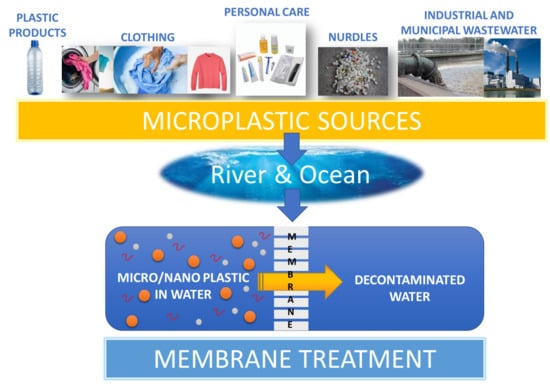
Membrane Processes for Microplastic Removal
Abstract
1. Introduction
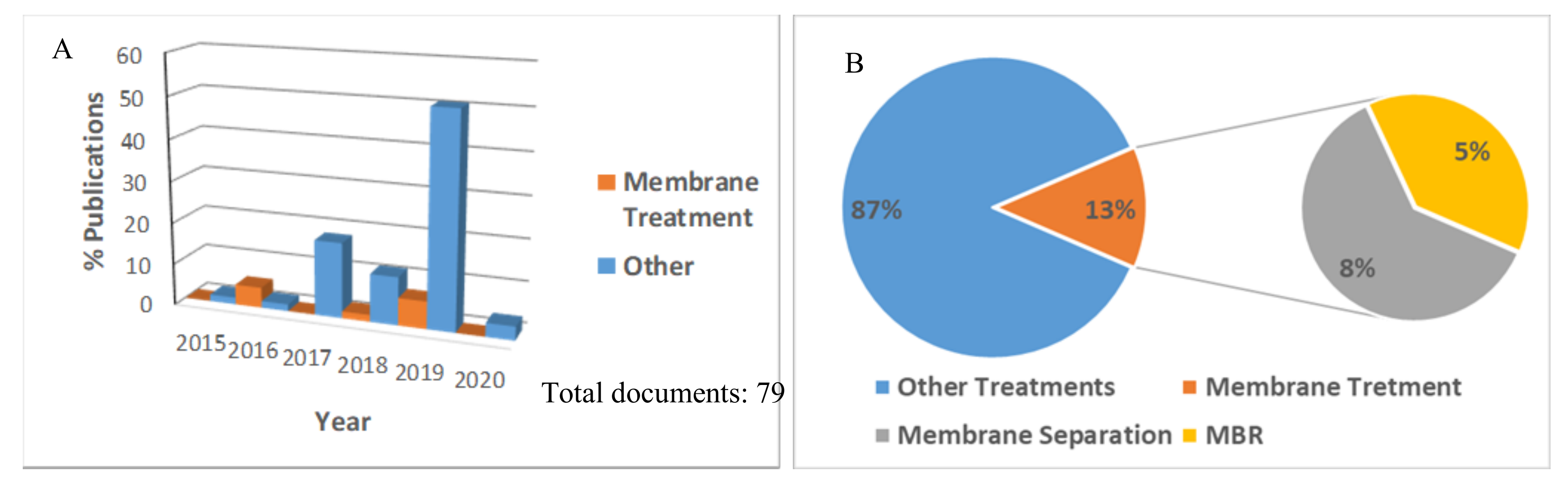
2. Literature Analysis on the Microplastic and Its Removal by Membrane Processes
2.1. Ultrafiltration
2.2. Dynamic Membranes Technology
- (i)
- it employs relatively lower-cost materials compared to traditional membranes (such as mesh, non-woven fabric, and woven filter cloth, and stainless-steel mesh);
- (ii)
- extra chemicals or other contaminants are not introduced considering that the filtration layer is formed by the contaminants of the influent;
- (iii)
- the experimental setup is generally more compact than the traditional membrane processes (e.g., for ultrafiltration (UF) and microfiltration (MF)), since DM permeation flux is much larger, the membrane module quantities are saved;
- (iv)
- the energy supply is lower since DM operates under gravity driving mode, and lower transmembrane pressure is required compared to traditional membranes.
2.3. Reverse Osmosis
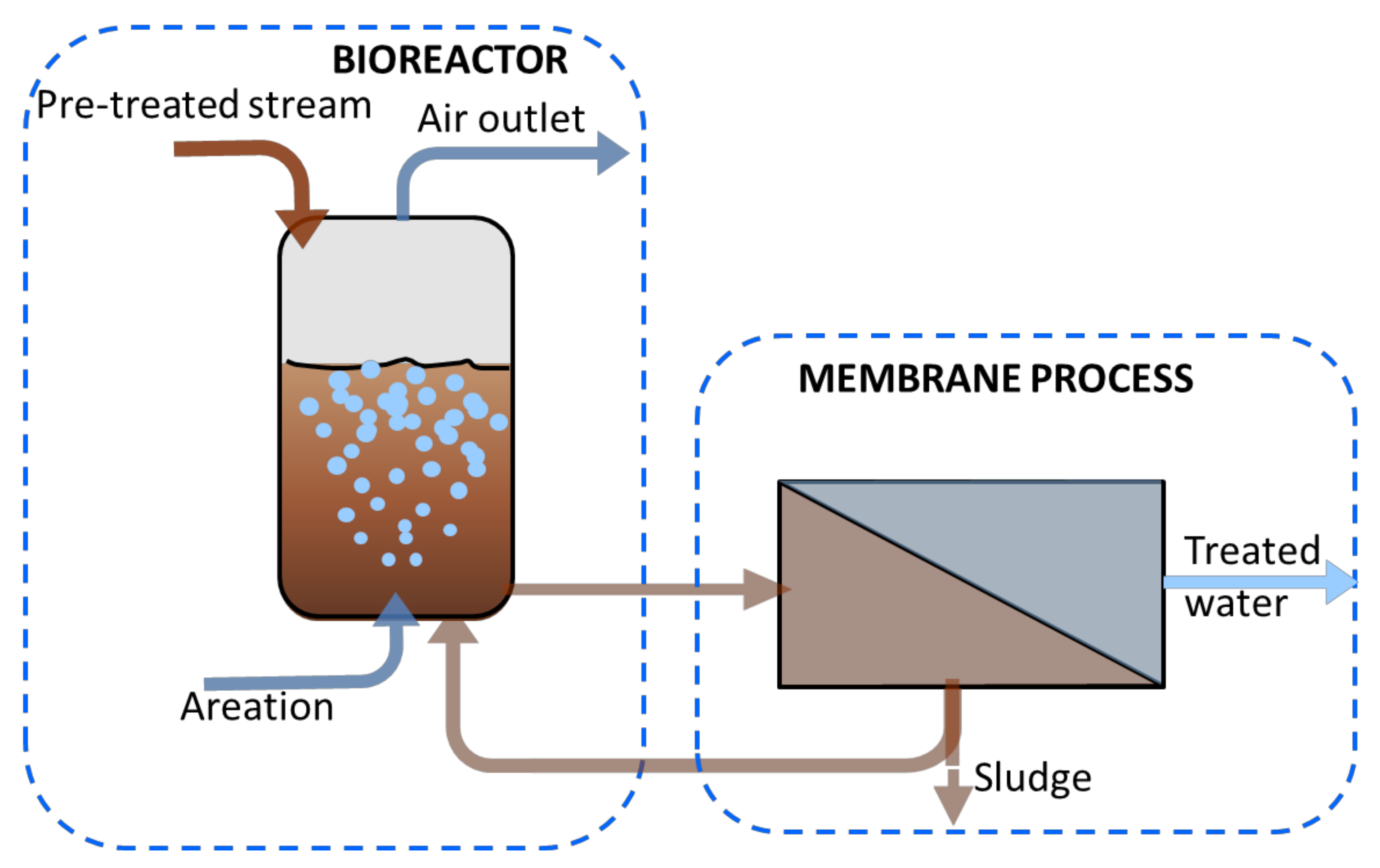
2.4. Membrane Bioreactor (MBR)
2.5. Polymeric Membranes as Source of Plastic Waste: Recent Advances in Their Reuse and Recycling
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plastics Europe Plastics-The Facts 2018: An Analysis of European Plastics Production, Demand and Waste Data. 2017. Available online: https://www.plasticseurope.org/en/resources/publications/619-plastics-facts-2018 (accessed on 21 May 2019).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sink. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.A.; de Carvalho-Souza, G.F. Are we eating plastic-ingesting fish? Mar. Pollut. Bull. 2016, 103, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Battaglia, P.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Luísa, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium (VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar]
- Khan, R.; Inam, M.A.; Khan, S.; Park, D.R.; Yeom, I.T. Interaction between Persistent Organic Pollutants and ZnO NPs in Synthetic and Natural Waters. Nanomaterials 2019, 9, 472. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8882. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, P.; Cachot, J.; Clérandeau, C.; Faure, F.; Van Arkel, K.; de Alencastro, L.F.; Levasseur, C.; Sciacca, F.; Bourgeois, J.P.; Morin, B. Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: Part I-adverse effects on fish cell line. Environ. Pollut. 2019, 248, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Rauwel, P.; Rauwel, E. Towards the Extraction of Radioactive Cesium-137 from Water via Graphene/CNT and Nanostructured Prussian Blue Hybrid Nanocomposites: A Review. Nanomaterials 2019, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Inter. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russel, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S. Micro-and Nano-plastics and Human Health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Germany, 2015; pp. 343–366. [Google Scholar]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro (nano) plastics: A threat to human health? Curr. Opin. Envi. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Celine, I.L.; da Costa, J.J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry-A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpaa, M.; Sillanpaa, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
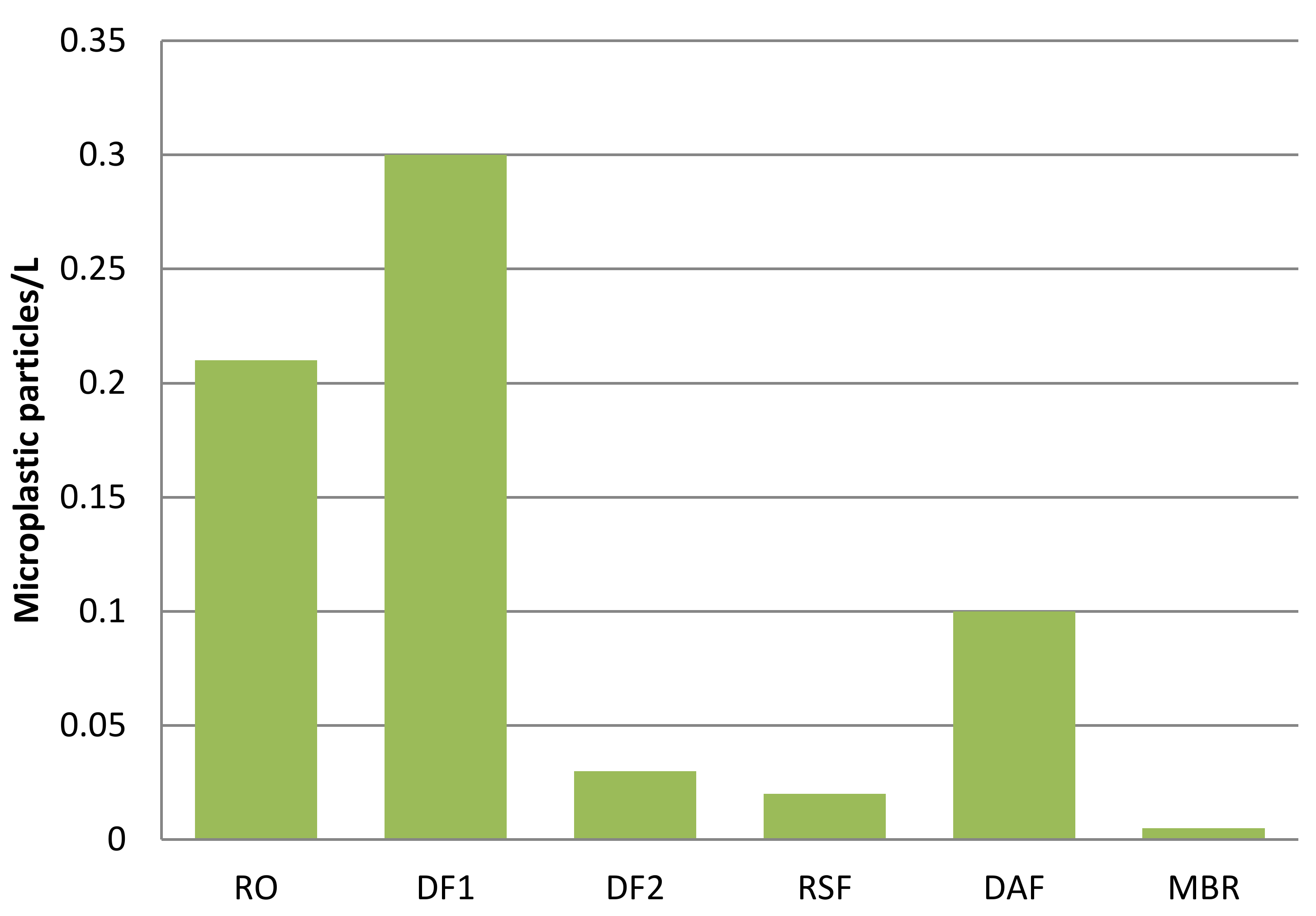
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setala, O. Solutions to microplastic pollution, Removal of microplastics fromwastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 401–407. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quin, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, I.V.; Manickam, V. Wastewater Treatment Technologies. In Environmental Management; Elsevier Butterworth-Heinemann: Oxford, UK, 2017; pp. 249–293. [Google Scholar]
- Talvitie, J.; Heinonen, M.; Paaakkonen, J.P.; Vahtera, E.; Mikola, A.; Setala, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastic in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Krzan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. Int. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano) plastics in the environment–Sources, fates and effects. Sci. Tot. Environ. 2016, 566, 15–26. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro (nano) plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Env. Sci Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioral disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 11452, 1–7. [Google Scholar]
- Mattsson, K.; Hansson, L.A.; Cedervall, T. Nano-plastics in the aquatic environment. Environ. Sci. Process. Impacts 2015, 17, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Gatidou, G.; Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence and fate of microplastics in Sewage Treatment Plant. J. Hazard. Mater. 2019, 367, 504–551. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Lavender Law, K. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Moslehyani, A.; Ismail, A.F.; Matsuura, T.; Rahman, M.A.; Goh, P.S. Recent Progresses of Ultrafiltration (UF) Membranes and Processes in Water Treatment. In Membrane Separation Principles and Applications.; Ismail, A.F., Rahman, M.A., Othman, M.H.D., Matsuura, T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 85–109. [Google Scholar]
- Molinari, R.; Argurio, P.; Poerio, T. Membrane Processes Based on Complexation Reactions of Pollutants as Sustainable Wastewater Treatments. Sustainability 2009, 1, 978–993. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Ultrafiltration of Polymer-Metal Complexes for Metal Ion Removal from Wastewaters. Macromol. Symp. 2006, 235, 206–221. [Google Scholar] [CrossRef]
- Hirata, T.; Hashimoto, A. Experimental assessment of the efficacy of microfiltration and ultrafiltration for Cryptosporidium removal. Water Sci. Technol. 1998, 38, 103–107. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Rose, J.B. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Veterinary 2004, 126, 219–234. [Google Scholar] [CrossRef]
- Mason, S.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsalainen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar]
- Sillanpää, M. Natural Organic Matter Water Characterization and Treatment Methods; Elsevier Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–367. [Google Scholar]
- Xia, S.J.; Liu, Y.N.; Xing, L.I.; Yao, J.J. Drinking water production by ultrafiltration of Songhuajiang River with PAC adsorption. J. Environ. Sci. 2007, 19, 536–539. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decade. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Li, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zou, X.; Feng, J.; Wu, Z. Microbial communities in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Comparison of bulk sludge and cake layer. Process Biochem. 2013, 48, 510–516. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Xu, Y.; Wang, Q.; Wu, Z.; Grasmick, A. Organic matter recovery from municipal wastewater by using dynamic membrane separation process. Chem. Eng. J. 2013, 219, 190–199. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, S.; He, F.; Xu, D.; Kong, L.; Wu, Z. Phosphate removal of acid wastewater from high-phosphate hematite pickling process by in-situ self-formed dynamic membrane technology. Desalin. Water Treat. 2012, 37, 77–83. [Google Scholar]
- Yang, T.; Ma, Z.; Yang, Q. Formation and performance of Kaolin/MnO2 bi-layer composite dynamic membrane for oily wastewater treatment: Effect of solution conditions. Desalin 2011, 270, 50–56. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Jiang, B.; Qiu, X.; Gao, B. A hybrid system combining self-forming dynamic membrane bioreactor with coagulation process for advanced treatment of bleaching effluent from straw pulping process. Water Treat. 2012, 18, 212–216. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yin, B.; Zhu, Y.; Fu, B.; Liu, H. Improving volatile fatty acid yield from sludge anaerobic fermentation through self-forming dynamic membrane separation. Bioresour. Technol. 2016, 218, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Alibardi, L.; Cossu, R.; Lavagnolo, M.C.; Spagni, A. Analysis of fouling development under dynamic membrane filtration operation. Chem. Eng. J. 2017, 312, 136–143. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors. Sci. Tot. Environ. 2018, 627, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalin. 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.J.; Goh, P.S.; Ismail, A.F.; Lai, S.O. Ultrafiltration as a pretreatment for seawater desalination: A review. Membr. Water Treat. 2014, 5, 15–29. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Qiblawey, H.; Zouari, N.; Rodrigues, D.F.; Hu, Y. Use of DPSIR Framework to Analyze Water Resources in Qatar and Overview of Reverse Osmosis as an Environment Friendly Technology. Environ. Progr. Sustain. Ener. 2019, 38, 1–13. [Google Scholar] [CrossRef]
- Xiao, K.; Lianga, S.; Wanga, X.; Chena, C.; Huanga, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef]
- Judd, S. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Mazzei, R.; Piacentini, E.; Gebreyohannes, Y.A.; Giorno, L. Membrane Bioreactors in Food, Pharmaceutical and Biofuel Applications: State of the Art, Progresses and Perspectives. Curr. Org. Chem. 2017, 21, 1–31. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setala, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.W.; Zhang, T.; Fang, H.H.P.; He, J.Z. Phthalates biodegradation in the environment. Appl. Microbiol. Biotechnol. 2008, 80, 183–198. [Google Scholar] [CrossRef]
- Gao, D.-W.; Wen, Z.-D. Phtalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Tot. Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef]
- Gong, J.; Kong, T.; Li, Y.; Li, Q.; Li, Z.; Zhang, J. Biodegradation of Microplastic Derived from Poly(ethylene terephthalate) with Bacterial Whole-Cell Biocatalysts. Polymers 2018, 10, 1326. [Google Scholar] [CrossRef]
- Camacho-Munoz, D.; Martin, J.; Santos, J.L.; Alonso, E.; Aparicio, I.; De la Torre, T. Effectiveness of three configurations of membrane bioreactors on the removal of priority and emergent organic compounds from wastewater: Comparison with conventional wastewater treatments. J. Environ. Monit. 2012, 14, 1428–1436. [Google Scholar] [CrossRef]
- Balabanic, D.; Hermosilla, D.; Merayo, N.; Klemencic, A.K.; Blanco, A. Comparison of different wastewater treatments for removal of selected endocrine-disruptors from paper mill wastewaters. J. Environ. Sci. Health Part A 2012, 47, 1350–1363. [Google Scholar] [CrossRef]
- Boonyaroj, V.; Chiemchaisri, C.; Chiemchaisri, W.; Theepharaksapan, S.; Yamamoto, K. Toxic organic micro-pollutants removal mechanisms in long-term operated membrane bioreactor treating municipal solid waste leachate. Bioresour. Technol. 2012, 113, 174–180. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades an assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Nash, S.M.B. Turning microplastics into nanoplastics through digestive fragmentation by Antartic krill. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Wei, R.; Oeser, T.; Then, J.; Schmidt, J.; Wohlgemuth, F.; Zimmermann, W. Enzymatic hydrolysis of polyethylene films in an ultrafiltration membrane reactor. J. Memb. Sci. 2015, 494, 182–187. [Google Scholar] [CrossRef]
- Global Membrane Filtration Market 2019 by Manufacturers. Regions, Type and Application, Forecast to 2024. Available online: https://www.globalmarketers.biz/report/chemicals-and-materials/global-membrane-filtration-market-2019-by-manufacturers,-regions,-type-and-application,-forecast-to-2024 (accessed on 5 November 2019).
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Achievements and Conclusions of the Life Transfomem. Project on the Recycling of Disposed Membranes. Available online: https://www.water.imdea.org/news/2018/achievements-and-conclusions-life-transfomem-project-recycling-disposed-membranes (accessed on 4 November 2019).
- RO Membrane Recycling. We Are your Global Partner for your Membrane Recycling. Available online: https://www.memre.de/ (accessed on 5 November 2019).
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2019, 21, 109–120. [Google Scholar] [CrossRef]


| Influencing Factors | Membrane Process Parameters | |
|---|---|---|
| Membrane process | Membrane material | -Flux -Transmembrane pressure (TMP) -Polarization concentration -Cake layer formation and fouling -Rejection/Removal -Specific energy consumption (SEC) |
| Membrane pore size | ||
| Membrane thickness | ||
| Membrane surface properties | ||
| Source of polluted water (Seawater, surface water, municipal water, industrial wastewater etc.) | ||
| Micro-Nanoplastic | Shape | |
| Size | ||
| Mass | ||
| Chemical composition | ||
| Concentration |
| Treatment Processes | Microplastic Removal (%) | WWTP location |
|---|---|---|
| Primary, Secondary | 99.9 | Sweden |
| Primary, Secondary (Biofilter) | 88.1 | France |
| Primary, Secondary | 99.9 | United States |
| Primary, Secondary | 98.4 | Scotland |
| Primary, Secondary | 11–94 | Netherlands |
| Primary, Secondary | 95.6 | United States |
| Primary, Secondary | 98.3 | Finland |
| Primary/AnMBR | 99.4 | United States |
| Primary/MBR | 99.3 | Finland |
| Primary, Secondary, Tertiary (GF) | 97.2 | United States |
| Primary, Secondary, Tertiary (BAF) | 97.8 | Finland |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. https://doi.org/10.3390/molecules24224148
Poerio T, Piacentini E, Mazzei R. Membrane Processes for Microplastic Removal. Molecules. 2019; 24(22):4148. https://doi.org/10.3390/molecules24224148
Chicago/Turabian StylePoerio, Teresa, Emma Piacentini, and Rosalinda Mazzei. 2019. "Membrane Processes for Microplastic Removal" Molecules 24, no. 22: 4148. https://doi.org/10.3390/molecules24224148
APA StylePoerio, T., Piacentini, E., & Mazzei, R. (2019). Membrane Processes for Microplastic Removal. Molecules, 24(22), 4148. https://doi.org/10.3390/molecules24224148