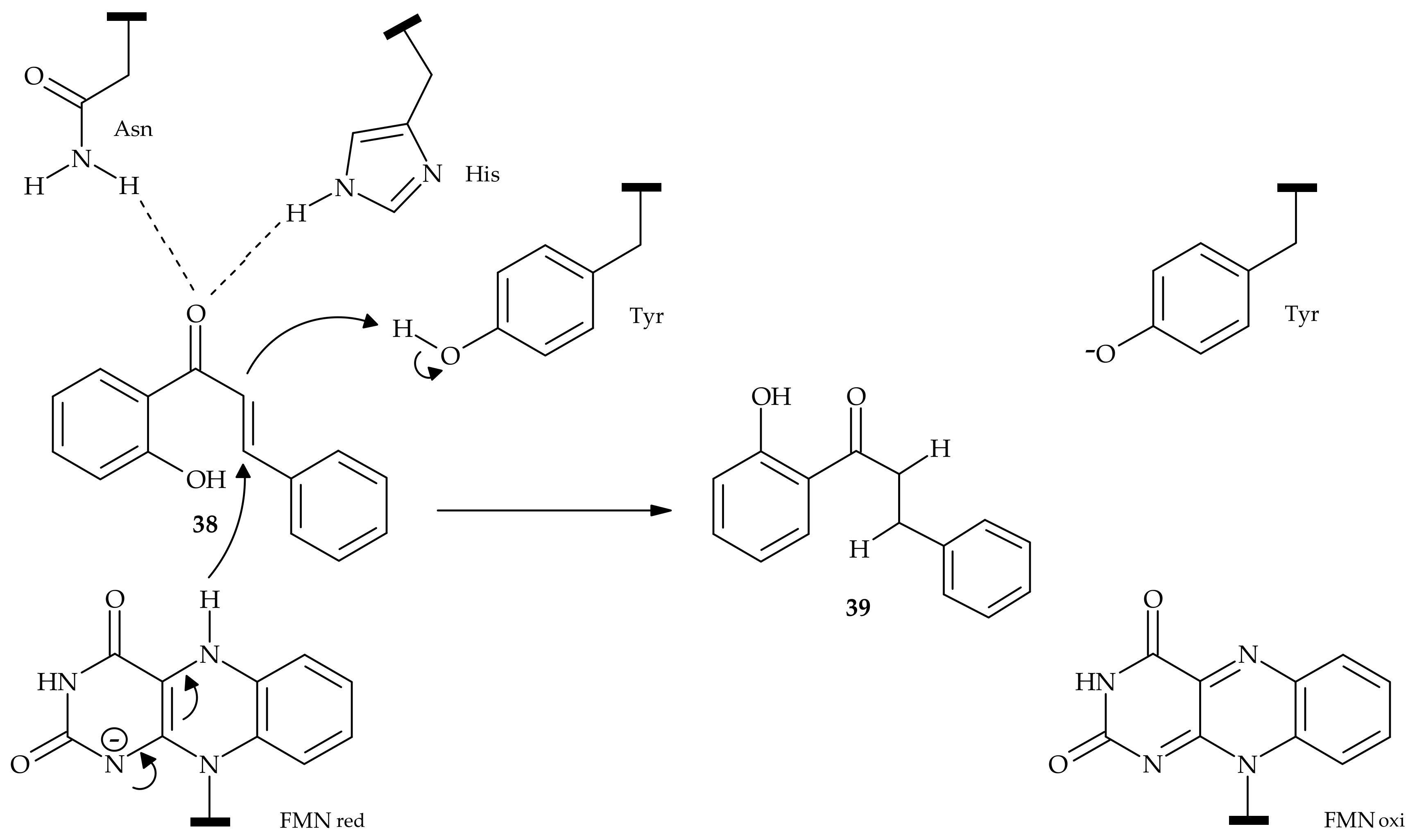
5. Microbiological and Enzymatic Hydrogenation of Chalcones
6. Pharmacological Properties of Dihydrochalcones
7. Dihydrochalcones as the Scaffolds for Drug Development
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, W.; Li, B.; Li, R. Solubilities of phloretin in 12 solvents at different temperatures. J. Chem. Eng. Data 2011, 56, 1459–1462. [Google Scholar]
- Tang, N.; Yan, W. Solubilities of naringin dihydrochalcone in pure solvents and mixed solvents at different temperatures. J. Chem. Eng. Data 2016, 61, 4085–4089. [Google Scholar] [CrossRef]
- Kim, M.; Shin, B.-K.; Won, D.; Han, J. Synthesis of 2′-hydroxydihydrochalcone from flavone. J. Appl. Biol. Chem. 2007, 50, 85–87. [Google Scholar]
- Miyakoshi, S.; Azami, S.; Kuzuyama, T. Microbial glucosylation of flavonol by Cunninghamella echinulate. J. Biosci. Bioeng. 2010, 110, 320–321. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M.S.M. Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 253–260. [Google Scholar] [CrossRef]
- Human, C.; De Beer, D.; Van Der Rijst, M.; Aucamp, M.; Joubert, E. Electrospraying as a suitable method for nanoencapsulation of the hydrophilic bioactive dihydrochalcone, aspalathin. Food Chem. 2019, 276, 467–474. [Google Scholar] [CrossRef]
- Dubois, G.E.; Crosby, G.A.; Stephenson, R.A.; Wingard, R.E.J. Dihydrochalcone sweeteners. Synthesis and sensory evaluation of sulfonate derivatives. J. Agric. Food Chem. 1977, 25, 763–772. [Google Scholar]
- Dubois, G.E.; Crosby, G.A.; Stephenson, R.A. Dihydrochalcone sweeteners. A study of the atypical temporal phenomena. J. Med. Chem. 1981, 24, 408–428. [Google Scholar] [CrossRef]
- Zhang, T.T.; Yang, L.; Jiang, J.G. Effects of thonningianin Ain natural foods on apoptosis and cell cycle arrest of HepG-2 human hepatocellular carcinoma cells. Food Funct. 2015, 6, 2588–2597. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical basis of anti-cancer-effects of phloretin—A natural dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef]
- Ding, B.; Ding, Q.; Zhang, S.; Jin, Z.; Wang, Z.; Li, S.; Dou, X. Characterization of the anti-Staphylococcus aureus fraction from Penthorum chinense Pursh stems. BMC Complement. Altern. Med. 2019, 19, 219. [Google Scholar] [CrossRef]
- Huang, D.; Jiang, Y.; Chen, W.; Yao, F.; Huang, G.; Sun, L. Evaluation of hypoglycemic effects of polyphenols and extracts from Penthorum chinense. J. Ethnopharmacol. 2015, 163, 256–263. [Google Scholar] [CrossRef]
- Alsanea, S.; Gao, M.; Liu, D. Phloretin prevents high-fat diet-induced obesity and improves metabolic homeostasis. AAPS J. 2017, 19, 797–805. [Google Scholar] [CrossRef]
- Shimamura, N.; Miyase, T.; Umehara, K.; Warashina, T.; Fujii, S. Phytoestrogen from Aspalathus linearis. Biol. Pharm. Bull. 2006, 29, 1271–1274. [Google Scholar] [CrossRef]
- Kang, B.C.; Kim, M.J.; Lee, S.; Choi, Y.A.; Park, P.H.; Shin, T.Y. Nothofagin sprresses mast cell-mediated allergic inflammation. Chem. Biol. Inter. 2019, 298, 1–7. [Google Scholar] [CrossRef]
- Ku, S.K.; Lee, W.; Kang, M.; Bae, J.S. Antithrombotic activities of aspalathin and nothofagin via inhibiting platelet aggregation and FIIa/FX. Arch. Pharm. Res. 2015, 38, 1080–1089. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Hamdy, A.H.; El-Fiky, N.M.; Mettwally, W.S.; El-Beih, A.A.; Kobayashi, N. Anti-influenza A virus activity of a new dihydrochalcone diglycoside isolated from the Egyptian seagrass Thalassodendron ciliatum (Forsk.) den Hartog. Nat. Prod. Res. 2014, 28, 377–382. [Google Scholar] [CrossRef]
- Phrutivorapongkul, A.; Lipipun, V.; Ruangrungsi, N.; Kirtikara, K.; Nishikawa, K.; Maruyama, S.; Watanabe, T.; Ishikawa, T. Studies on the chemical constituents of stem bark of Millettia leucantha: Isolation of new chalcones with cytotoxic, anti-herpes simplex virus and anti-inflammatory activities. Chem. Pharm. Bull. 2003, 51, 187–190. [Google Scholar] [CrossRef]
- Ghumatkar, P.J.; Patil, S.P.; Jain, P.D.; Tambe, R.M.; Sathaye, S. Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol. Biochem. Behav. 2015, 135, 182–191. [Google Scholar] [CrossRef]
- Barreca, D.; Curro, M.; Bellocco, E.; Ficarra, S.; Lagana, G.; Tellone, E.; Laura Giunta, M.; Visalli, G.; Caccamo, D.; Galtieri, A.; et al. Neuroprotective effects of phloretin and its glycosylated derivative on rotenone-induced toxicity in human SH-SY5Y neuronal-like cells. Biofactors 2017, 43, 549–557. [Google Scholar] [CrossRef]
- Lin, C.C.; Chu, C.L.; Ng, C.S.; Lin, C.Y.; Chen, D.Y.; Pan, I.H.; Huang, K.J. Immunomodulation of phloretin by impairing dendritic cell activation and function. Food Funct. 2014, 5, 997–1006. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef]
- Ibdah, M.; Martens, S.; Gang, D.R. Biosynthesis pathway and metabolic engineering of plant dihydrochalcones. J. Agric. Food Chem. 2018, 66, 2273–2280. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Jung, S.N.; Chang, H.; Yun, J.; Lee, C.W.; Lee, J.; Choi, S.; Nash, O.; Han, D.C.; Kwon, B.M. Artocarpus altilis (Parkinson) fosberg extracts and geranyl dihydrochalcone inhibit STAT3 activity in prostatę cancer DU145 cells. Phytother. Res. 2015, 29, 749–756. [Google Scholar] [CrossRef]
- Pompermaier, L.; Schwaiger, S.; Mawunu, M.; Lautenschlaeger, T.; Stuppner, H. Development and validation of a UHPLC-DAD method for the quantitative analysis of major dihydrochalcone glucosides from Thonningia sanguinea VAHL. Planta Med. 2019, 85, 911–916. [Google Scholar] [CrossRef]
- Adamu, G.N.; Gosh, C.; Molitor, C.; Kampatsikas, I.; Hutabarat, O.; Miosic, S.; Rompel, A.; Halbwirth, H.; Spornberger, A.; Stich, K. Investigations on the formation of dihydrochalcones in apple (Malus sp.) leaves. Acta Horticulturae 2019, 1242, 415–420. [Google Scholar] [CrossRef]
- Stanišić, M.; Ćosić, T.; Savić, J.; Krstić-Milošević, D.; Mišić, D.; Smagocki, A.; Niković, S.; Banjac, N. Hairy root culture as a valuable tool for allelopathic studies in apple. Tree Physiol. 2019, 39, 888–905. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Schneider, B.; Hölscher, D.; Stich, K. Cloning and heterologous expression of glycosyltransferases from Malusdomestica and Pyrus communis, which convert phloretin to phloretin 2′-O-glucoside (phloridzin). Plant Sci. 2010, 178, 299–306. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Gutierrez, B.L.; Zhong, G.Y.; Brown, S.K. Genetic diversity of dihydrochalcone content in Malus germplasm. Genet. Resour. Crop Evol. 2018, 65, 1485–1502. [Google Scholar] [CrossRef]
- Birtic, S.; Régis, S.; Le Bourvellec, C.; Renard, C.M.G.C. Impact of air-drying on polyphenol extractability from apple pomace. Food Chem. 2019, 296, 142–149. [Google Scholar] [CrossRef]
- Dixit, S.; Mautya, P.; Srivastava, M.; Shanker, K.; Bawankule, D.U.; Gupta, M.M.; Rai, L.K. Quantitation of dietary dihydrochalcones in Indian crabapple (Malus sikkimensis) using validated high-performance liquid chromatography. J. Chromatogr. Sci. 2019, bmz040. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Comparison of bioactive potential of cranberry fruit and fruit-based products versus leaves. J. Funct. Foods. 2016, 22, 232–242. [Google Scholar] [CrossRef]
- Schulze, A.E.; Beelders, T.; Koch, I.; Erasmus, L.M.; De Beer, D.; Joubert, E. Honeybush herbal teas (Cyclopia spp.) contribute to high levels of dietary exposure to xanthones, benzophenones, dihydrochalcones and other bioactive phenolics. J. Food Compos. Anal. 2015, 44, 139–148. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Verheul, M.J. The flavonoids of tomatoes. J. Agric. Food Chem. 2008, 56, 2436–2441. [Google Scholar] [CrossRef]
- Trani, M.; Carbonetti, A.; Monache, G.D.; Monache, F.D. Dihydrochalcones and coumarins of Esenbeckia grandiflora subsp. Brevipetiolata. Fitoterapia. 2004, 75, 99–102. [Google Scholar] [CrossRef]
- Müller, A.H.; Vieira, P.C.; da Silva, F.G.F.; Fernandes, J.B. Dihydrochalcones, coumarins and alkaloids from Metrodorea nigra. Phytochemistry 1995, 40, 1797–1800. [Google Scholar] [CrossRef]
- Singh, D.; Siew, Y.Y.; Chong, T.I.; Yew, H.C.; Ho, S.S.; Lim, C.S.E.; Tan, W.X.; Neo, S.Y.; Koh, H.L. Identification of phytoconstituents in Leea indica (Burm. F.) Merr. leaves by high performance liquid chromatography micro time-of-flight mass spectrometry. Molecules 2019, 24, 714. [Google Scholar] [CrossRef]
- Somsrisa, J.; Meepowpan, P.; Krachodnok, S.; Thaisuchat, H.; Punyanitya, S.; Nantasaen, N.; Pompimon, W. Dihydrochaloces with anti-inflammatory activity from leaves and twigs of Cyathostemma argenteum. Molecules 2013, 18, 6898–6907. [Google Scholar] [CrossRef]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.J.; Kim, S.Y.; et al. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorg. Chem. 2019, 103145. [Google Scholar] [CrossRef]
- Çevik, D.; Kan, Y.; Kirmizibekmez, H. Mechanisms of action of cytotoxic phenolic compounds from Glycyrrhiza iconica roots. Phytomedicine 2019, 58, 152872. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, N.T.; Awale, S. Prenylated dihydrochalcones from Artocarpus altilis as antiausterity agents. Enzymes 2015, 37, 95–110. [Google Scholar] [PubMed]
- Feng, J.; Peng, Y.; Zhao, W.W.; Fan, H.J.; Sun, Z.L.; Lu, Y. Chemical constituents of Balanophora involucrata. Chem. Nat. Compd. 2018, 54, 646–648. [Google Scholar] [CrossRef]
- Asakawa, Y.; Toyota, M.; Takemoto, T. Seven new bibenzyls and a dihydrochalcone from Radula variabilis. Phytochemistry 1978, 17, 2005–2010. [Google Scholar] [CrossRef]
- Hufford, C.D.; Oguntimein, B.O. New dihydrochalcones and flavanones from Uvaria angolensis. J. Nat. Prod. 1982, 45, 337–342. [Google Scholar] [CrossRef]
- Yang, W.M.; Liu, J.K.; Qin, X.D.; Wu, W.L.; Chen, Z.H. Antioxidant activities of three dihydrochalcone glucosides from leaves of Lithocarpus pachyphyllus. Z. Naturforsch C J. Biocsci. 2004, 59, 481–484. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.; Liu, Z. Preparative isolation, quantification and antioxidant activity of dihydrochalcones from Sweet Tea (Lithocarpus polystachyus Rehd.). J. Chromatogr. B 2015, 1002, 372–378. [Google Scholar] [CrossRef]
- Orjala, J.; Wright, A.D.; Behrends, H.; Folkers, G.; Sticher, O.; Rüegger, H.; Rali, T. Cytotoxic and antibaterial dihydrochalcones from Piper aduncum. J. Nat. Prod. 1994, 57, 18–26. [Google Scholar] [CrossRef]
- Dan, Z.; Wang, D.; Wang, G.H.; Guo, Z.J.; Zou, X.H.; Lin, T.; Chen, H.F. Chemical constituents in higher polar substances from Desmodium caudatum. Zhongguo Zhongyao Zazhi. 2014, 39, 3112–3116. [Google Scholar]
- Amorim, M.S.; Serain, A.F.; Salvador, M.J.; Stefanello, M.E.A. Chemical constituents of Sinningia hatschbachii. Nat. Prod. Commun. 2017, 12, 1763–1764. [Google Scholar] [CrossRef]
- Mohamed, E.I.; Zaki, M.A.; Chaurasiya, N.D.; Owis, A.I.; AbouZid, S.; Wang, Y.-H.; Avula, B.; Seida, A.A.; Tekwani, B.L.; Ross, S.A. Monoamine oxidases inhibitors from Colvillea racemosa: Isolation, biological evaluation, and computational study. Fitoterapia 2018, 124, 217–223. [Google Scholar] [CrossRef]
- Cho, C.S.; Shim, S.C. A ruthenium-catalyzed one-pot method for α-alkylation of ketones with aldehydes. J. Organomet. Chem. 2006, 691, 4329–4332. [Google Scholar] [CrossRef]
- Kose, O.; Saito, S. Cross-coupling reaction of alcohols for carbon-carbon bond formation using pincer-type NHC/palladium catalysts. Org. Biomol. Chem. 2010, 8, 896–900. [Google Scholar] [CrossRef]
- Jiang, Q.; Guo, T.; Wang, Q.; Wu, P.; Yu, Z. Rhodium (I)-catalyzed arylation of β-chloro ketones and related derivatives through domino dehydrochlorination/conjugate addition. Adv. Synth. Catal. 2006, 355, 1874–1880. [Google Scholar] [CrossRef]
- Bagal, D.B.; Qureshi, Y.S.; Dhake, K.P.; Khan, S.R.; Bhanage, B.M. An efficient and heterogeneous recyclable palladium catalyst for chemoselective conjugate of α,β-unsaturated carbonyls in aqueous medium. Green Chem. 2011, 13, 1490–1494. [Google Scholar] [CrossRef]
- Chen, P.; Li, W.; Wang, Y. Atmospheric hydrogenation of α,β-unsaturated ketones catalyzed by highly efficient and recyclable Pd nanocatalyst. Catal. Commun. 2019, 125, 10–14. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, J.; Feng, Y.; Ma, T.; Niu, M. First enantioselective synthesis of brosimacutins H and I. Chin. J. Org. Chem. 2016, 36, 547–554. [Google Scholar] [CrossRef]
- Reddy, M.R.; Aidhen, A.; Reddy, U.A.; Reddy, G.B.; Ingle, K.; Mukhopadhyay, S. Synthesis of 4-C-β-D-glucosylated isoliquiritigenin and analogues for aldose reductase inhibition studies. Eur. J. Org. Chem. 2019, 2019, 3937–3948. [Google Scholar] [CrossRef]
- Sum, T.H.; Sum, T.J.; Stokes, J.E.; Galloway, W.R.J.D.; Spring, D.R. Divergent and concise total syntheses of dihydrochalcones and 5-deoxyflavones recently isolated from Tacca species and Mimosa diplotricha. Tetrahedron 2015, 71, 26–27. [Google Scholar] [CrossRef]
- Tsunekawa, R.; Hanaya, K.; Higashibayashi, S.; Sugai, T. Synthesis of fisetin and 2′,4′,6′-trihydroxydihyrochalcone 4′-O-β-neohesperidoside based on site-selective deacetylation and deoxygenation. Org. Chem. 2018, 82, 1316–1322. [Google Scholar] [CrossRef]
- Cho, J.S.; Yoo, S.S.; Cheong, T.K.; Kim, M.J.; Kim, Y.; Park, K.H. Transglycosylation of neohesperidin dihydrochalcone by Bacillus stearothermophilus maltogenic amylase. J. Agric. Food Chem. 2000, 48, 152–154. [Google Scholar] [CrossRef]
- Eichenberger, M.; Lehka, B.J.; Folly, C.; Fischer, D.; Martens, S.; Simón, E.; Naesby, M. Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab. Eng. 2017, 39, 80–89. [Google Scholar] [CrossRef]
- Gutmann, A.; Bungaruang, L.; Weber, H.; Leypold, M.; Breinbauer, R.; Nidetzky, B. Toward the synthesis of glucosylated dihydrochalcone natural products using glucosyltransferase-catalysed cascade reactions. Green Chem. 2014, 16, 4417–4425. [Google Scholar] [CrossRef]
- Jia, W.; Xi, Q.; Liu, T.; Yang, M.; Chen, Y.; Yin, D.; Wang, X. One-pot synthesis of O-heterocycles or aryl ketones using an InCl3/Et3SiH system by switching the solvent. J. Org. Chem. 2019, 84, 5141–5149. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Engst, W.; Blaut, M. Degradation of neohesperidin dihydrochalcone by human intestinal bacetrai. J. Agric. Food Chem. 2005, 53, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Monge, P.; Solheim, E.; Scheline, R.R. Dihydrochalcone metabolism in the rat: Phloretin. Xenobiotica 1984, 14, 917–924. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The C-glycosyl flavonoid, aspalathin, is absorbed methylated and glucuronidated in humans. Mol. Nutr. Food Res. 2009, 53, 1104–1111. [Google Scholar] [CrossRef]
- Kreuz, S.; Joubert, E.; Waldmann, K.-H.; Ternes, W. Aspalathin, a flavonoid in Aspalathus linearis (rooibos), is absorbedby pig intestine as C-glycoside. Nutr. Res. 2008, 28, 690–701. [Google Scholar] [CrossRef]
- Ohta, H.; Konishi, J.; Tsuchihashi, G. Selective hydrogenation of carbon-carbon double bonds of chalcones by Corynebacterium equi IFO 3730. Agric. Biol. Chem. 1985, 49, 665–669. [Google Scholar] [CrossRef]
- Silva, V.D.; Stambuk, B.U.; Nascimento, M.G. Effecient chemoselective biohydrogenation of 1,3-diaryl-2-propen-1-ones catalyzed by Saccharomyces cerevisiae yeasts in biphasic system. J. Mol. Catal. B. Enzym. 2010, 63, 157–163. [Google Scholar] [CrossRef]
- Pathan, N.B.; Rahatgaonkar, A.M.; Chorghade, M.S. Stereoselective bioreduction of chalcone and β-diketone by Saccharomyces cerevisiae in biphasic solvent system: A mechanistic study. Indian J. Chem. 2012, 51B, 992–1001. [Google Scholar]
- Corrêa, M.J.C.; Nunes, F.M.; Bitencourt, H.R.; Borges, F.C.; Guilhon, G.M.S.P.; Arruda, M.S.P.; Marinho, A.M.R.; Santos, A.S.; Alves, C.N.; Brasil, D.S.B.; et al. Biotransformation of chalcones by the endophytic fungus Aspergillus flavus isolated from Paspalum maritimum trin. J. Braz. Chem. Soc. 2011, 22, 1333–1338. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Białońska, A.; Ciunik, Z. Microbial transformations of flavanone by Aspergillus niger and Penicillium chermesinum cultures. J. Mol. Catal. B. Enzym. 2008, 52–53, 34–39. [Google Scholar] [CrossRef]
- De Matos, I.L.; Nitschke, M.; Porto, A.L.M. Hydrogenation of halogenated 2′-hydroxychalcones by mycelia of marine-derived fungus Penicillium raistrickii. Marine Biotechnol. 2019, 21, 430–439. [Google Scholar] [CrossRef]
- Stompor, M.; Potaniec, B.; Szumny, A.; Zieliński, P.; Żołnierczyk, A.; Anioł, M. Microbiological reduction of xanthohumol and 4-methoxychalcone. Przem. Chem. 2013, 92, 574–578. [Google Scholar]
- Łużny, M.; Krzywda, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Effective hydrogenation of 3-(2″-furyl)- and 3-(2″-thienyl)-1-(2′-hydroxyphenyl)-prop-2-en-1-one in selected yeast cultures. Molecules 2019, 24, 3185. [Google Scholar] [CrossRef]
- Żyszka-Haberecht, B.; Poliwoda, A.; Lipok, J. Biocatalytic hydrogenation of the C=C bond in the enone unit of hydroxylated chalcones—process arising from cyanobacterial adaptations. Appl. Microbiol. Biotechnol. 2018, 102, 7097–7111. [Google Scholar] [CrossRef]
- Stompor, M.; Potaniec, B.; Szumny, A.; Zieliński, P.; Żołnierczyk, A.K.; Anioł, M. Microbial synthesis of dihydrochalcones using Rhodococcus and Gordonia species. J. Mol. Catal. B Enzym. 2013, 97, 283–288. [Google Scholar] [CrossRef]
- Stompor, M.; Kałużny, M.; Żarowska, B. Biotechnological methods for chalcone reduction using whole cells of Lactobacillus, Rhodococcus and Rhodotorula strains as a way to produce new derivatives. Appl. Microbiol. Biotechnol. 2016, 100, 8371–8384. [Google Scholar] [CrossRef]
- Klingel, T.; Hadamjetz, M.; Fischer, A.; Wefers, D. Glucosylation of flavonoids and flawonoid glycosides by mutant dextransucrase from Lactobacillus reuteri TMW 1.106. Carbohydr. Res. 2019, 483, 107741. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, B.; Liu, A.; Lu, Y.J.; Zhou, J.L.; Zhang, J.; Wong, W.L. Enzymatic production of natural sweetener trilobatin from citrus flavanone naringin using immobilised α-l-rhamnosidase as the catalyst. Int. J. Food Sci. Technol. 2018, 53, 2097–2103. [Google Scholar]
- Wang, J.; Chun, M.H.; Xue, B.; Ma, H.; Ma, C.; Hattori, M. Estrogenic and antiestrogenic activities of phloridzin. Biol. Pharm. Bull. 2010, 33, 592–597. [Google Scholar] [CrossRef]
- Viet, M.H.; Chen, C.Y.; Hu, C.K.; Chen, Y.R.; Li, M.S. Discovery of dihydrochalcones as potential lead for Alzheimer’s disease: In silico and in vitro study. PLoS ONE 2013, 8, e79151. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.M.; Gentili, B. Taste and structure in phenolic glycosides. J. Agric. Food Chem. 1969, 17, 696–700. [Google Scholar] [CrossRef]
- Janvier, S.; Goscinny, S.; Le Donne, C.; Van Loco, J. Low calorie sweeteners in food and food supplements on the Italian market. Food Addit. Contam. Part B 2015, 8, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-S.; Zhang, Y.; Hu, W.-Z.; Zhang, X.-F.; Fu, X.; Lv, X. Dihydrochalcones and diterpenoids form Pteris ensiformis and their bioactivities. Molecules 2017, 22, 1413. [Google Scholar] [CrossRef]
- Shabala, S.C.; Dludla, P.V.; Muller, C.J.F.; Nxele, X.; Kappo, A.P.; Louw, J.; Johnson, R. Aspalathin ameliorates doxorubicin-induced oxidative stress in H9c2 cardiomyoblasts. Toxicol. In Vitro 2019, 55, 134–139. [Google Scholar] [CrossRef]
- Nascimento, Y.M.; Abreu, L.; Lima, R.L.; Silva, A.D.S.; Costa, V.C.O.; Melo, J.I.M.; Scotti, M.T.; Sobral, M.V.; Araujo, S.S.; Filho, M.A.; et al. Zornioside, a dihydrochalcone C-glycoside, and other compounds from Zornia brasiliensis. Rev. Bras. Farmacogn. 2018, 28, 192–197. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, N.T.; Dau, H.T.; Nguyen, H.X.; Dang, P.H.; Le, T.M.; Nguyen Phan, T.H.; Tran, A.H.; Nguyen, B.D.; Ueda, J.Y.; et al. Geranyl dihydrochalcones from Artocarpus altilis and their antiausteric activity. Planta Med. 2014, 80, 193–200. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Paradysz, A.; Król, W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 2010, 15, 5336–5353. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Zhu, J.; Chang, Q.; Zhou, H.; Shi, Z.; Min, L.; Cai, Y.; Guan, H. Alpha, 2′-dihydroxy-4,4′-dimethoxydihydrochalcone inhibits cell proliferation, invasion, and migration in gastric cancer in part via autophagy. Biomed. Pharmacother. 2018, 98, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.N.; Badwaik, V.D.; Waghwani, H.K.; Moolani, H.V.; Tockstein, S.; Thompson, D.H.; Dakshinamurthy, R. Development of dihydrochalcone-functionalized gold nanoparticles for augmented antineoplastic activity. Int. J. Nanomed. 2018, 13, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Hsieh, M.J.; Yang, S.F.; Chen, S.P.; Tsai, W.C.; Chen, P.N. Phloretin suppresses metastasis by targeting protease and inhibits cancer stemness and angiogenesis in human cervical cancer cells. Phytomedicine 2019, 62, 152964. [Google Scholar] [CrossRef]
- Kobori, M.; Shinmoto, H.; Tsushida, T.; Shinohara, K. Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett. 1997, 119, 207–212. [Google Scholar] [CrossRef]
- Kim, U.; Kim, C.Y.; Lee, J.M.; Oh, H.; Ryu, B.; Kim, J.; Park, J.H. Phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathol. Oncol. Res. 2019, 1–8. [Google Scholar]
- Duan, H.; Wang, R.; Yan, X.; Liu, H.; Zhang, Y.; Mu, D.; Han, J.; Li, X. Phloretin induces apoptosis of human esophageal cancer via a mitochondria-dependent pathway. Oncol. Lett. 2017, 14, 6763–6768. [Google Scholar] [CrossRef]
- Xu, M.; Gu, W.; Shen, Z.; Wang, F. Anticancer activity of phloretin against human gastric cancer cell lines involves apoptosis, cell cycle arrest, and inhibition of cell invasion and JNK signalling pathway. Med. Sci. Monit. 2018, 24, 6551–6558. [Google Scholar] [CrossRef]
- Qin, X.; Xing, Y.F.; Zhou, Z.; Yao, Y. Dihydrochalcone compounds isolated from crabapple leaves showed anticancer effects on human cancer cell lines. Molecules 2015, 20, 21193–21203. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Filip-Psurska, B.; Wietrzyk, J.; Popłoński, J.; Hyszcza, E. Fungal metabolities of xanthohumol with potent antiproliferative activity on human cancer cell lines in vitro. Bioorg. Med. Chem. 2013, 21, 2001–2006. [Google Scholar] [CrossRef]
- Prawat, U.; Chairerk, O.; Phupornprasert, U.; Salae, A.W.; Tuntiwachwuttikul, P. Two new C-benzylated dihydrochalcone derivatives from the leaves of Melodorum siamensis. Planta Med. 2013, 79, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Potipiranun, T.; Adisakwattana, S.; Worawalai, W.; Ramadhan, R.; Phuwapraisirisan, P. Identification of pinocembrin as an anti-glycation agent and α-glucosidase inhibitor from fingerroot (Boesenbergia rotunda): The tentative structure–activity relationship towards MG-trapping activity. Molecules 2018, 23, 3365. [Google Scholar] [CrossRef]
- Pérez, G.R.M.; García, C.A.H.; Paredes, C.S.P.; Muniz, R.A.; Mota, F.J.M.; Flores, V.S.O. 3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy- dihydrochalcone, from bark of Eysenhardtia polystachya prevents diabetic nephropathy via inhibiting protein glycation in STZ-nicotinamide induced diabetic mice. Molecules 2019, 24, 1214. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Z.; Wang, A.; Jia, L.; Zhang, X.; Fang, M.; Yi, K.; Li, Q.; Hu, H. Comparative oral and intravenous pharmacokinetics of phlorizin in rats having type 2 diabetes and in normal rats based on phase II metabolism. Food Funct. 2019, 10, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.W.; Zhu, H.; Yuan, X.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Eight new bioflavonoids with lavandulyl units from the roots of Sophora flavescens and their inhibitory effect on PTP1B. Bioorg. Chem. 2019, 86, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Dludla, P.V.; Gabuza, K.B.; Muller, C.J.F.; Joubert, E.; Louw, J.; Johnson, R. Aspalathin, a C-glucosyl dihydrochalcone from rooibos improves the hypoglycemic potential of metformin in type 2 diabetic (db/db) mice. Physiol. Res. 2018, 67, 813–818. [Google Scholar] [CrossRef]
- Schloms, L.; Storbeck, K.H.; Swart, P.; Gelderblom, C.A.; Swart, A.C. The influence of Aspalathus linearis (Rooibos) and dihydrochalcones on adrenal steroidogenesis: Quantification of steroid intermediates and end products in H295R cells. J. Steroid. Biochem. Mol. Biol. 2012, 128, 128–138. [Google Scholar] [CrossRef]
- Mazibuko, S.E.; Joubert, E.; Johnson, R.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspatathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol. Nutr. Food Res. 2015, 59, 2199–2208. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, P.; Opoku, A.R.; Muller, C.J.F. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012, 39, 5299–5306. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, C.; Lee, B.-S.; Park, E.K.; Kim, K.-M.; Bae, J.-S. Renal protective effects of aspalathin and nothofagin from rooibos (Aspalathus linearis) in a mouse model of sepsis. Pharmacol. Rep. 2018, 70, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Legault, J.; Simard, F.; Chiasson, E.; Pichette, A. New antibacterial dihydrochalcone derivatives from buds of Populus balsamifera. Tetrahedron Lett. 2013, 54, 1631–1633. [Google Scholar] [CrossRef]
- Daus, M.; Chaithada, P.; Phongpaichit, S.; Watanapokasin, R.; Carroll, A.R.; Mahabusarakam, W. New prenylated dihydrochalcones from the leaves of Artocarpus elasticus. Phytochem. Lett. 2017, 19, 226–230. [Google Scholar] [CrossRef]
- Montenegro, I.; Madrid, A. Synthesis of dihydroisorcordoin derivatives and their in vitro anti-oomycete activities. Nat. Prod. Res. 2019, 33, 1214–1217. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef]
- Jamil, S.; Lathiff, S.M.A.; Abdullah, A.; Jemaon, N.; Sirat, H.M. Antimicrobial flavonoids from Artocarpus anisophyllus Miq. and Arocarpus lowii king. Journal Teknologi. 2014, 71, 95–99. [Google Scholar]
- Chowdhury, A.; Mukherjee, N.; Adityachaudhury, N. Sensitivity of some plant pathogenic fungi towards plant metabolites: Antifungal activity of some chalcones, dihydrochalcones and flavanones. Experientia 1974, 30, 1022–1024. [Google Scholar] [CrossRef]
- Takahashi, T.; Kokubo, R.; Sakaino, M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef]
- Koudokpon, H.; Armstrong, N.; Daugnon, T.V.; Fah, L.; Hounsa, E.; Bankolé, H.S. Antibacterial activity of chalcone and dihydrochalcone compounds from Uvaria chamae roots against multidrug-resistant bacteria. BioMed Res. Int. 2018, 2018, 1453173. [Google Scholar] [CrossRef]
- Simpson, M.J.; Hjelmqvist, D.; Lopez-Alarcon, C.; Karamehmedocic, N.; Minehan, T.G.; Yepremyan, A.; Salehani, B.; Lissi, E.; Joubert, E.; Udekwu, K.I. Anti-peroxyl radical quality and antibacterial properties of Rooibos infusions and their pure glucosylated polyphenolic constituents. Molecules 2013, 18, 11264–11280. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Song, X.; Fu, J.; Su, C.; Xia, X.; Song, E.; Song, Y. Artificial sweetener neohesperidin dihydrochalcone showed antioxidative, anti-inflammatory and anti-apoptosis effects against paraquat-induced liver injury in mice. Int. Immunopharmacol. 2015, 29, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, Y.; Chen, X.; Wang, Y.; Chen, W.; Xu, Q.; Li, P.; Ma, F. Extraction, identification, and antioxidant and anticancer tests of seven dihydrochalcones from Malus ‘Red Splendor’ fruit. Food Chem. 2017, 231, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, Y.; Wang, J.; Li, P.; Ma, F. Structure-antioxidant capacity relationship of dihydrochalcone compounds in Malus. Food Chem. 2019, 275, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant structure–activity relationship analysis of five dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.C.; Ding, Y.; Green, I.R.; Golderblom, W.C. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other Rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Liu, A.; Huang, B.; Lei, L.; Lu, Y.J.; Zhou, J.L.; Wong, W.L. Production of high antioxidant activity flavonoid monoglucosides from citrus flavanone with immobilised α-L-rhamnosidase in one step. Int. J. Food Sci. Technol. 2019, 54, 2854–2862. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as novel radical scavenging antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.; Yasmin, A. Inhibition of oxidation of aqueous emulsions of omega-3 fatty acids and fish oil by phloretin and phloridzin. Molecules 2010, 15, 251–257. [Google Scholar] [CrossRef]
- Sartor, L.; Pezzato, E.; DellÁica, I.; Caniato, R.; Biggin, S.; Garbisa, S. Inhibition of matrix-proteases by polyphenols: Chemical insights for anti-inflammatory and anti-invasion drug design. Biochem. Pharmacol. 2002, 64, 229–237. [Google Scholar] [CrossRef]
- Kwak, S.; Han, M.S.; Bae, J.S. Aspalathin and nothofagin from rooibos (Aspalathus linearis) inhibit endothelial protein C receptor shedding in vitro and in vivo. Fitoterapia 2015, 100, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, B.R.M.; Hung, H.Y.; Kuo, P.C.; Huang, G.J.; Chan, Y.Y.; Huang, S.C.; Wu, S.J.; Morris-Natschke, S.L.; Lee, K.H.; Wu, T.S. Synthesis and biological evaluation of chalcone, dihydrochalcone, and 1,3-diarylpropane analogs as anti-inflammatory agents. Bioorg Med. Chem. 2017, 27, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, C.; Zhang, C.; Cai, L.; Chen, H. The protective effect of phloretin in osteoarthritis: An In vitro and in vivo study. Food Funct. 2018, 9, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Bae, J.-S. Anti-inflammatory effects of aspalathin and nothofagin from Rooibos (Aspalathus linearis) in vitro and in vivo. Inflammation 2015, 38, 1502–1516. [Google Scholar] [CrossRef]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of apple polyphenols on inflammatory gene expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef]
- Natanzi, A.R.E.; Mahmoudian, S.; Minaeie, B.; Sabzevari, O. Hepatoprotective activity of phloretin and hydroxychalcones against acetaminophen induced hepatotoxicity in mice. Iran. J. Pharm. Sci. 2011, 7, 89–97. [Google Scholar]
- Cheng, M.; Ding, L.; Kan, H.; Zhang, H.; Jiang, B.; Sun, Y.; Cao, S.; Li, W.; Koike, K.; Qiu, F. Isolation, structural elucidation and in vitro hepatoprotective activity of flavonoids from Glycyrrhiza uralensis. J. Nat. Med. 2019, 73, 847–854. [Google Scholar] [CrossRef]
- Hermoso, A.; Jiménez, I.A.; Mamani, Z.A.; Bazzocchi, I.L.; Piñero, J.E.; Ravelo, A.G.; Valladares, B. Antileishmanial activities of dihydrochalcones from Piper elongatum and synthetic related compounds. Structural requirements for activity. Bioorg. Med. Chem. 2003, 11, 3975–3980. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Lai, F.; Liang, F.; Wen, J.; Lin, W.; Qiu, J.; Liu, S.; Li, L. Trilobatin as an HIV-1 entry inhibitor targeting the HIV-1 Gp41 envelope. FEBS Lett. 2018, 592, 2361–2377. [Google Scholar] [CrossRef]
- Johnson, R.; Dludla, P.V.; Muller, C.J.F.; Huisamen, B.; Essop, M.F.; Louw, J. The transcription profile unveils the cardio-protective effect of aspalathin against lipid toxicity in an in vitro H9c2 model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of selected polyphenols—A short report. Pol. J. Food Nutr. Sci. 2013, 64, 59–64. [Google Scholar] [CrossRef]
- Ardiansah, B. Chalcones bearing N, O, and S-heterocycles: Recent notes on their biological activity. J. Appl. Pharm Sci. 2019, 9, 117–129. [Google Scholar]
- Ivaković, B.M.; Nikolic, K.; Ilić, B.B.; Žižak, Ž.S.; Novaković, R.B.; Čudina, O.A.; Vladimirov, S.M. Phenylpropiophenone derivatives as potential anticancer agents: synthesis, biological evaluation and quantitative structure-activity relationship study. Eur. J. Med. Chem. 2013, 63, 239–255. [Google Scholar]
- Irshad, M.; Ali, Q.; Iram, F.; Ahamad, S.A.; Saleem, M.; Saadia, M.; Batool, M.; Kanwal, A.; Tabassum, S. Aurones and analogues: Promising heterocyclic scaffolds for development of antioxidant and antimicrobial agents. Rus. J. Gen. Chem. 2019, 89, 1519–1527. [Google Scholar] [CrossRef]
- Dal Picolo, C.R.; Bezerra, M.P.; Gomes, K.S.; Passero, L.F.D.; Laurenti, M.D.; Martins, E.G.A.; Sartorelli, P.; Lago, J.H.G. Antileishmanial activity evaluation of adunchalcone, a new prenylated dihydrochalcone from Piper aduncum L. Fitoterapia 2014, 97, 28–33. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rakshit, J.; Bandyopadhyay, J.; Basu, S. Multi-functional neuroprotective activity of neohesperidin dihydrochalcone: A novel scaffold for Alzheimer’s disease therapeutics identified via drug repurposing screening. New. J. Chem. 2018, 42, 11755–11769. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, Y.; Lei, J.; Wei, G. Dihydrochalcone molecules destabilize Alzheimer’s amyloid-β protofibrils through binding to the protofibril cavity. Phys. Chem. Chem. Phys. 2018, 20, 17208–17217. [Google Scholar] [CrossRef]
- Krbechek, L.; Inglett, G.E.; Holik, M.; Dowling, B.; Wagner, R.; Riter, R. Dihydrochalcones. Synthesis of potential sweetening agents. J. Agric. Food Chem. 1968, 16, 108–112. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin. Pharmacokinet. 2015, 54, 691–708. [Google Scholar] [CrossRef]
- Jesus, A.R.; Vila-Viçosa, D.; Machugueiro, M.; Margues, A.P.; Dore, T.M.; Rauter, A.P. Targeting type 2 diabetes with C-glucosyl dihydrochalcones as selective sodium glucose co-transporter 2 (SGLT2) inhibitors: synthesis and biological evaluation. J. Med. Chem. 2017, 60, 568–579. [Google Scholar] [CrossRef]

| Therapeutics | Compound | Treatment and Methods | Targets and Effects | Experimental Model | Ref. |
|---|---|---|---|---|---|
| Anticancer | evelynin A | SRB assay | antiproliferative activity IC50 = 6.3 ± 0.7µM (HeLa) IC50 = 4.7 ± 0.4 µM (PC-3) | In vitro, HeLa (cervical carcinoma), A549 (lung cancer), and PC-3 (prostate cancer) | [87] |
| evelynin B | antiproliferative activity IC50 =8.8 ± 0.5 µM (HeLa) IC50 = 8.3 ± 0.6 µM (A549) IC50 = 5.0 ± 0.8 µM (PC-3) inhibits tubulin polymerization | ||||
| aspalathin | 0.2–1.0 µM | Co-treatment with aspalathin attenuates doxorubicin-induced cardiotoxicity by improving endogenous antioxidant levels and mitochondrial membrane potential while inhibiting reactive oxygen species production and cellular apoptosis. | In vitro, H9c2 cardiomyoblasts | [88] | |
| zornioside | 0.39–50 µg/mL MTT assay | IC50 = 37.26 µM | In vitro, HL60 (leukemia cells) | [89] | |
| sakenins F and H | PC50 values of 8.0 µM and 11.1 µM | In vitro, PANC-1 human pancreatic cancer cells | [90] | ||
| 2′,6′-dihydroxy- -4′4-dimethoxydihydrochalcone, 2′,6′-dihydroxy- -4′-methoxydihydrochalcone, and 2′,4′,6′-trihydroxydihydrochalcone | MTT assay 50–100 μM | Augment TRAIL (tumor necrosis factor-related apoptosis-inducing ligand)-induced apoptosis and cytotoxicity | In vitro, LNCaP (human hormone-sensitive prostate cancer) | [91] | |
| 2′-dihydroxy-4,4′-dimethoxydihydrochalcone | 8–32 µM 12–48 mg/kg | Antiproliferative activity, suppresses cell proliferation, induces excess of reactive oxygen species (ROS) accumulation, and inhibits the invasion and migration ability of MKN45 cells. Upregulates the autophagy-related proteins Beclin-1, Atg5, and Atg7; induces formation of Autophagosomes and regulatory role of ROS/MEK/ERK signaling pathways. In vivo inhibits the growth of MKN45 xenograft tumors in nude mice and suppresses Ki67 expression. | In vitro, MKN45 (human gastric cancer) In vivo, BALB/C nude mice | [92] | |
| conjugates of phloretin (Pht) and phlorizin (Phl) with gold nanoparticles (AuNPs) | Fourty-five-fold increase in the efficacy in the antineoplastic activity of Pht-AuNPs over pure phloretin and 4.49-fold increase in efficacy of Phl-AuNP over pure phlorizin. | In vitro, HeLa (cervical carcinoma) | [93] | ||
| phloretin | 0–60 µM 10–20 mg/kg | Marked suppression of invasion and migration through downregulation of matrix metalloproteinase (MMP)-2, MMP-3, and cathepsin Sin human SiHa cervical cancer cells. Suppresses lung metastasis and tumor growth. | In vitro, SiHa (cervical cancer), HeLa, and CaSki (cervical cancer) In vivo, BALB/c AnN.CgFoxnnu/Crl Narl mice | [94] | |
| 0.1–0.2 mM | Induces apoptosis by the inhibition of glucose transmembrane transport and internucleosomal DNA fragmentation | In vitro, B16 (mouse melanoma 4A5 cells) | [95] | ||
| 20–100 µM | Inhibits cancer cell proliferation and migration by increasing the ROS production in the cells | In vitro, PC-3 and DU-145 (human prostate cancer) | [96] | ||
| 60–100 µg/mL | Induces cancer cellapoptosis via a mitochondrial-dependent pathway. Downregulates the antiapoptosis-associated molecule B-cell lymphoma 2 (bcl-2) and increases the levels of the apoptosis-associated molecules bcl-2-like protein 4 and tumor protein p53. Affects the expression of apoptotic protease activating factor-1. | In vitro, EC-109 (human esophageal cancer) | [97] | ||
| MTT assay, DAPI, annexin V/PI, Transwell assay, and Western blotting. 0–16 µM | Inhibits cell proliferation. IC50 = 8.0 µM (AGS) IC50 = 16.0 µM (MGC80-3, BGC-823, SGC-7901, SNU-5) IC50 = 32.0 µM (SNU-1, SNU-5, SNU-16, RF-1) IC50 > 60 µM (SNU-16, GES- 1) | In vitro, MGC80-3, BGC-823, SGC-7901, AGS, SNU-1, SNU-5, SNU-16, RF-1, and GES-1 (gastric cancers) | [98] | ||
| 3‴-methoxy-6″-O-feruloy-4′-O- -glucopyranosyl-phloretin | MTT assay | IC50 = 39.79 ± 5.72 µg/mL (A549) IC50 = 59.28 ± 5.06 µg/mL (BEL7402) IC50 = 49.36 ± 3.04 µg/mL (HepG2) IC50 = 65.09 ± 2.77 µg/mL (HT-29) Glycoside moiety bound to the phloretin structure decreases its anticancer activity (e.g., in trilobatin) | In vitro, A549 (lung cancer), BEL 7402 (liver cancer), HepG2 human ileocecal cancer cell line, and HT-29 (colon cancer) | [99] | |
| 3-hydroxyphloretin | IC50 = 39.83 ± 4.23 µg/mL (A549) IC50 = 45.17 ± 8.02 µg/mL (BEL7402) IC50 = 37.79 ± 4.04 µg/mL (HepG2) IC50 = 35.37 ± 2.53 µg/mL (HT-29) | ||||
| α,β-dihydroxanthohumol | SRB assay 0.1–100 µg/mL | IC50 = 9.15 ± 0.62 µM (MCF-7)) IC50 = 14.73 ± 3.88 µM (PC-3) IC50 = 74.41 ± 23.44 µM (HT-29) | In vitro, MCF-7 (breast cancer), PC-3 (prostate cancer), and HT-29 (colon cancer) | [100] | |
| 4,2′,4′-trihydroxy-6′-methoxy-3′(2″-hydroxybenzyl)dihydrochalcone, 2′,4′-dihydroxy-4,6′-dimethoxy-3′(2″-hydroxybenzyl)dihydrochalcone | Antitumor activity IC50 = 2.02–20.03 µg/mL | In vitro, KB (epimermoid carcinoma), MCF-7 (breast cancer, and NCl-H187 (lung cancer) | [101] | ||
| Antidiabetic | panduratin isopanduratin | MG–BSA assay | Antiglycation agents, α-glucosidase inhibition | In vitro, bovine serum albumin and α-glucosidase (from rat intestine) | [102] |
| 3′-O-β-d-glucopyranosyl α,4,2′,4′,6′-pentahydroxy-dihydrochalcone | 25, 50, and 100 mg/kg diabetic nephropathy | Antiglycation compound inhibits protein glycation and decreases accumulation of advanced glycation end products (AGEs). | In vivo, STZ (streptozotocin)-induced diabetic mice (C57BL/6) | [103] | |
| phlorizin | Improves the symptoms of diabetes and diabetic complications. | [104] | |||
| dihydrochalcones from the roots of Sophora flavescens | Inhibitors of PTP1B | PTP1B inhibition assay | [105] | ||
| hydroxydihydrochalcones from Artemisia (Astraceae) | spectrophotometric method | Have inhibitory activity against principal enzymes of carbohydrate metabolism, such as α-amylase (IC50 = 150.24–384.14 μg/mL) and α-glucosidase (IC50 = 214.42–754.12 μg/mL). May become a complement to synthetic antidiabetic drugs for controlling blood glucose level. | In vitro, α-glucosidase from Saccharomyces cerevisiae, α-amylase from Aspergillus oryzae | [106] | |
| aspalathin | 130 mg/kg | Protects against diabetes-associated symptoms in mice. | In vivo, type-2 diabetic mouse model | [107] | |
| complications associated with hepatic insulin resistance and metabolic disease-relate | Plays a significant role in the maintenance of hormonal homeostasis. Influences the steroid hormone biosynthesis and the flux through the mineralocorticoid, glucocorticoid, and androgen pathways, thus possibly contributes to the alleviation of negative effects arising from elevated glucocorticoid levels. | In vitro, H295R | [108] | ||
| 10 µM | Reverses the palmitate-induced insulin resistance. Suppresses nuclear factor kappa beta (NF-κB), insulin receptor substrate one (serine 307) (IRS1 (Ser (307) and AMP-activated protein kinase (AKT) phosphorylation and increases serine/threonine kinase AKT activation. Increases the peroxisome proliferator-activated receptor alpha and gamma (PPARα and γ) and carnitine palmitoyltransferase one (CPT1) expression. | In vitro, 3T3-L1 adipocytes exposed to palmitate | [109] | ||
| 10 µM | Regulates hepatic cellular metabolism, increases energy expenditure, and modulates PI3K/AKT and AMPK signaling pathways. | In vitro, C3A (liver cells exposed to palmitate) | [110] | ||
| phlorizin | 5–40 mg/kg | Competitive inhibitor of sodium/glucose cotransporters in the intestine (SGLT1) and kidney (SGLT2) involved in intestinal glucose absorption and renal glucose reabsorption. | In vivo, Wistar rats | [111] | |
| Kidney diseases | aspalathin and nothofagin | Induce renal damage. Inhibits nuclear factor (NF)-κB activation and reduces the induction of NO synthase and excessive production of nitric acid. Reduce the plasma levels of NO, TNF-α, and IL-6; increases lipid peroxidation; and markedly enhances the antioxidant defence system by restoring the levels of superoxide dismutase, glutathione peroxidase, and catalase. | In vivo, mice model sepsis | [112] | |
| Antimicrobial activity | balsacones A, B, and C | MIC = 3.1–6.6 μM (S. aureus) resistance (E. coli) | In vitro, Staphylococcus aureus Escherichia coli | [113] | |
| Elastichalcone C | In vitro, methicillin-resistant Staphylococcus aureus SK1, Saprolegnia parasitica Saprolegnia diclina | [114] | |||
| dihydroisorcordoin | In vitro, Saprolegnia parasitica Saprolegnia diclina | [115] | |||
| phloretin, phlorizin,3’,5’-di-C-glucoside | microdilution method | phloretin: MIC = 7.81–125 μM (S. aureus) MIC = 62.5 μM (L. monocytogenes) MIC = 125 μM (S. typhimurium) MIC > 1000 μM (P. aeruginosa, E. coli) | In vitro, Staphylococcus aureus ATCC 6538 Listeria monocytogenes ATCC 13932 methicyllin-resistant Staphylococcus aureus (MRSA, clinical strains: 526, 588, 550, 541, 531, 530, 543, 581) Salmonella typhimurium ATCC 13311 Pseudomonas aeruginosa ATCC 27853 Pseudomonas aeruginosa ATCC 15442 E. coli ATCC 10536 | [116] | |
| 2′,4′-dihydroxy-4- methoxy-3′-prenyldihydrochalcone 2′,4′-dihydroxy-3,4-(2”,2”-dimethylchromeno)-3′-prenyldihydrochalcone | Inhibition zone: 7.25–10.75 mm MIC = 0.9–1.8 mg/mL | In vitro, Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 29737), Escherichia coli (ATCC 10536), Pseudomonas putida (ATCC 49128) | [117] | ||
| 2′,4’-dihydroxychalcone | 105 µg/mL | Inhibits spore germination of plant pathogenic fungi | In vitro, Heiminthosporium oryzae, Aspergillus niger, Rhizopus nigricans | [118] | |
| 2′,6′-dihydroxy-3′-methyl-4′-methoxydihydrochalcone | MIC = 1.0–7.8 mg/L | In vitro, B. cereus (IFO3001) Pseudomonas putida (IFO3738) S. aureus (IFO12732) methicillin-resistant S. aureus (MRSA; RIM0310925) Enterococcus faecalis (IFO12970) Alicyclobacillus acidoterrestris (ATCC49025) Propionibacterium acnes (ATCC6919) Trichophyton mentagrophytes (IFO5466) | [119] | ||
| dihydrochalcones identified in ethanol extracts from Uvaria chamae roots (uvaretin, uvangoletin, diuvaretin) | Inhibition diameter: 9–30.7 mm | S. aureus ATCC 259223 S. aureus (clinical isolate) E. faecalis (clinical isolate) | [120] | ||
| aspalathin, nothofagin | Antibacterial effect | In vitro, Escherichia coli (CFT073) Staphylococcus epidermis (Se19) Staphylococcus aureus (ATCC 25923) | [121] | ||
| Antioxidant activity | neohesperidin dihydrochalcone | Downregulates cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expressions. Inhibites PQ-induced nuclear factor-kappa B (NF-κB) expression and mitochondrial-driven apoptotic signaling | In vivo, mice with paraquat (PQ)-induced acute liver injury | [122] | |
| phlorizin, trilobatin, 3-hydroxyphlorizin, sieboldin, phloretin 2′-xyloglucosid | 3-Hydroxyphloretin was the best antioxidant among the seven compounds. Both glycosylation of the A ring and the ortho phenolic hydroxyl groups of the B ring were important for the cytotoxicity of dihydrochalcone molecules. | DPPH and ABTS assays | [123] | ||
| phloretin, phlorizin, trilobatin, sieboldin, 3-hydroxyphloretin 3-hydroxyphlorizin | Presence of an o-dihydroxyl group in the B-ring increased activity, whereas glycosylation in the A-ring decreased antioxidant potential of dihydrochalcones. Glycosylation at the 2′-position enhanced the dissociation ability of the 4′-hydroxyl group and increased the antioxidant activity of dihydrochalcones containing o-dihydroxyl. | DPPH and ABTS assays | [124] | ||
| phloretin, phloridzin, trilobatin, naringin dihydrochalcone, neohesperidin dihydrochalcone | 1 mg/mL (2–10 µL) FRAP, DPPH 0.25 mg/mL (2–10 µL) ABTS 5 mg/mL (2–10 µL) superoxide radical scavenging | Antioxidant structure-activity relationship. In FRAP assay, antioxidant activity of pairs of dihydrochalcones had the following relationship: phloretin > phloridzin, phloretin > trilobatin, trilobatin > phloridzin, trilobatin > naringin dihydrochalcone, neohesperidin dihydrochalcone > naringin dihydrochalcone. | FRAP, DPPH, ABTS, and superoxide radical scavenging assays | [125] | |
| aspalathin, nothofagin | Inhibitor of Fe(II)-induced lipid peroxidation: IC50 = 50.2 µM (aspalathin) IC50 = 1388 µM (aspalathin) ABTS assay: IC50 = 3.33 µM (aspalathin) IC50 = 4.04 µM (nothofagin) | ABTS, metal chelating, and Fe(II)-induced microsomal lipid peroxidation assays | [126] | ||
| trilobatin, hesperetin dihydrochalcone-7-O-glucoside, prunin and hesperetin-7-O-glucoside (obtained in the selective enzymatic hydrolysis of parental flavonoid glycosides using an immobilised α-L-rhamnosidase) | 0.4–1.0 mM (DPPH) 0.025–0.200 mM (FRAP) 20–80 µM (ORAC) | Some of the flavonoid monoglucosides showed significant improvement in the antioxidant activity. | DPPH, FRAP, and ORAC assays | [127] | |
| flavanones and dihydrochalcones | Dihydrochalcones exhibited higher antioxidant activities than the corresponding flavanones. The hydroxyl group at the 2‘-position in dihydrochalcone A ring is an essential pharmacophore for its radical scavenging potential. | DPPH assay and lipid peroxidation in the erythrocyte membrane assays | [128] | ||
| phloretin | 1–5 mM | Supresses lipid oxidation in PUFA % of inhibition of the peroxyl radical-induced oxidation in oil-in-water emulsion containing methyl linolenate = 72.5 ± 5.5 | In vitro, oil–in–water emulsion system, TBARS and fish oil system | [129] | |
| Antiinflammatory activity | phloretin | 125 µM | IC50 = 20 µM (MMP-2) does not inhibit LE and MMP-9 | In vitro, matrix-proteases, leukocyte elastase (LE), and gelatinase A (MMP-2; human neuroblastoma cells) gelatinase B (MMP-9; HT-1080 human fibrosarcoma cells) | [130] |
| aspalathin nothofagin | Inhibits HG-mediated vascular hyperpermeability, adhesion of monocytes toward HUVECs, and expression of CAMs. Suppresses the formation of ROS and the activation of NFκB. | In vivo, male C57BL/6 mice In vitro, primary human umbilical vein endothelial cells (HUVECs) | [131] | ||
| α,β-dihydroxantohumol | 1–15 µM | IC50 (COX-1) = 124.50 ± 7.61 IC50 (COX-2) = 103.8 ± 6.11 | In vitro, binding to human serum albumin (HSA), COX-1, and COX-2 activity | [132] | |
| phloretin | Osteoarthritis (OA) | Inhibits the IL-1β-induced production of NO, PGE2, TNF-α, and IL-6; the expression of COX-2, iNOS, MMP-3, MMP-13, and ADAMTS-5; and the degradation of aggrecan and collagen-II in human chondrocytes. Suppresses the IL-1β-stimulated phosphorylation of PI3K/Akt and activation of NF-κB. Decreass the expression of MMP-13 and increases the expression of collagen-II in mice. | In vivo, mice In vitro, human OA chondrocytes | [133] | |
| aspalathin nothofagin | MTT assay 9.1–27.1 µg/mouse (aspalathin) 8.7–26.2 µg/mouse (nothofagin) | Inhibit LPS-induced barrier disruption, expression of cell adhesion molecules (CAMs), and adhesion/transendothelial migration of neutrophils to human endothelial cells. Suppress LPS-induced hyperpermeability and leukocyte migration. Suppress the production of tumor necrosis factor-α (TNF-α) or interleukin (IL)-6 and the activation of nuclear factor-κB (NF-κB) or extracellular regulated kinases (ERK) 1/2 by LPS | In vitro, HUVECs (primary human umbilical vein endothelial cells) In vivo, male C57BL/c mice | [134] | |
| apple polyphenol | Significantly inhibits proinflammatory gene expression and represses NF-jB-, IP-10-, IL-8-promoter-, and STAT1-dependent signal transduction in a dose-dependent manner. | In vitro, T84 cells (colon epithelial cells), the human colon adenocarcinoma 1264 cell line DLD-1 (DSMZ ACC278), the human leukemia T-cell line Jurkat (DSMZ ACC282), and the human monocyticleukemia cell lineMonoMac6 (DSMZ ACC124) | [135] | ||
| Hepatoprotective | phloretin | 50 mg/kg | Reduces mortality rate in mice, resulting in protection against lethal effect of acetaminophen. Downregulates SGOT serum transaminases. | In vivo, mice with hepatotoxicity induced by acetaminophen | [136] |
| 2′,4′-dihydroxydihydrochalcone-4-O-β-D-glucopyranoside | MTT assay 10 µM | Cell survival rate (% of normal) 74.63 ± 11.11 | In vitro, D-galactosamine-induced toxicity inhuman hepatoma HepG2 cells | [137] | |
| Antileishmanial activity | IC50 = 2.98 µg/mL IC50 = 3.65 µg/mL | In vitro, promastigotes of Leishmania braziliensis | [138] | ||
| Anti-HIV, anti-herpes simplex virus type 2 | trilobatin | Exhibits broad anti-HIV activity and displays synergistic anti-HIV activities combined with antiretroviral agents (e.g., maraviroc, zidovudine, and raltegravir). Effective in inhibiting HSV-2 (at the concetration of 458 µM) | In vitro, MT-2 and Vero cells | [139] | |
| Cardioprotective | aspalathin | 1 µM 13 and 130 mg/kg | Protects cardiomyocytes against hyperglycemia-induced shifts in substrate preference and subsequent apoptosis; prevents myocardium apoptosis; modulates key regulators associated with lipid metabolism (Adipoq, Apob, CD36, Cpt1, Pparγ, Srebf1/2, Scd1, and Vldlr), insulin resistance (Igf1, Akt1, Pde3, and Map2k1), inflammation (Il3, Il6, Jak2, Lepr, Socs3, and Tnf13), and apoptosis (Bcl2 and Chuk) | In vitro, H9c2 cardiomiocytes In vivo, C57BLKS/J homozygous Leprdb/db mice | [140] |
| Anticholinesterase and antibutyrylcholinesterases activity | phloretin phlorizin | 0.2 mM | Exhibit anti-AChE and anti-BChEs activity. Aglycons were more effective than corresponding glucosides. | In vitro, acetylcholinesterase (AChE, C3389), butyrylcholinesterase (BChE, C7512) | [141] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review. Molecules 2019, 24, 4468. https://doi.org/10.3390/molecules24244468
Stompor M, Broda D, Bajek-Bil A. Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review. Molecules. 2019; 24(24):4468. https://doi.org/10.3390/molecules24244468
Chicago/Turabian StyleStompor, Monika, Daniel Broda, and Agata Bajek-Bil. 2019. "Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review" Molecules 24, no. 24: 4468. https://doi.org/10.3390/molecules24244468
APA StyleStompor, M., Broda, D., & Bajek-Bil, A. (2019). Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review. Molecules, 24(24), 4468. https://doi.org/10.3390/molecules24244468