Solid-Phase Insertion of N-mercaptoalkylglycine Residues into Peptides
Abstract
1. Introduction
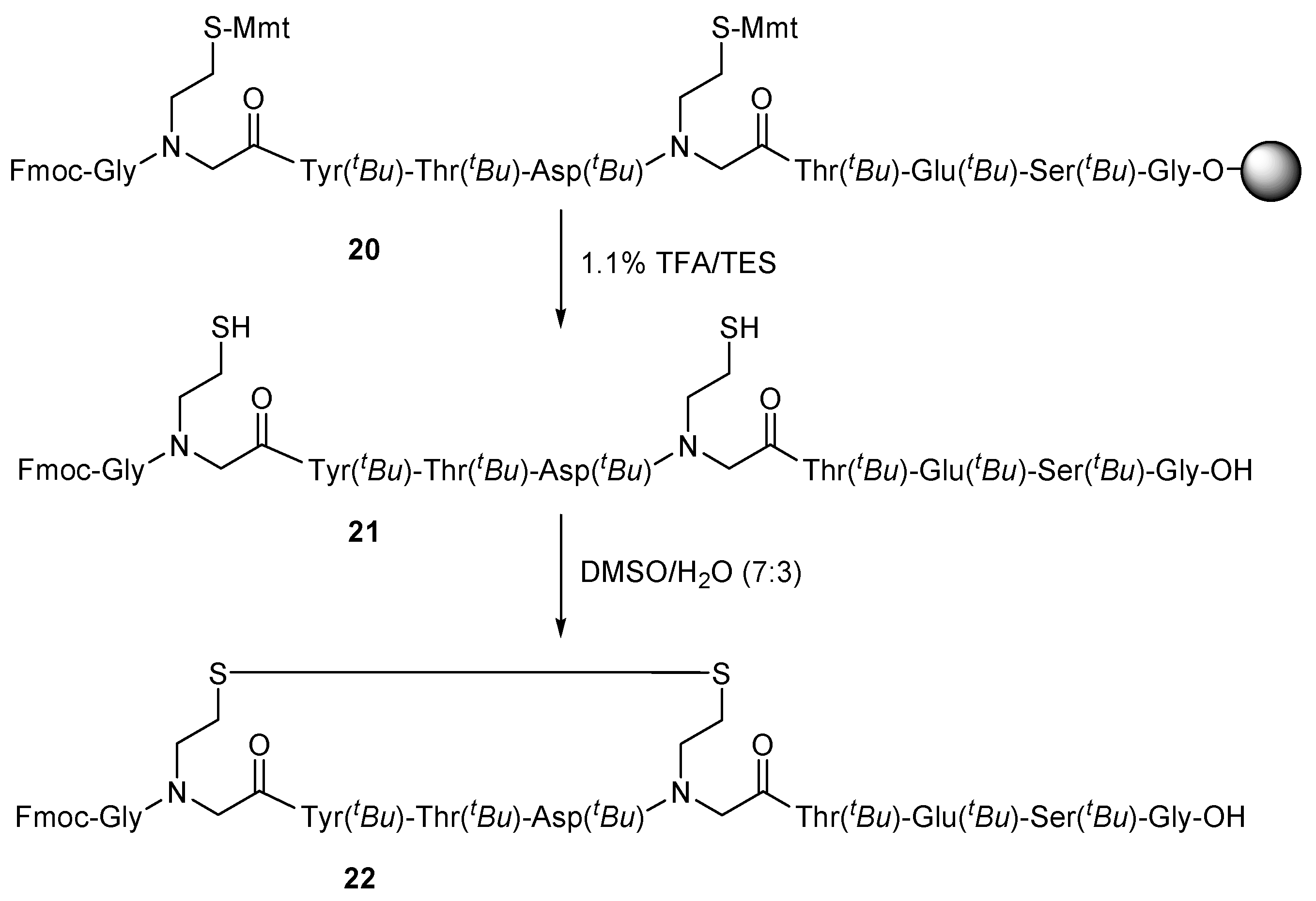
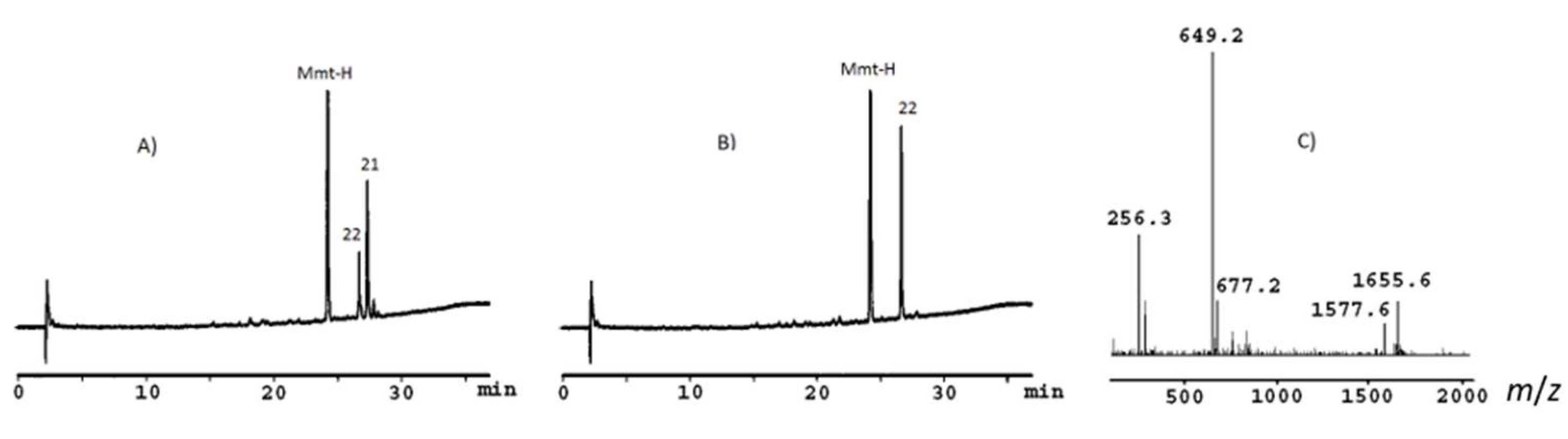
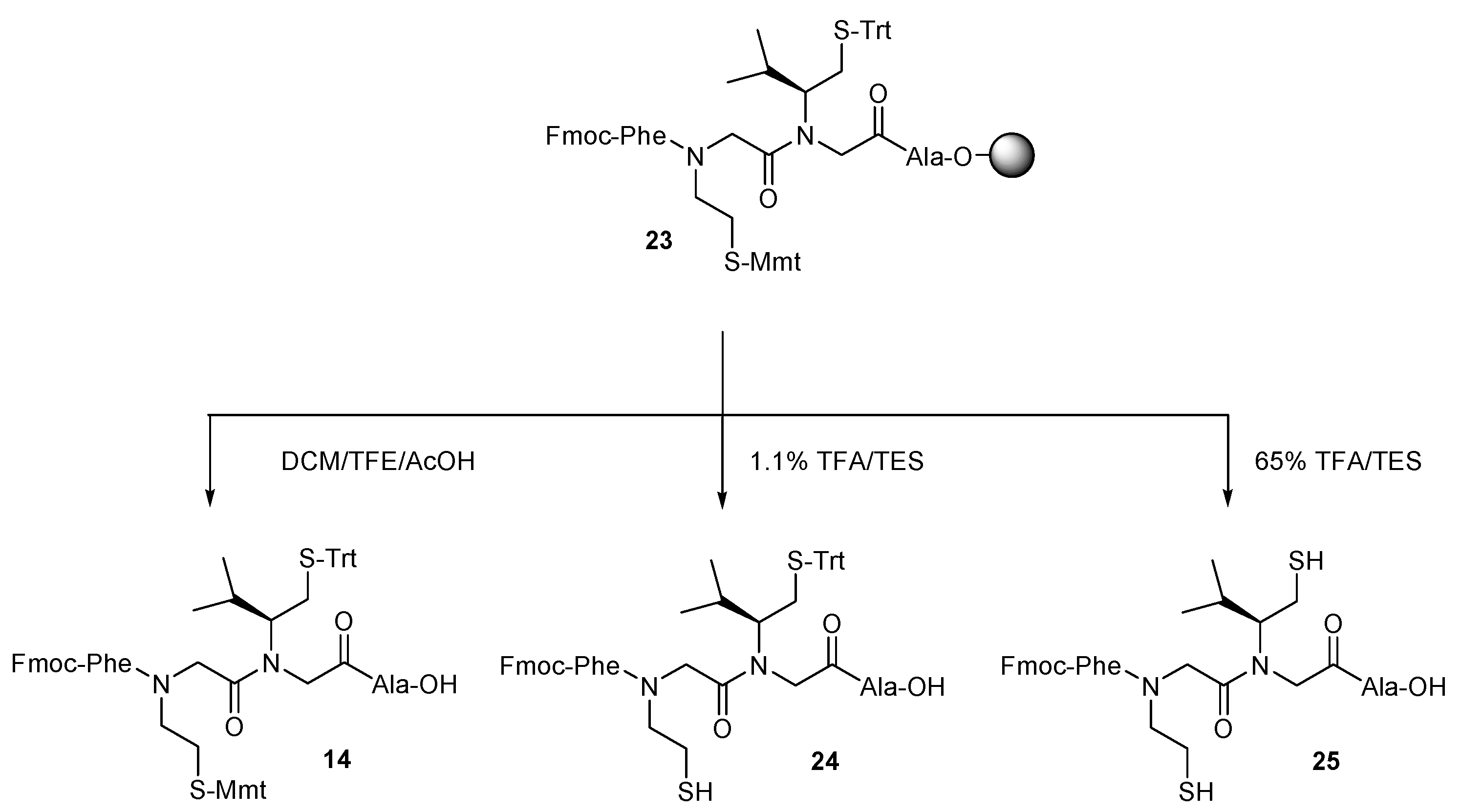
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthetic Procedures
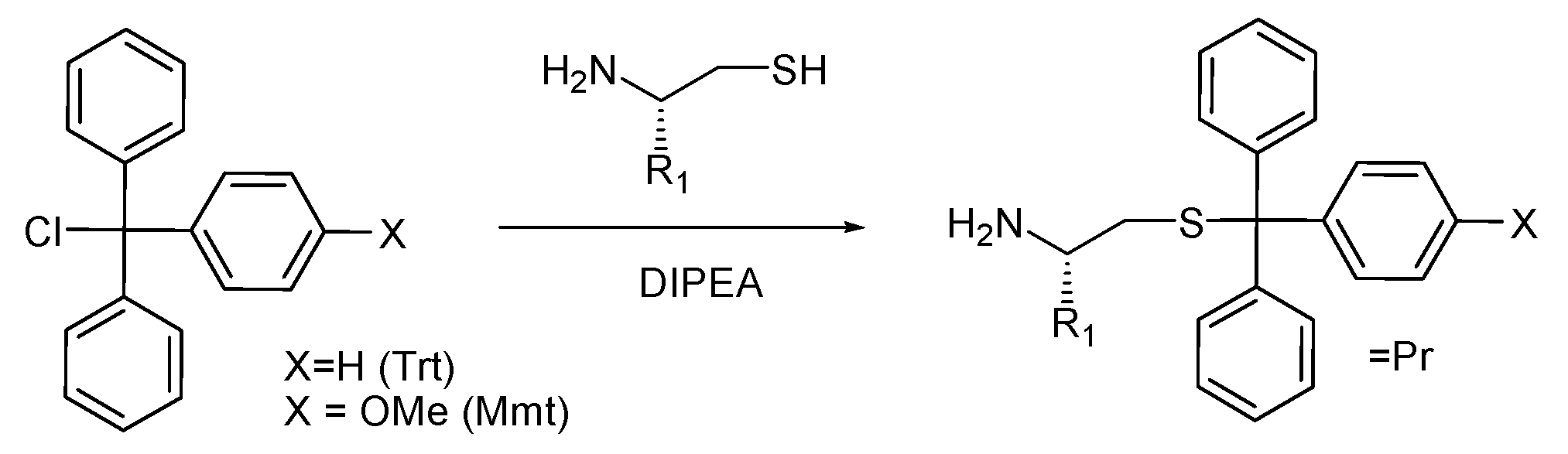
3.3.1. General Protocol for the Synthesis of S-trityl (Trt) and S-4-methoxytrityl (Mmt) Aminothiols
3.3.2. Solid-Phase Peptide Assembly, General Protocol
Pre-Activation of Fmoc-Amino Acids
Coupling
Fmoc-Group Removal and Fmoc-Removal Test
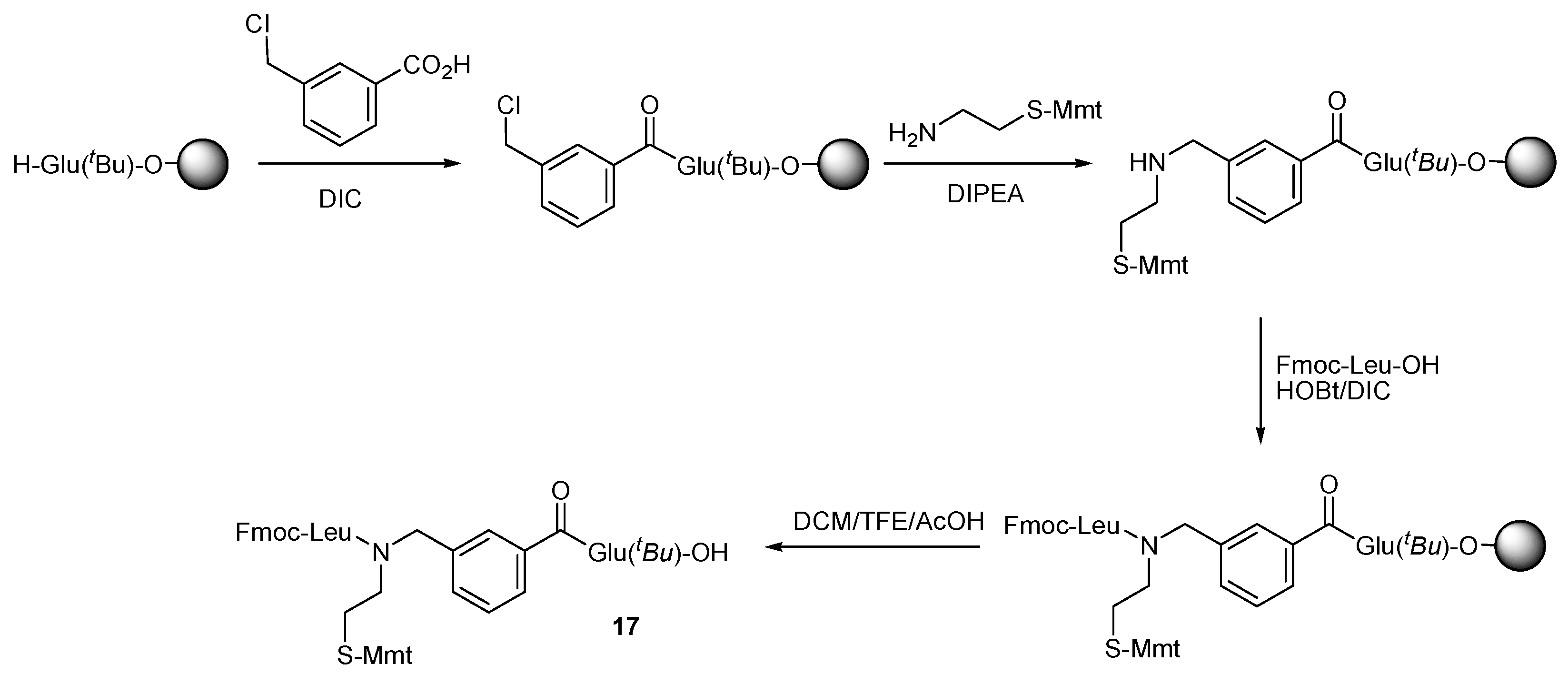
3.3.3. General Procedure for the Solid-Phase Synthesis of Bromoacylated Peptides
3.3.4. General Procedure for Peptoid Formation
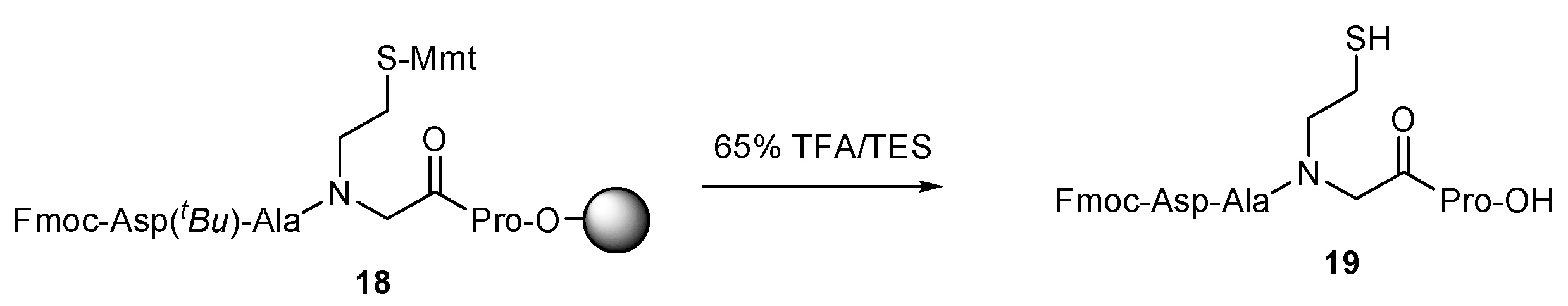
3.3.5. General Procedure for the Cleavage of the Peptide–Peptoid Hybrids from the Resin
3.3.6. General Procedure for the Oxidation of Mercaptoalkyl Peptoid Side Chains
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-Methylation of Peptides and Proteins: An Important Element for Modulating Biological Functions. Angew. Chem. Int. Ed. 2013, 52, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.L.; Leggio, A.; Malagrinò, F.; Romio, E.; Siciliano, C.; Liguori, A. N-Methylated α-Amino Acids and Peptides: Synthesis and Biological Activity. Mini Rev. Med. Chem. 2016, 16, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient method for the preparation of peptoids oligo(N-substituted glycines) by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Simon, R.J.; Kania, R.S.; Zuckermann, R.N.; Huebner, V.D.; Jewell, D.A.; Banville, S.; Ng, S.; Wang, L.; Rosenberg, S.; Marlowe, C.K. Peptoids: A modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 1992, 89, 9367–9371. [Google Scholar] [CrossRef]
- Miller, S.M.; Simon, R.J.; Ng, S.; Zuckermann, R.N.; Kerr, J.M.; Moos, W.H. Comparison of the proteolytic susceptibilities of homologous l-amino acid, d-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev. Res. 1995, 35, 20–32. [Google Scholar] [CrossRef]
- Fowler, S.A.; Blackwell, H.E. Structure-function relationships in peptoids: Recent advances toward deciphering the structural requirements for biological function. Org. Biomol. Chem. 2009, 7, 1508–1524. [Google Scholar] [CrossRef]
- Goodman, M.; Bhumralkar, M.; Jefferson, E.A.; Kwak, J.; Locardi, E. Collagen mimetics. Biopolymers 1998, 47, 127–142. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Cruz, L.J.; Mora, P.; Francesch, A.; Messeguer, A.; Pérez-Paya, E.; Albericio, F. Smallest peptoids with antiproliferative activity on human neoplastic cells. J. Med. Chem. 2007, 50, 2443–2449. [Google Scholar] [CrossRef]
- Czyzewski, A.M.; McCaig, L.M.; Dohm, M.T.; Broering, L.A.; Yao, L.J.; Brown, N.J.; Didwania, M.K.; Lin, J.S.; Lewis, J.F.; Veldhuizen, R.; et al. Effective in vivo treatment of acute lung injury with helical, amphipathic peptoid mimics of pulmonary surfactant proteins. Sci. Rep. 2018, 8, 6795. [Google Scholar] [CrossRef]
- Young, S.C. A Systematic Review of Antiamyloidogenic and Metal-Chelating Peptoids: Two Structural Motifs for the Treatment of Alzheimer’s Disease. Molecules 2018, 23, 296. [Google Scholar] [CrossRef]
- Song, D.; Park, H.; Lee, S.H.; Kim, M.J.; Kim, E.J.; Lim, K.M. PAL-12, a new anti-aging hexa-peptoid, inhibits UVB-induced photoaging in human dermal fibroblasts and 3D reconstructed human full skin model, Keraskin-FT™. Arch. Derm. Res. 2017, 309, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Gomes Von Borowski, R.; Gnoatto, S.C.B.; Macedo, A.J.; Gillet, R. Promising Antibiofilm Activity of Peptidomimetics. Front. Microbiol. 2018, 9, 2157. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Basu, M.; Ghosh, M.K. Design, synthesis and use of peptoids in the diagnosis and treatment of cancer. Front. Biosci. (Elite Ed) 2017, 9, 101–108. [Google Scholar] [PubMed]
- Mándity, I.M.; Fülöp, F. An overview of peptide and peptoid foldamers in medicinal chemistry. Expert Opin. Drug Discov. 2015, 10, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Dohm, M.T.; Kapoor, R.; Barron, A.E. Peptoids: Bio-inspired polymers as potential pharmaceuticals. Curr. Pharm. Des. 2011, 17, 2732–2747. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. 2009, 11, 299–307. [Google Scholar]
- Sun, J.; Zucherman, R.N. Peptoid Polymers: A Highly Designable Bioinspired Material. Acs Nano 2013, 7, 4715–4732. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y.; Wang, M.; Jian, T.; Ding, S.; Mu, P.; Liao, Z.; Shi, Q.; Cai, X.; Jin, H.; et al. Bioinspired Peptoid Nanotubes for Targeted Tumor Cell Imaging and Chemo-Photodynamic Therapy. Small 2019, 15, e1902485. [Google Scholar] [CrossRef]
- Battigelli, A. Design and preparation of organic nanomaterials using self-assembled peptoids. Adv. Sci. 2019, 110, e23265. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z. Peptoid applications in biomedicine and nanotechnology (Chapter 7). In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Koutsopoulos, S., Ed.; Woodhead: Duxford, UK, 2018; pp. 183–213. [Google Scholar]
- Schneider, J.A.; Craven, T.W.; Kasper, A.C.; Yun, C.; Haugbro, M.; Briggs, E.M.; Svetlov, V.; Nudler, E.; Knaut, H.; Bonneau, R.; et al. Design of Peptoid-peptide Macrocycles to Inhibit the β-catenin TCF Interaction in Prostate Cancer. Nat. Commun. 2018, 9, 4396. [Google Scholar] [CrossRef]
- Olsen, C.A.; Montero, A.; Leman, L.J.; Ghadiri, M.R. Macrocyclic Peptoid–Peptide Hybrids as Inhibitors of Class I Histone Deacetylases. Acs Med. Chem. Lett. 2012, 3, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Stawikowski, M.J. Peptoids and peptide-peptoid hybrid biopolymers as peptidomimetics. Methods Mol. Biol. 2013, 1081, 47–60. [Google Scholar] [PubMed]
- Patch, J.A.; Kirshenbaum, K.; Seurynck, S.L.; Zuckermann, R.N.; Barron, A.E. The Many Roles of Peptoids in Drug Discovery. In Pseudopeptides in Drug Development; Nielsen, P.E., Ed.; Wiley-VCH: Weinheim, Germany, 2004; pp. 1–31. [Google Scholar]
- Culf, A.S.; Ouellette, R.J. Solid-Phase Synthesis of N-Substituted Glycine Oligomers (α-Peptoids) and Derivatives. Molecules 2010, 15, 5282–5335. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, B.-C.; Zuckermann, R.N. Peptoids: Synthesis, Characterization, and Nanostructures. Compr. Biomater. 2011, 2, 53–76. [Google Scholar]
- Tran, H.; Gael, S.L.; Connolly, M.D.; Zuckermann, R.N. Solid-phase submonomer synthesis of peptoid polymers and their self-assembly into highly-ordered nanosheets. J. Vis. Exp. 2011, 57, e3373. [Google Scholar] [CrossRef]
- Webster, A.M.; Cobb, S.L. Recent Advances in the Synthesis of Peptoid Macrocycles. Chemistry 2018, 24, 7560–7573. [Google Scholar] [CrossRef]
- Barlos, K.; Gatos, D.; Kallitsis, J.; Papaphotiu, G.; Sotiriu, P.; Wenqing, Y.; Schiifer, W. Darstellung geschützter peptid-fragmente unter einsatz substituierter triphenylmethyl-harze. Telrahedron Lett. 1989, 30, 3943–3946. [Google Scholar] [CrossRef]
- Lee, B.-C.; Zuckermann, R.N.; Dill, K.A. Folding a Nonbiological Polymer into a Compact Multihelical Structure. J. Am.Chem. Soc. 2005, 127, 10999–11009. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Odaka, C.; Mizuochi, T. Angiotensin-converting enzyme inhibitor captopril prevents activation-induced apoptosis by interfering with T cell activation signals. Clin. Exp. Immunol. 2000, 121, 515–522. [Google Scholar] [CrossRef]
- Llorens, C.; Gacel, G.; Swerts, J.P.; Perdrisot, R.; Fournié-Zaluski, M.C.; Schwartz, J.C.; Roques, B.P. Rational design of enkephalinase inhibitors: Substrate specificity of enkephalinase studied from inhibitory potency of various dipeptides. Biochem. Biophys. Res. Commun. 1980, 96, 1710–1716. [Google Scholar] [CrossRef]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The first selective-target and broad-spectrum radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kodadek, T. Synthesis, Screening and Hit Optimization of Stereochemically Diverse Combinatorial Libraries of Peptide Tertiary Amides. Chem. Biol. 2013, 20, 360–369. [Google Scholar]
- Suwal, S.; Kodadek, T. Solid-phase synthesis of peptoid-like oligomers containing diverse diketopiperazine units. Org. Biomol. Chem. 2014, 12, 5831–5834. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef]
- Mourtas, S.; Katakalou, C.; Nicolettou, A.; Tzavara, C.; Gatos, D.; Barlos, K. Resin-bound aminothiols: Synthesis and application. Tetrahedron Lett. 2003, 44, 179–182. [Google Scholar] [CrossRef]
- Barlos, K.; Gatos, D.; Kallitsis, J.; Papaphotiu, G.; Sotiriu, P.; Wenqing, Y.; Schäfer, W. Darstellung geschützter peptid-fragmente unter einsatz substituierter triphenylmethyl-harze. Tetrahedron Lett. 1989, 30, 3943–3946. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef]
- Joullié, M.M.; Lassen, K.M. Evolution of amide bond formation. Arkivoc 2010, 8, 189–250. [Google Scholar]
- Trivedi, M.V.; Laurence, J.S.; Siahaan, T.J. The role of thiols and disulfides on protein stability. Curr. Protein Pept. Sci. 2009, 10, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and Protocols of Modern Solid-Phase Peptide Synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available. |


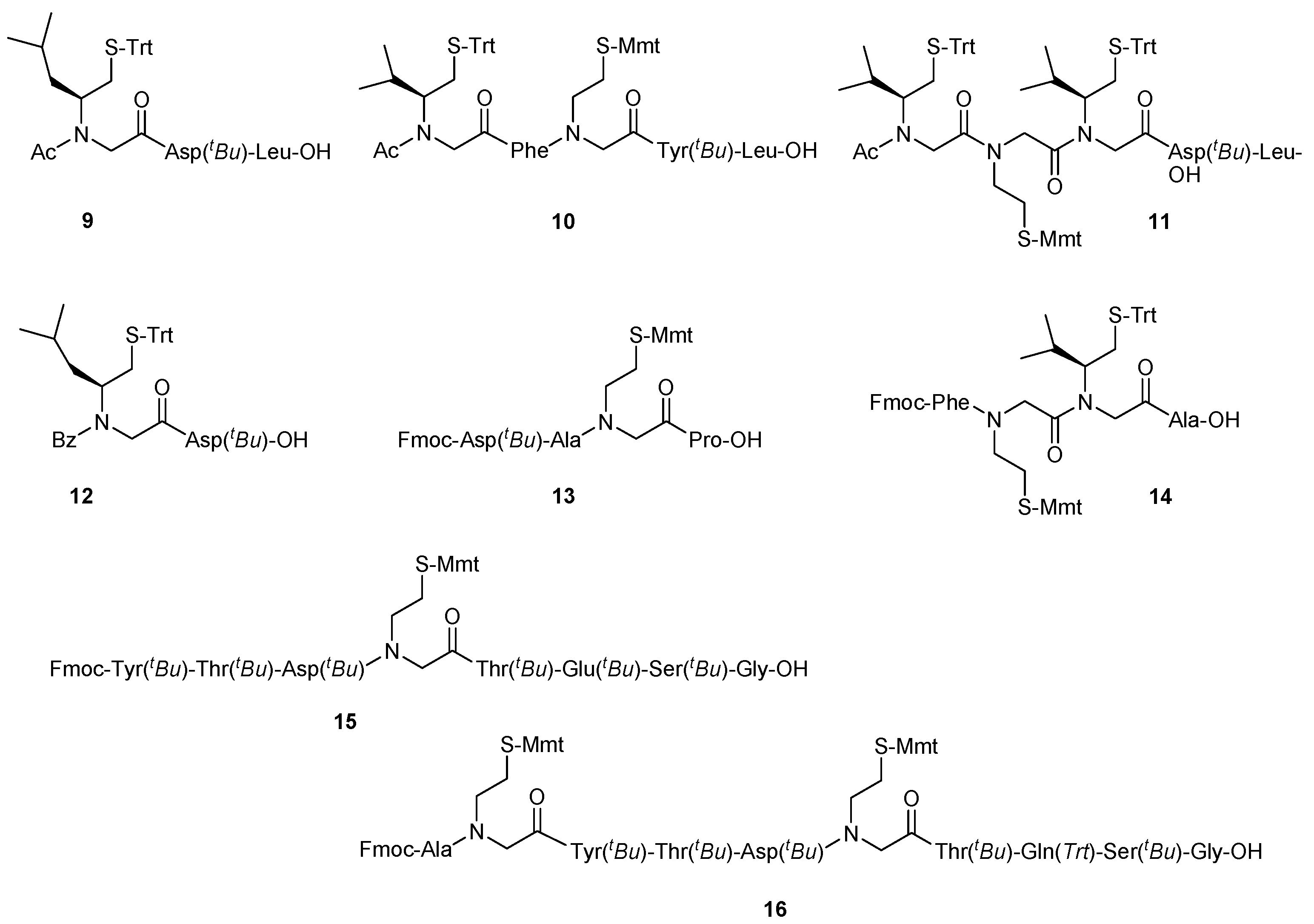
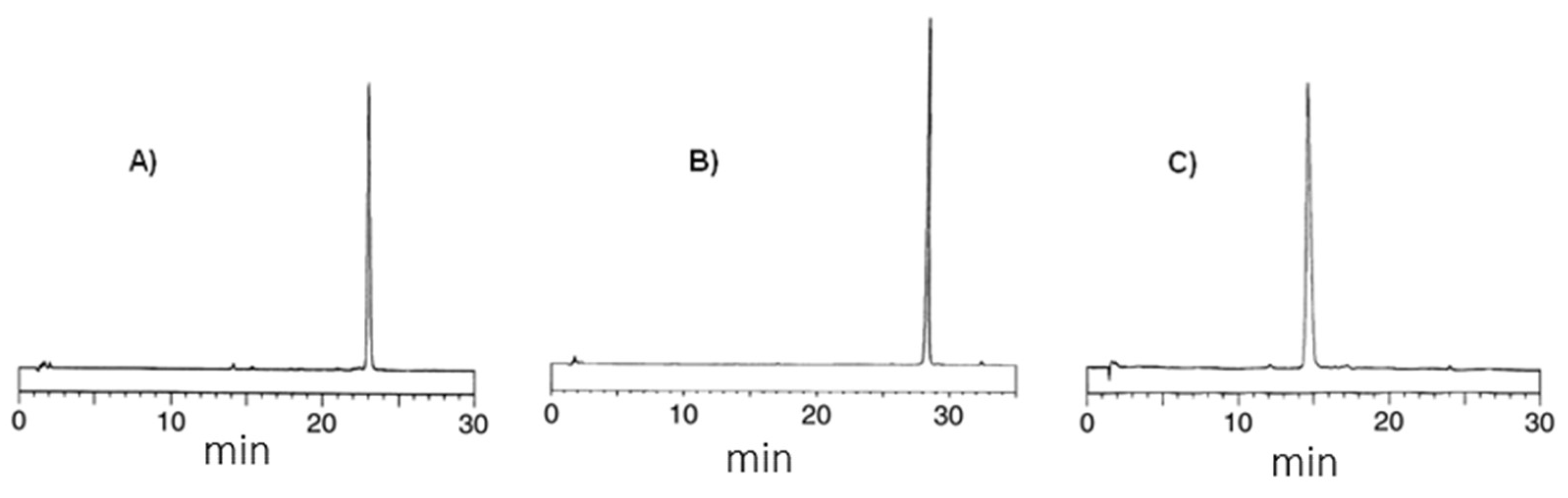
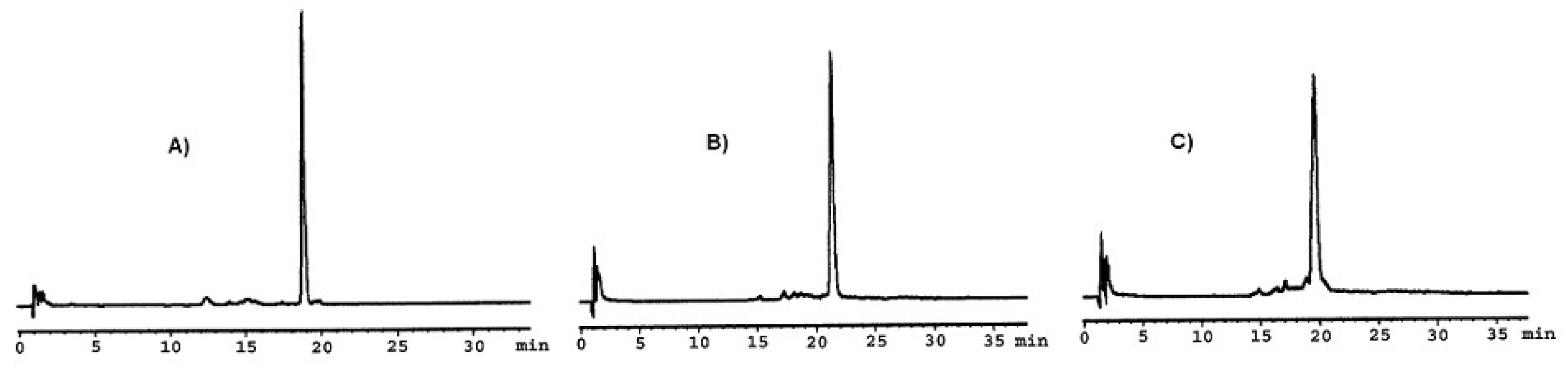
| a/a | tR | Crude Yield | HPLC Purity c | m/z | |
|---|---|---|---|---|---|
| (min) | (%) | (%) | Calculated | Found | |
| 9 | 26.7 b | 92 | 91 | 760.4 d | 760.6 d |
| 10 | 23 a | 94 | 93 | 1330.63 d | 665.9 e |
| 11 | 34 b | 92 | 89 | 1536.71 d | 768.9 a |
| 12 | 27.2 b | 89 | 88 | 709.33 d | 709.3 d |
| 13 | 32 b | 95 | 93 | 969.41 d | 969.4 d |
| 14 | 18.8 a | 93 | 90 | 1249.52 d | 625.4 e |
| 15 | 19.3 a | 93 | 89 | 1719.89 d | 855.0 e |
| 16 | 26.9 a | 90 | 92 | 2365.13 d | - |
| 17 | 28.3 b | 96 | 92 | 1004.45 d | 1004.4 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourtas, S.; Gatos, D.; Barlos, K. Solid-Phase Insertion of N-mercaptoalkylglycine Residues into Peptides. Molecules 2019, 24, 4261. https://doi.org/10.3390/molecules24234261
Mourtas S, Gatos D, Barlos K. Solid-Phase Insertion of N-mercaptoalkylglycine Residues into Peptides. Molecules. 2019; 24(23):4261. https://doi.org/10.3390/molecules24234261
Chicago/Turabian StyleMourtas, Spyridon, Dimitrios Gatos, and Kleomenis Barlos. 2019. "Solid-Phase Insertion of N-mercaptoalkylglycine Residues into Peptides" Molecules 24, no. 23: 4261. https://doi.org/10.3390/molecules24234261
APA StyleMourtas, S., Gatos, D., & Barlos, K. (2019). Solid-Phase Insertion of N-mercaptoalkylglycine Residues into Peptides. Molecules, 24(23), 4261. https://doi.org/10.3390/molecules24234261








