Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening
Abstract
1. Introduction
2. Results and Discussion
2.1. Literature Review
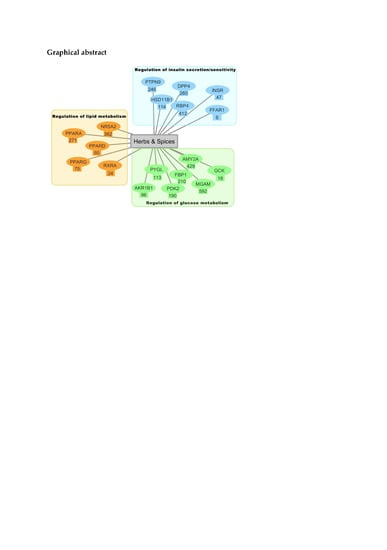
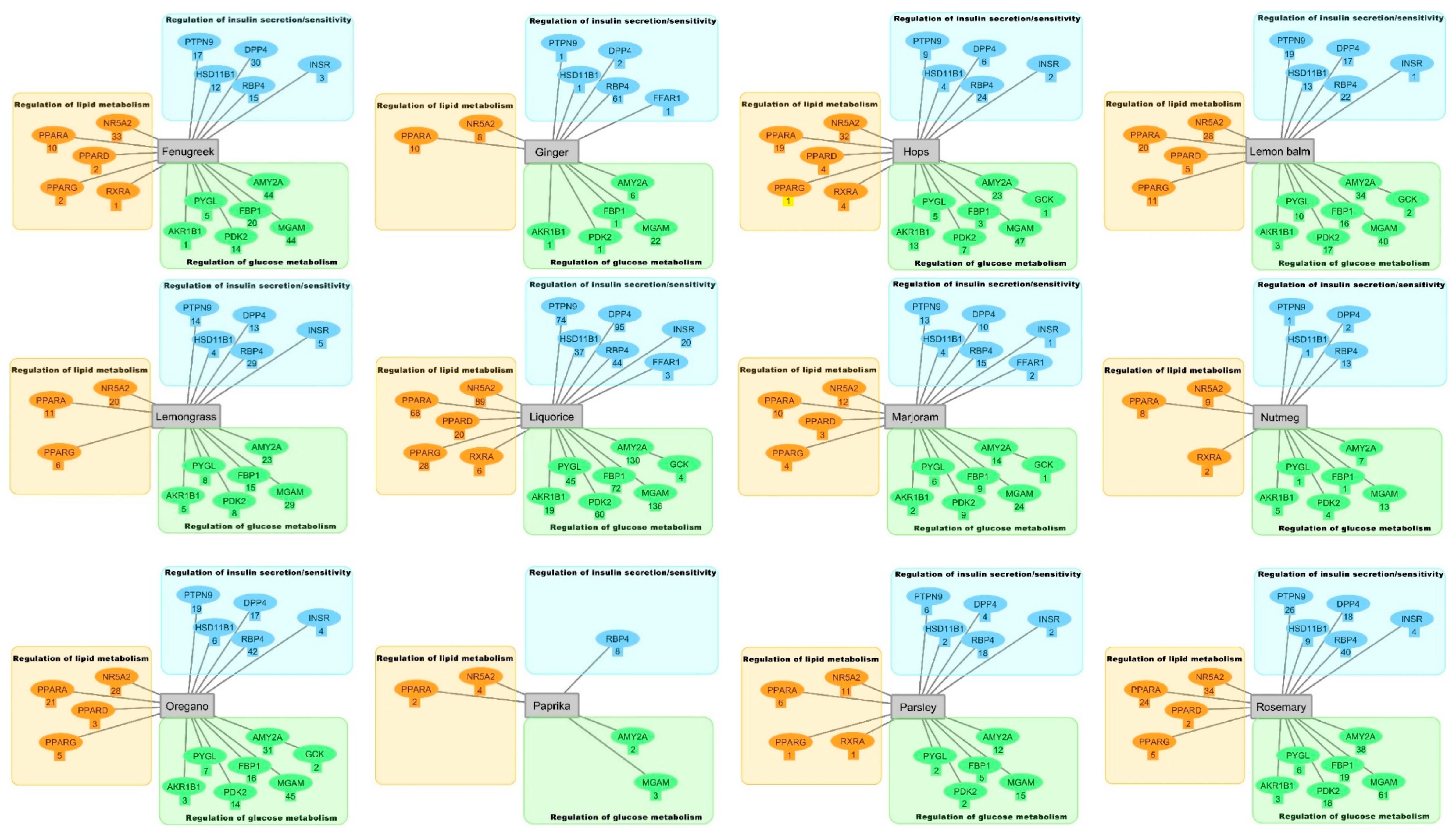
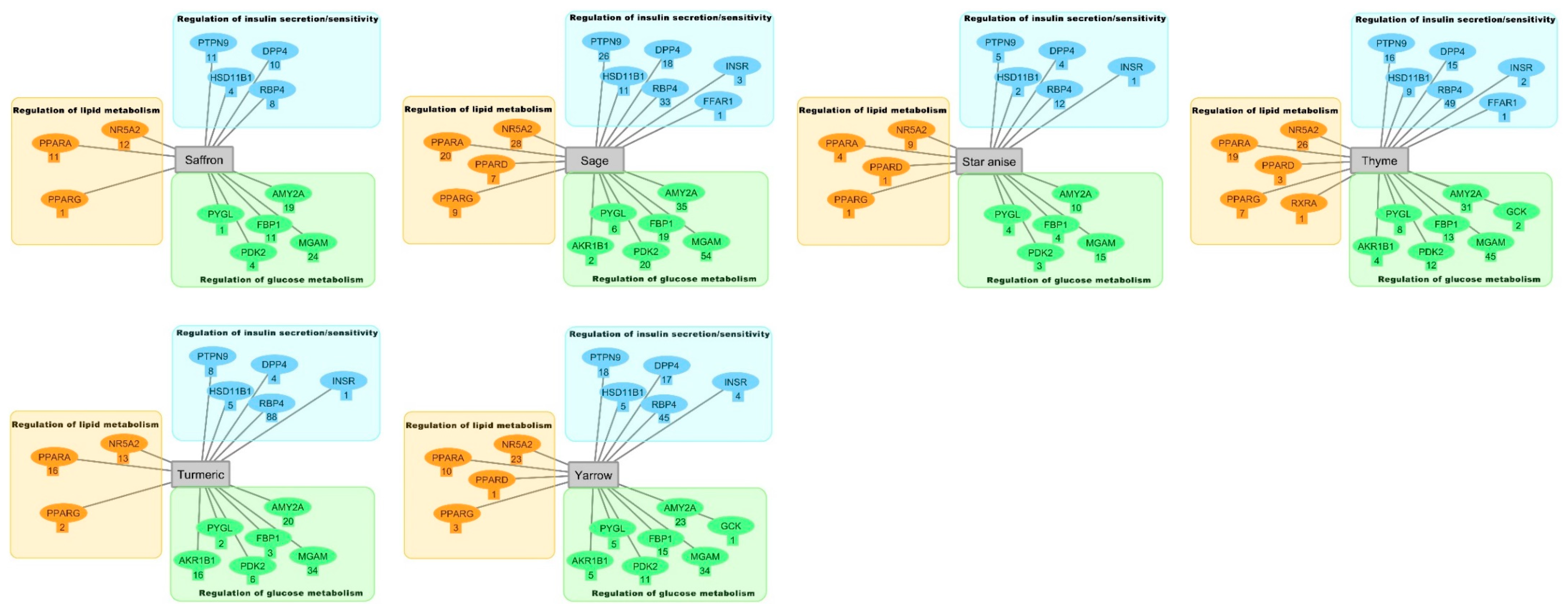
2.2. Inverse Virtual Screening with the DIA-DB Web Server
2.3. Hierarchical Clustering Analysis
3. Materials and Methods
3.1. Literature Review
3.2. Preparation of Compound Structures and Inverse Virtual Screening of Potential Anti-Diabetic Activity.
3.3. Hierarchical Clustering Analysis of the Bioactive Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, X.; Li, H.; Xu, H.; Woo, S.; Dong, H.; Lu, F.; Lange, A.J.; Wu, C. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sinica B 2012, 2, 358–367. [Google Scholar] [CrossRef]
- Fryirs, M.; Barter, P.J.; Rye, K.-A. Cholesterol metabolism and pancreatic β-cell function. Curr. Opin. Lipidol. 2009, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Meerza, D.; Naseem, I.; Ahmed, J. Pharmacology of signaling pathways: In type 2 diabetes. Diabetes Metab. Syndrome Clin. Res. Rev. 2013, 3, 180–185. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fact Sheet. 15 November 2017. Available online: http://www.who.int/newsroom/fact-sheet/detail/diabetes (accessed on 10 April 2019).
- DeFronzo, R.A.; Triplitt, C.L.; Abdul-Ghani, M.; Cersosimo, E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectrum 2014, 27, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gourgari, E.; Wilhelm, E.E.; Hassanzadeh, H.; Aroda, V.R.; Shoulson, I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J. Diabetes Complicat. 2017, 31, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Abo, K.; Fred-Jaiyesimi, A.; Jaiyesimi, A. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J. Ethnopharmacol. 2008, 115, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pacific J. Tropical Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef]
- Deutschländer, M.; Lall, N.; Van De Venter, M. Plant species used in the treatment of diabetes by South African traditional healers: An inventory. Pharm. Biol. 2009, 47, 348–365. [Google Scholar] [CrossRef]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Li, W.; Zheng, H.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef]
- Harlev, E.; Nevo, E.; Mirsky, N.; Ofir, R. Antidiabetic attributes of desert and steppic plants: A review. Planta Med. 2013, 79, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Abbaszadeh, S.; Shahsavari, S.; Alizadeh, M.; Beyranvand, F. The most useful medicinal herbs to treat diabetes. Biomed. Res. Therapy 2018, 5, 2538–2551. [Google Scholar] [CrossRef]
- Chen, J.; Mangelinckx, S.; Adams, A.; Wang, Z.-t.; Li, W.-l.; De Kimpe, N. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat. Prod. Commun. 2015, 10, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Krishna Mohan, G.; Sandhya Rani, M. Phytochemicals for diabetes management. Pharm. Crops 2014, 5, 11–28. [Google Scholar] [CrossRef]
- Putta, S.; Sastry Yarla, N.; Kumar Kilari, E.; Surekha, C.; Aliev, G.; Basavaraju Divakara, M.; Sridhar Santosh, M.; Ramu, R.; Zameer, F.; Prasad, M. Therapeutic potentials of triterpenes in diabetes and its associated complications. Curr. Topics Med. Chem. 2016, 16, 2532–2542. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, L.; Zhou, Y.; Tong, R.; Zeng, M.; Shi, J.; Lib, X. Polysaccharides from Chinese herbal medicine for anti-diabetes recent advances. Int. J. Biol. Macromol. 2019, 121, 1240–1253. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants–a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective herbs and foods from different traditional medicines and diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef]
- Kaefer, C.M.; Milner, J.A. The role of herbs and spices in cancer prevention. J. Nutr. Biochem. 2008, 19, 347–361. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.S.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G. Health benefits of herbs and spices: The past, the present, the future. Available online: https://ro.uow.edu.au/hbspapers/28/ (accessed on 23 September 2019).
- Chen, B.-W.; Li, W.-X.; Wang, G.-H.; Li, G.-H.; Liu, J.-Q.; Zheng, J.-J.; Wang, Q.; Li, H.-J.; Dai, S.-X.; Huang, J.-F. A strategy to find novel candidate anti-Alzheimer’s disease drugs by constructing interaction networks between drug targets and natural compounds in medical plants. PeerJ 2018, 6, e4756. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-X.; Li, W.-X.; Han, F.-F.; Guo, Y.-C.; Zheng, J.-J.; Liu, J.-Q.; Wang, Q.; Gao, Y.-D.; Li, G.-H.; Huang, J.-F. In silico identification of anti-cancer compounds and plants from traditional Chinese medicine database. Sci. Rep. 2016, 6, 25462. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Q.; Dai, S.-X.; Zheng, J.-J.; Guo, Y.-C.; Li, W.-X.; Li, G.-H.; Huang, J.-F. The identification and molecular mechanism of anti-stroke traditional Chinese medicinal compounds. Sci. Rep. 2017, 7, 41406. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, C.L.; Polansky, M.M.; Anderson, R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000, 48, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Sicari, V.; Tenuta, M.C.; Leporini, M.R.; Falco, T.; Pellicanò, T.M.; Menichini, F.; Tundis, R. Phytochemicals content, antioxidant and hypoglycaemic activities of commercial nutmeg mace (Myristica fragrans L.) and pimento (Pimenta dioica (L.) Merr.). Int. J. Food Sci. Technol. 2016, 51, 2057–2063. [Google Scholar] [CrossRef]
- Yogalakshmi, K.; Vaidehi, J. Free radical scavenging activity of methanolic leaf extract of Pimenta dioica on streptozotocin-induced diabetic rats. Available online: http://www.jipbs.com/VolumeArticles/FullTextPDF/119_JIPBSV2I404.pdf (accessed on 1 August 2019).
- Shobha, R.; Rajeshwari, C.; Andallu, B. Anti-peroxidative and anti-diabetic activities of aniseeds (Pimpinella anisum L.) and identification of bioactive compounds. Amer. J. Phytomed. Clin. Therap. 2013, 1, 516–527. [Google Scholar]
- Farzaneh, V.; Gominho, J.; Pereira, H.; Carvalho, I.S. Screening of the Antioxidant and Enzyme Inhibition Potentials of Portuguese Pimpinella anisum L. Seeds by GC-MS. Food Analy. Methods 2018, 11, 2645–2656. [Google Scholar] [CrossRef]
- Shobha, R.I.; Andallu, B. Antioxidant, Anti-Diabetic and Hypolipidemic Effects of Aniseeds (Pimpinella anisum L.): In vitro and in vivo Studies. J. Complement Med. Alt. Healthcare 2018, 5, 1–12. [Google Scholar]
- El-Beshbishy, H.; Bahashwan, S. Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of α-glucosidase and α-amylase activities: An in vitro study. Toxicol. Ind. Health 2012, 28, 42–50. [Google Scholar] [CrossRef]
- Bhatti, H.A.; Tehseen, Y.; Maryam, K.; Uroos, M.; Siddiqui, B.S.; Hameed, A.; Iqbal, J. Identification of new potent inhibitor of aldose reductase from Ocimum basilicum. Bioorganic Chem. 2017, 75, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oyeleye, S.I.; Oboh, G. Biological activities, antioxidant properties and phytoconstituents of essential oil from sweet basil (Ocimum basilicum L.) leaves. Comparative Clin. Pathol. 2016, 25, 169–176. [Google Scholar] [CrossRef]
- Ezeani, C.; Ezenyi, I.; Okoye, T.; Okoli, C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. J. Int. Ethnopharmacol. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Irondi, E.A.; Agboola, S.O.; Oboh, G.; Boligon, A.A. Inhibitory effect of leaves extracts of Ocimum basilicum and Ocimum gratissimum on two key enzymes involved in obesity and hypertension in vitro. J. Int. Ethnopharmacol. 2016, 5, 396. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Bio. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Analy. 2015, 23, 124–129. [Google Scholar] [CrossRef]
- Ugwu, M.; Umar, I.; Utu-Baku, A.; Dasofunjo, K.; Ukpanukpong, R.; Yakubu, O.; Okafor, A. Antioxidant status and organ function in streptozotocin-induced diabetic rats treated with aqueous, methanolic and petroleum ether extracts of Ocimum basilicum leaf. J. Appl. Pharm. Sci. 2013, 3, S75–S79. [Google Scholar]
- Saraswat, M.; Muthenna, P.; Suryanarayana, P.; Petrash, J.M.; Reddy, G.B. Dietary sources of aldose reductase inhibitors: Prospects for alleviating diabetic complications. Asia Pacific J. Clin. Nutri. 2008, 17, 55–564. [Google Scholar]
- Basak, S.S.; Candan, F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iranian J. Pharm. Res. IJPR 2013, 12, 367–379. [Google Scholar]
- Aljamal, A. Effect bay leaves on the patients with diabetes mellitus. Res. J. Med. Plants 2011, 5, 471–476. [Google Scholar] [CrossRef][Green Version]
- Khan, A.; Zaman, G.; Anderson, R.A. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J. Clin. Biochem. Nutri. 2009, 44, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, N.; Jaggi, A.S. Evaluation of in vitro aldose reductase inhibitory potential of alkaloidal fractions of Piper nigrum, Murraya koenigii, Argemone mexicana, and Nelumbo nucifera. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Rameshkannan, D.M.; Mani, D.P. Analysis of antioxidant and antidiabetic activity of Piper nigrum leaf extract by in-vitro assay. J. Pharm. Bio. Sci. 2018, 13, 53–56. [Google Scholar]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepper. Adv. Pharm. Sci. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Beck, V.; Jungbauer, A. PPARα activation by culinary herbs and spices. Planta Medica 2011, 77, 497–504. [Google Scholar] [CrossRef]
- Atal, S.; Agrawal, R.P.; Vyas, S.; Phadnis, P.; Rai, N. Evaluation of the effect of piperine per se on blood glucose level in alloxan-induced diabetic mice. Acta Pol. Pharm. 2012, 69, 965–969. [Google Scholar]
- Onyesife, C.O.; Ogugua, V.N.; Anaduaka, E.G. Hypoglycemic potentials of ethanol leaves extract of black pepper (Piper nigrum) on alloxan-induced diabetic rats. Annals Biol. Res. 2014, 5, 26–31. [Google Scholar]
- Kaleem, M.; Sheema, S.H.; Bano, B. Protective effects of Piper nigrum and Vinca rosea in alloxan induced diabetic rats. Indian J. Physiol. Pharmacol. 2005, 49, 65–71. [Google Scholar]
- Sarfraz, M.; Khaliq, T.; Khan, J.A.; Aslam, B. Effect of aqueous extract of black pepper and ajwa seed on liver enzymes in alloxan-induced diabetic Wister albino rats. Saudi Pharm. J. 2017, 25, 449–452. [Google Scholar] [CrossRef]
- Veeresham, C.; Sujatha, S.; Rani, T.S. Effect of piperine on the pharmacokinetics and pharmacodynamics of glimepiride in normal and streptozotocin-induced diabetic rats. Nat. Prod. Commun. 2012, 7, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M.; Haeri Rohani, A.; Basati, F. Hypoglycemic effect of ethanolic extract of Carum carvi L. seeds in normal and streptozotocin-induced diabetic rats. J. Med. Plants 2010, 9, 106–113. [Google Scholar]
- Ene, A.; Nwankwo, E.; Samdi, L. Alloxan-induced diabetes in rats and the effects of Black caraway (Carum carvi L.) oil on their body weights. J. Pharmacol. Toxicol. 2008, 3, 141–146. [Google Scholar]
- Erjaee, H.; Rajaian, H.; Nazifi, S.; Chahardahcherik, M. The effect of caraway (Carum carvi L.) on the blood antioxidant enzymes and lipid peroxidation in streptozotocin-induced diabetic rats. Comparative Clin. Pathol. 2015, 24, 1197–1203. [Google Scholar] [CrossRef]
- Haidari, F.; Seyed-Sadjadi, N.; Taha-Jalali, M.; Mohammed-Shahi, M. The effect of oral administration of Carum carvi on weight, serum glucose, and lipid profile in streptozotocin-induced diabetic rats. Saudi Med. J. 2011, 32, 695–700. [Google Scholar] [PubMed]
- Lemhadri, A.; Hajji, L.; Michel, J.-B.; Eddouks, M. Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J. Ethnopharmacol. 2006, 106, 321–326. [Google Scholar] [CrossRef] [PubMed]
- El-Yamani, M. Cinnamon, cardamom and ginger impacts as evaluated on hyperglycemic rats. Res. J. Specific Educ. 2011, 20, 665–678. [Google Scholar]
- Winarsi, H.; Sasongko, N.; Purwanto, A.; Nuraeni, I. Effect of cardamom leaves extract as antidiabetic, weight lost and hypocholesterolemic to alloxan-induced Sprague Dawley diabetic rats. Int. Food Res. J. 2014, 21, 2253–2261. [Google Scholar]
- Bhat, G.N.; Nayak, N.; Vinodraj, K.; Chandralekha, N.; Mathai, P.; Cherian, J. Comparison of the efficacy of cardamom (Elettaria cardamomum) with pioglitazone on dexamethasone-induced hepatic steatosis, dyslipidemia, and hyperglycemia in albino rats. J. Adv. Pharm. Technol. Res. 2015, 6, 136–140. [Google Scholar] [CrossRef]
- Bhaswant, M.; Poudyal, H.; Mathai, M.; Ward, L.; Mouatt, P.; Brown, L. Green and black cardamom in a diet-induced rat model of metabolic syndrome. Nutrients 2015, 7, 7691–7707. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Fallah, H.; Sharifi-far, F.; Mirtajaddini, M. The inhibitory effect of some Iranian plants extracts on the alpha glucosidase. Iranian J. Basic Med. Sci. 2008, 11, 1–9. [Google Scholar]
- Hamza, A.; Ksiksi, T.; Shamsi, O.; Balfaqh, S. α-Glucosidase Inhibitory Activity of Common Traditional Medicinal Plants Used for Diabetes Mellitus. J. Dev. Drugs 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Medagama, A.B. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutri. J. 2015, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Ravindran, R.; Zinjarde, S.; Bhargava, S.; Ravi Kumar, A. Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid. Based Complemen. Alter. Med. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Seetaloo, A.; Aumeeruddy, M.; Kannan, R.R.; Mahomoodally, M. Potential of traditionally consumed medicinal herbs, spices, and food plants to inhibit key digestive enzymes geared towards diabetes mellitus management—A systematic review. South African J. Botany 2019, 120, 3–24. [Google Scholar] [CrossRef]
- Salehi, P.; Asghari, B.; Esmaeili, M.A.; Dehghan, H.; Ghazi, I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plants Res. 2013, 7, 257–266. [Google Scholar]
- Tahir, H.U.; Sarfraz, R.A.; Ashraf, A.; Adil, S. Chemical composition and antidiabetic Activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int. J. Food Propert. 2016, 19, 2156–2164. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water extractable phytochemicals from some tropical spices. Pharm. Biol. 2012, 50, 857–865. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pacific J. Tropical Biomed. 2012, 2, 774–781. [Google Scholar]
- Prasad, R.C.; Herzog, B.; Boone, B.; Sims, L.; Waltner-Law, M. An extract of Syzygium aromaticum represses genes encoding hepatic gluconeogenic enzymes. J. Ethnopharmacol. 2005, 96, 295–301. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Ohtomo, T.; Yamada, J.; Nishiyama, T.; Mae, T.; Kishida, H.; Kawada, T. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-A y mice and identification of the active ingredients. J. Nat. Med. 2012, 66, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M.; Zari, T.A. Modulatory effects of ginger and clove oils on physiological responses in streptozotocin-induced diabetic rats. Int. J. Pharmacol. 2007, 3, 34–40. [Google Scholar]
- Khathi, A.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Effects of Syzygium aromaticum-derived triterpenes on postprandial blood glucose in streptozotocin-induced diabetic rats following carbohydrate challenge. PLoS ONE 2013, 8, e81632. [Google Scholar] [CrossRef] [PubMed]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Renal Failure 2014, 36, 419–431. [Google Scholar] [CrossRef]
- Ngubane, P.S.; Masola, B.; Musabayane, C.T. The effects of Syzygium aromaticum-derived oleanolic acid on glycogenic enzymes in streptozotocin-induced diabetic rats. Renal Failure 2011, 33, 434–439. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Adefegha, O.M.; Boligon, A.A.; Athayde, M.L. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. J. Sci. Food Agric. 2014, 94, 2726–2737. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresource Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Hemlata, B.; Pornima, G.; Tukaram, K.; Pankaj, B. In vitro anti-amylase activity of some Indian dietary spices. J. Appl. Biol. Biotechnol. Vol. 2019, 7, 70–74. [Google Scholar]
- Lee, H.-S. Cuminaldehyde: Aldose reductase and α-glucosidase inhibitor derived from Cuminum cyminum L. seeds. J. Agric. Food Chem. 2005, 53, 2446–2450. [Google Scholar] [CrossRef]
- Srivsatava, R.; Srivastava, S.P.; Jaiswal, N.; Mishra, A.; Maurya, R.; Srivastava, A.K. Antidiabetic and antidyslipidemic activities of Cuminum cyminum L. in validated animal models. Med. Chem. Res. 2011, 20, 1656–1666. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Die Pharmazie An Int. J. Pharm. Sci. 2006, 61, 952–956. [Google Scholar]
- Jagtap, A.; Patil, P. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem. Toxicol. 2010, 48, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, E.; Khedri, M.R.; Zangeneh, M.M.; Abiari, M.; Zangeneh, A.; Amiri-Paryan, A.; Tahvilian, R.; Moradi, R. Effect of Anethum graveolens aqueous extract on blood fasting glucose and hematological parameters in diabetic male BALB/c mice. Onl. J. Vet. Res. 2017, 21, 784–793. [Google Scholar]
- Goodarzi, M.T.; Khodadadi, I.; Tavilani, H.; Abbasi Oshaghi, E. The role of Anethum graveolens L. (Dill) in the management of diabetes. J. Tropical Med. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Mishra, N. Haematological and hypoglycemic potential Anethum graveolens seeds extract in normal and diabetic Swiss albino mice. Veterinary World 2013, 6, 502–507. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Tavilani, H.; Khodadadi, I.; Goodarzi, M.T. Dill tablet: A potential antioxidant and anti-diabetic medicine. Asian Pacific J. Tropical Biomed. 2015, 5, 720–727. [Google Scholar] [CrossRef]
- Abu-Zaiton, A.; Alu’datt, M.; Wafa, M. Evaluating the effect of Foeniculum vulgare extract on enzymes related with blood pressure and diabetes (in vitro study). Int. J. Chem. Eng. Biol. Sci. 2015, 2, 77–80. [Google Scholar]
- Godavari, A.; Amutha, K.; Moorthi, N.M. In-vitro hypoglycemic effect of Foeniculum vulgare Mill. Seeds on the carbohydrate hydrolysing enzymes, alpha-amylase and alpha-glucosidase. Int. J. Pharm. Sci. Res. 2018, 9, 4441–4445. [Google Scholar]
- Al-Aridhi, D.T. The Effect of Foeniculum Vulgare Alcoholic Extract on Some Metabolic Changes in Liver and Kidneys of Alloxan Diabetic Mice. Iraqi J. Med. Sci. 2014, 12, 119–125. [Google Scholar]
- Anitha, T.; Balakumar, C.; Ilango, K.; Jose, C.B.; Vetrivel, D. Antidiabetic activity of the aqueous extracts of Foeniculum vulgare on streptozotocin-induced diabetic rats. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 487–494. [Google Scholar]
- El-Soud, N.; El-Laithy, N.; El-Saeed, G.; Wahby, M.; Khalil, M.; Morsy, F.; Shaffie, N. Antidiabetic activities of Foeniculum vulgare Mill. essential oil in streptozotocin-induced diabetic rats. Macedonian J. Med. Sci. 2011, 4, 139–146. [Google Scholar]
- Parsaeyan, N. The effect of Foeniculum vulgare (fennel) extract on lipid profile, lipid peroxidation and liver enzymes of diabetic rat. Iranian J. Diabetes Obesity 2016, 8, 24–29. [Google Scholar]
- Ganeshpurkar, A.; Diwedi, V.; Bhardwaj, Y. In vitro α-amylase and α-glucosidase inhibitory potential of Trigonella foenum-graecum leaves extract. Ayu 2013, 34, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Bansode, T.S.; Gupta, A.; Chaphalkar, S.; Salalkar, B. Integrating in-silico and in-vitro approaches to screen the antidiabetic drug from Trigonella foenum graecum Linn. Int. J. Biochem. Res. Rev. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Gad, M.Z.; El-Sawalhi, M.M.; Ismail, M.F.; El-Tanbouly, N.D. Biochemical study of the anti-diabetic action of the Egyptian plants Fenugreek and Balanites. Mol. Cell. Biochem. 2006, 281, 173–183. [Google Scholar] [CrossRef]
- Kamtekar, S.; Keer, V.; Patil, V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharm. Sci. 2014, 4, 61–65. [Google Scholar]
- Hamden, K.; Keskes, H.; Elgomdi, O.; Feki, A.; Alouche, N. Modulatory effect of an isolated triglyceride from fenugreek seed oil on of α-amylase, lipase and ace activities, liver-kidney functions and metabolic disorders of diabetic rats. J. Oleo Sci. 2017, 66, 633–645. [Google Scholar] [CrossRef][Green Version]
- Buchholz, T.; Melzig, M.F. Medicinal plants traditionally used for treatment of obesity and diabetes mellitus–screening for pancreatic lipase and α-Amylase inhibition. Phytotherapy Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Christensen, K.B.; Minet, A.; Svenstrup, H.; Grevsen, K.; Zhang, H.; Schrader, E.; Rimbach, G.; Wein, S.; Wolffram, S.; Kristiansen, K. Identification of plant extracts with potential antidiabetic properties: Effect on human peroxisome proliferator-activated receptor (PPAR), adipocyte differentiation and insulin-stimulated glucose uptake. Phytotherapy Res. 2009, 23, 1316–1325. [Google Scholar] [CrossRef]
- Hamden, K.; Jaouadi, B.; Carreau, S.; Bejar, S.; Elfeki, A. Inhibitory effect of fenugreek galactomannan on digestive enzymes related to diabetes, hyperlipidemia, and liver-kidney dysfunctions. Biotechnol. Bioproc. Eng. 2010, 15, 407–413. [Google Scholar] [CrossRef]
- Hamden, K.; Jaouadi, B.; Salami, T.; Carreau, S.; Bejar, S.; Elfeki, A. Modulatory effect of fenugreek saponins on the activities of intestinal and hepatic disaccharidase and glycogen and liver function of diabetic rats. Biotechnol. Bioproc. Eng. 2010, 15, 745–753. [Google Scholar] [CrossRef]
- Hamden, K.; Keskes, H.; Belhaj, S.; Mnafgui, K.; Allouche, N. Inhibitory potential of omega-3 fatty and fenugreek essential oil on key enzymes of carbohydrate-digestion and hypertension in diabetes rats. Lipids Health Disease 2011, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tharaheswari, M.; Kumar, J.S.P.; Reddy, N.J.; Subhashree, S.; Rani, S.S. Fenugreek seed extract stabilize plasma lipid levels in type 2 diabetes by modulating PPARs and GLUT4 in insulin target tissues. AJPCT 2014, 2, 587–602. [Google Scholar]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Gammoh, S.; Ereifej, K.; Johargy, A.; Kubow, S.; Almajwal, A.M.; Rawashdeh, M. Optimization of Phenolic Content, Antioxidant, and Inhibitory Activities of α-Glucosidase and Angiotensin Converting (AC) Enzymes from Zingiber officinale Z. Int. J. Food Propert. 2016, 19, 1303–1316. [Google Scholar] [CrossRef]
- Elya, B.; Handayani, R.; Sauriasari, R.; Hasyyati, U.S.; Permana, I.T.; Permatasari, Y.I. Antidiabetic activity and phytochemical screening of extracts from Indonesian plants by inhibition of alpha amylase, alpha glucosidase and dipeptidyl peptidase IV. Pakistan J. Bio. Sci. 2015, 18, 279–284. [Google Scholar] [CrossRef]
- Adefegha, A. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. J. Food Nutri. Res. 2010, 49, 14–20. [Google Scholar]
- Priya Rani, M.; Padmakumari, K.; Sankarikutty, B.; Lijo Cherian, O.; Nisha, V.; Raghu, K. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int. J. Food Sci. Nutri. 2011, 62, 106–110. [Google Scholar] [CrossRef]
- Kato, A.; Higuchi, Y.; Goto, H.; Kizu, H.; Okamoto, T.; Asano, N.; Hollinshead, J.; Nash, R.J.; Adachi, I. Inhibitory effects of Zingiber officinale Roscoe derived components on aldose reductase activity in vitro and in vivo. J. Agric. Food Chem. 2006, 54, 6640–6644. [Google Scholar] [CrossRef]
- Son, M.J.; Miura, Y.; Yagasaki, K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology 2015, 67, 641–652. [Google Scholar] [CrossRef]
- Keskin, Ş.; Şirin, Y.; Çakir, H.; Keskin, M. An investigation of Humulus lupulus L.: Phenolic composition, antioxidant capacity and inhibition properties of clinically important enzymes. South African J. Botany 2019, 120, 170–174. [Google Scholar] [CrossRef]
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzyme Inhibit. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Liu, G.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J. Agric. Food Chem. 2014, 62, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Lima-Fontes, M.r.; Costa, R.; Rodrigues, I.; Soares, R. Xanthohumol restores hepatic glucolipid metabolism balance in type 1 diabetic wistar rats. J. Agric. Food Chem. 2017, 65, 7433–7439. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, D.; Song, X.; Zhang, Y.; Ding, W.; Peng, X.; Zhang, X.; Li, Y.; Ma, Y.; Wang, R. Natural prenylchalconaringenins and prenylnaringenins as antidiabetic agents: α-glucosidase and α-amylase inhibition and in vivo antihyperglycemic and antihyperlipidemic effects. J. Agric. Food Chem. 2017, 65, 1574–1581. [Google Scholar] [CrossRef]
- Yajima, H.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Fujiwara, D.; Odai, H.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor α and γ and reduce insulin resistance. J. Bio. Chem. 2004, 279, 33456–33462. [Google Scholar] [CrossRef]
- Nozawa, H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-Ay mice. Biochem. Biophys. Res. Commun. 2005, 336, 754–761. [Google Scholar] [CrossRef]
- Takahashi, K.; Osada, K. Effect of dietary purified xanthohumol from hop (Humulus lupulus L.) pomace on adipose tissue mass, fasting blood glucose level, and lipid metabolism in KK-Ay mice. J. Oleo Sci. 2017, 66, 531–541. [Google Scholar] [CrossRef]
- Kwon, Y.-I.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pacific J. Clin. Nutri. 2006, 15, 107–118. [Google Scholar]
- Weidner, C.; Wowro, S.J.; Freiwald, A.; Kodelja, V.; Abdel-Aziz, H.; Kelber, O.; Sauer, S. Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol. Nutri. Food Res. 2014, 58, 903–907. [Google Scholar] [CrossRef]
- Hasanein, P.; Riahi, H. Antinociceptive and antihyperglycemic effects of Melissa officinalis essential oil in an experimental model of diabetes. Med. Prin. Practice 2015, 24, 47–52. [Google Scholar] [CrossRef]
- Khodsooz, S.; Moshtaghian, J.; Eivani, M. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Melissa officinalis (Lemon Balm) in alloxan-induced diabetic rats. Physiol. Pharmacol. 2016, 20, 24–30. [Google Scholar]
- Tashakor, A.; Kelishadi, M.R.; Ghasemi, A.; Daylami, F.; Rahimi, A.; Doabi, S.Z.; Abdolusefi, N.N. Effects of hydroalcoholic extract in Mellissa officinalis plant on fat profiles and glucose level in diabetic rats induced by streptozotocin. J. Chem. Pharm. Res 2016, 8, 402–406. [Google Scholar]
- Boaduo, N.K.K.; Katerere, D.; Eloff, J.N.; Naidoo, V. Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in South Africa using in vitro methods. Pharm. Biol. 2014, 52, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, A.D.; Kamble, S.P.; Nalawade, M.L.; Arvindekar, A.U. Citronellol: A potential antioxidant and aldose reductase inhibitor from Cymbopogon citratus. Int. J. Pharm. Pharm. Sci. 2015, 7, 203–209. [Google Scholar]
- Santoso, F.; Winarno, J.; Gunawan-Puteri, M.D. Application of Lemongrass (Cymbopogon Citratus) As A Functional Food Ingredient With Alpha-Glucosidase Inhibitory Activity. Adv. Eng. Res. 2018, 172, 205–209. [Google Scholar]
- Jumepaeng, T.; Prachakool, S.; Luthria, D.L.; Chanthai, S. Determination of antioxidant capacity and α-amylase inhibitory activity of the essential oils from citronella grass and lemongrass. Int. Food Res. J. 2013, 20, 481–485. [Google Scholar]
- Bharti, S.; Kumar, A.; Prakash, O.; Krishnan, S.; Gupta, A. Essential oil of cymbopogon citratus against diabetes: Validation by in vivo experiments and computational studies. J Bioanal. Biomed. 2013, 5, 194–203. [Google Scholar]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-γ ligand-binding activity. Bioorganic Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef]
- Ko, B.-S.; Jang, J.S.; Hong, S.M.; Sung, S.R.; Lee, J.E.; Lee, M.Y.; Jeon, W.K.; Park, S. Changes in components, glycyrrhizin and glycyrrhetinic acid, in raw Glycyrrhiza uralensis Fisch, modify insulin sensitizing and insulinotropic actions. Biosci. Biotechnol. Biochem. 2007, 6, 1452–1461. [Google Scholar] [CrossRef][Green Version]
- Yoon, G.; Lee, W.; Kim, S.-N.; Cheon, S.H. Inhibitory effect of chalcones and their derivatives from Glycyrrhiza inflata on protein tyrosine phosphatase 1B. Bioorganic Med. Chem. Lett. 2009, 19, 5155–5157. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.H.; Jung, S.H.; Kim, J.K.; Pan, C.-H.; Lim, S.S. Aldose reductase inhibitory compounds from Glycyrrhiza uralensis. Bio. Pharm. Bullet. 2010, 33, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.Z.; El-Shehabi, M. Antidiabetic activity of caffeic acid and 18β-glycyrrhetinic acid and its relationship with the antioxidant property. Asian J. Pharm. Clin. Res. 2015, 8, 229–235. [Google Scholar]
- Kalaiarasi, P.; Kaviarasan, K.; Pugalendi, K.V. Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Europ. J. Pharmacol. 2009, 612, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef] [PubMed]
- El-Ghffar, E.A.A. Ameliorative effect of glabridin, a main component of Glycyrrhiza glabra L. roots in streptozotocin induced Type 1 diabetes in male albino rats. Ind. J. Tradition. Knowledge 2016, 15, 570–579. [Google Scholar]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H.; Kumar, S. Evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats. Europ. J. Pharm. Sci. 2017, 106, 220–230. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kishida, H.; Arai, N.; Nishiyama, T.; Mae, T. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK-Ay mice. Bio. Pharm. Bullet. 2004, 27, 1775–1778. [Google Scholar] [CrossRef]
- Kataya, H.H.; Hamza, A.A.; Ramadan, G.A.; Khasawneh, M.A. Effect of licorice extract on the complications of diabetes nephropathy in rats. Drug Chem. Toxicol. 2011, 34, 101–108. [Google Scholar] [CrossRef]
- Sen, S.; Roy, M.; Chakraborti, A.S. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J. Pharm. Pharmacol. 2011, 63, 287–296. [Google Scholar] [CrossRef]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Ind. J. Experimental Biol. 2013, 51, 129–138. [Google Scholar]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; De Mejia, E.G. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, J.; Mizuhata, K.; Sato, E.; Nishioka, T.; Aoyama, Y.; Kasai, T. 6-Hydroxyflavonoids as α-glucosidase inhibitors from marjoram (Origanum majorana) leaves. Biosci. Biotechnol. Biochem. 2003, 67, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Pimple, B.; Kadam, P.; Patil, M. Comparative antihyperglycaemic and antihyperlipidemic effect of Origanum majorana extracts in NIDDM rats. Oriental Pharm. Experimental Med. 2012, 12, 41–50. [Google Scholar] [CrossRef]
- Soliman, M.M.; Nassan, M.A.; Ismail, T.A. Origanum Majoranum extract modulates gene expression, hepatic and renal changes in a rat model of type 2 diabetes. Iranian J. Pharm. Res. IJPR 2016, 15, 45. [Google Scholar]
- Tripathy, B.; Satyanarayana, S.; Khan, K.A.; Raja, K. Evaluation of anti-diabetic and anti-hyperlipidemic activities of ethanolic leaf extract of Origanum Majorana in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Res. 2018, 9, 1529–1536. [Google Scholar]
- Han, K.L.; Choi, J.S.; Lee, J.Y.; Song, J.; Joe, M.K.; Jung, M.H.; Hwang, J.-K. Therapeutic potential of peroxisome proliferators–activated receptor-α/γ dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes 2008, 57, 737–745. [Google Scholar] [CrossRef]
- Lestari, K.; Hwang, J.; Kariadi, S.H.; Wijaya, A.; Ahmad, T.; Subarnas, A.; Supriyatna, M.; Muchtaridi, M. Screening for PPAR γ agonist from Myristica fragrans Houtt seeds for the treatment of Type 2 diabetes by in vitro and in vivo. Med. Health Sci. J. 2012, 12, 7–15. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Le, T.V.T.; Kang, H.W.; Chae, J.; Kim, S.K.; Kwon, K.-i.; Seo, D.B.; Lee, S.J.; Oh, W.K. AMP-activated protein kinase (AMPK) activators from Myristica fragrans (nutmeg) and their anti-obesity effect. Bioorganic Med. Chem. Lett. 2010, 20, 4128–4131. [Google Scholar] [CrossRef]
- Patil, S.B.; Ghadyale, V.A.; Taklikar, S.S.; Kulkarni, C.R.; Arvindekar, A.U. Insulin secretagogue, alpha-glucosidase and antioxidant activity of some selected spices in streptozotocin-induced diabetic rats. Plant Foods Human Nutri. 2011, 66, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Shyni, G.; Sasidharan, K.; Francis, S.K.; Das, A.A.; Nair, M.S.; Raghu, K. Licarin B from Myristica fragrans improves insulin sensitivity via PPARγ and activation of GLUT4 in the IRS-1/PI3K/AKT pathway in 3T3-L1 adipocytes. RSC Adv. 2016, 6, 79859–79870. [Google Scholar] [CrossRef]
- Yang, S.; Min Kyun, N.; Jang, J.P.; Kim, K.A.; Kim, B.Y.; Sung, N.J.; Oh, W.K.; Ahn, J.S. Inhibition of protein tyrosine phosphatase 1B by lignans from Myristica fragrans. Phytotherapy Res. 2006, 20, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhi, D.; Kurian, R.; Veeranjaneyulu, A.; Bodhankar, S. Antidiabetic and antihyperlipidemic effects of Myristica fragrans. in animal models. Pharm. Biol. 2007, 45, 64–68. [Google Scholar] [CrossRef]
- Somani, R.; Singhai, A. Hypoglycaemic and antidiabetic activities of seeds of Myristica fragrans in normoglycaemic and alloxan-induced diabetic rats. Asian J. Exp. Sci. 2008, 22, 95–102. [Google Scholar]
- McCue, P.; Vattem, D.; Shetty, K. Inhibitory effect of clonal oregano extracts against porcine pancreatic amylase in vitro. Asia Pacific J. Clin. Nutri. 2004, 13, 401–408. [Google Scholar]
- Koukoulitsa, C.; Zika, C.; Geromichalos, G.D.; Demopoulos, V.J.; Skaltsa, H. Evaluation of aldose reductase inhibition and docking studies of some secondary metabolites, isolated from Origanum vulgare L. ssp. hirtum. Bioorganic Med. Chem. 2006, 14, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Koukoulitsa, C.; Zika, C.; Hadjipavlou–Litina, D.; Demopoulos, V.J.; Skaltsa, H. Inhibitory effect of polar oregano extracts on aldose reductase and soybean lipoxygenase in vitro. Phytotherapy Res. 2006, 20, 605–606. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Koga, K.; Shibata, H.; Yoshino, K.; Nomoto, K. Effects of 50% Ethanol Extract from Rosemary (Rosmarinus officinalis) on α-Glucosidase Inhibitory Activity and the Elevation of Plasma Glucose Level in Rats, and Its Active Compound. J. Food Sci. 2006, 71, S507–S512. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Evaluation of pepper (Capsicum annuum) for management of diabetes and hypertension. J. Food Biochem. 2007, 31, 370–385. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; De Luca, D.; de Cindio, B.; Menichini, F. Comparative study on the chemical composition, antioxidant properties and hypoglycaemic activities of two Capsicum annuum L. cultivars (Acuminatum small and Cerasiferum). Plant Foods Human Nutri. 2011, 66, 261–269. [Google Scholar] [CrossRef]
- Magied, M.M.A.; Salama, N.A.R.; Ali, M.R. Hypoglycemic and hypocholesterolemia effects of intragastric administration of dried red chili pepper (Capsicum annum) in alloxan-induced diabetic male albino rats fed with high-fat-diet. J. Food Nutri. Res. 2014, 2, 850–856. [Google Scholar] [CrossRef]
- Bolkent, S.; Yanardag, R.; Ozsoy-Sacan, O.; Karabulut-Bulan, O. Effects of parsley (Petroselinum crispum) on the liver of diabetic rats: A morphological and biochemical study. Phytotherapy Res. 2004, 18, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Abou Khalil, N.S.; Abou-Elhamd, A.S.; Wasfy, S.I.; El Mileegy, I.M.; Hamed, M.Y.; Ageely, H.M. Antidiabetic and antioxidant impacts of desert date (Balanites aegyptiaca) and parsley (Petroselinum sativum) aqueous extracts: Lessons from experimental rats. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Ozsoy-Sacan, O.; Yanardag, R.; Orak, H.; Ozgey, Y.; Yarat, A.; Tunali, T. Effects of parsley (Petroselinum crispum) extract versus glibornuride on the liver of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2006, 104, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.; Eltablawy, N.A.; Hamed, M.S. The ameliorative effect of Petroselinum crispum (parsley) on some diabetes complications. J. Med. Plants Studies 2015, 3, 92–100. [Google Scholar]
- Yanardağ, R.; Bolkent, Ş.; Tabakoğlu-Oğuz, A.; Özsoy-Saçan, Ö. Effects of Petroselinum crispum extract on pancreatic B cells and blood glucose of streptozotocin-induced diabetic rats. Bio. Pharm. Bullet. 2003, 26, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Camerotto, C.; Cestaro, B. Anti-oxidant, anti-glycant, and inhibitory activity against α-amylase and α-glucosidase of selected spices and culinary herbs. Int. J. Food Sci. Nutri. 2011, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Moss-Pierce, T.; Ford, P.; Jiang, T.A. Rosemary (Rosmarinus officinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J. Agric. Food Chem. 2013, 61, 2803–2810. [Google Scholar] [CrossRef]
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Almasri, I.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2010, 4, 2235–2242. [Google Scholar]
- Al-Jamal, A.-R.; Alqadi, T. Effects of rosemary (Rosmarinus officinalis) on lipid profile of diabetic rats. Jordan J. Bio. Sci. 2011, 147, 1–5. [Google Scholar]
- Emam, M.A. Comparative evaluation of antidiabetic activity of Rosmarinus officinalis L. and Chamomile recutita in streptozotocin induced diabetic rats. Agric. Biol. JN Am. 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Kensara, O.; ElSawy, N.; Altaf, F.; Header, E. Hypoglycemic and hepato-protective effects of Rosmarinus officinalis in experimental diabetic Rats. UQU Med. J. 2010, 1, 98–113. [Google Scholar]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.V.; Shirani, K.; Hosseinzadeh, H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn-Schmiedeberg’s Archives Pharmacol. 2016, 389, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lee, H.; Jung, E.-S.; Seyedian, R.; Jo, M.; Kim, J.; Kim, J.-S.; Kim, E. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 2012, 135, 2350–2358. [Google Scholar] [CrossRef]
- Elgazar, A.F.; Rezq, A.A.; Bukhari, H.M. Anti-hyperglycemic effect of saffron extract in alloxan-induced diabetic rats. Eur. J. Biol. Sci. 2013, 5, 14–22. [Google Scholar]
- Kianbakht, S.; Hajiaghaee, R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J. Med. Plants 2011, 3, 82–89. [Google Scholar]
- Mohajeri, D.; Mousavi, G.; Doustar, Y. Antihyperglycemic and pancreas-protective effects of Crocus sativus L.(Saffron) stigma ethanolic extract on rats with alloxan-induced diabetes. J. Biol. Sci. 2009, 9, 302–310. [Google Scholar] [CrossRef]
- Mohammad, R.; Daryoush, M.; Ali, R.; Yousef, D.; Mehrdad, N. Attenuation of oxidative stress of hepatic tissue by ethanolic extract of saffron (dried stigmas of Crocus sativus L.) in streptozotocin (STZ)-induced diabetic rats. African J. Pharm. Pharmacol. 2011, 5, 2166–2173. [Google Scholar]
- Samarghandian, S.; Borji, A.; Delkhosh, M.B.; Samini, F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J. Pharm. Pharm. Sci. 2013, 16, 352–362. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. BioMed. Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khacheba, I.; Djeridane, A.; Yousfi, M. Twenty traditional Algerian plants used in diabetes therapy as strong inhibitors of α-amylase activity. Int. J. Carbohydrate Chem. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Christensen, K.B.; Jørgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the nuclear receptor PPARγ by metabolites isolated from sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Moradabadi, L.; Kouhsari, S.M.; Sani, M.F. Hypoglycemic effects of three medicinal plants in experimental diabetes: Inhibition of rat intestinal α-glucosidase and enhanced pancreatic Insulin and cardiac Glut-4 mRNAs expression. Iranian J. Pharm. Res. IJPR 2013, 12, 387–397. [Google Scholar]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndrome Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Aljarah, A.K.; Hameed, I.H. In Vitro Anti-diabetic Properties of Methanolic Extract of Thymus vulgaris Using α-glucosidase and α-amylase Inhibition Assay and Determination of its Bioactive Chemical Compounds. Ind. J. Public Health Res. Develop. 2018, 9, 388–392. [Google Scholar] [CrossRef]
- El-Houri, R.B.; Kotowska, D.; Olsen, L.C.; Bhattacharya, S.; Christensen, L.P.; Grevsen, K.; Oksbjerg, N.; Færgeman, N.; Kristiansen, K.; Christensen, K.B. Screening for bioactive metabolites in plant extracts modulating glucose uptake and fat accumulation. Evid. Based Complemen. Alter. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Ekoh, S.N.; Akubugwo, E.I.; Ude, V.C.; Edwin, N. Anti-hyperglycemic and anti-hyperlipidemic effect of spices (Thymus vulgaris, Murraya koenigii, Ocimum gratissimum and Piper guineense) in alloxan-induced diabetic rats. Int. J. Biosci. 2014, 4, 179–187. [Google Scholar]
- Hanna, E.T.; Aniess, W.; Khalil, A.F.; Abdalla, E.S.; Hassanin, E.A.; Nagib, E.W. The effect of ginger and thyme on some biochemical parameters in diabetic rats. IOSR J. Pharm. Biol. Sci. 2014, 9, 54–61. [Google Scholar] [CrossRef]
- Ozkol, H.; Tuluce, Y.; Dilsiz, N.; Koyuncu, I. Therapeutic potential of some plant extracts used in Turkish traditional medicine on streptozocin-induced type 1 diabetes mellitus in rats. J. Membrane Biol. 2013, 246, 47–55. [Google Scholar] [CrossRef]
- Lekshmi, P.; Arimboor, R.; Indulekha, P.; Nirmala Menon, A. Turmeric (Curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. Int. J. Food Sci. Nutri. 2012, 63, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, P.; Arimboor, R.; Raghu, K.; Menon, A.N. Turmerin, the antioxidant protein from turmeric (Curcuma longa) exhibits antihyperglycaemic effects. Nat. Prod. Res. 2012, 26, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, S.; McFarlane, J.R. An aqueous extract of Curcuma longa (turmeric) rhizomes stimulates insulin release and mimics insulin action on tissues involved in glucose homeostasis in vitro. Phytotherapy Res. 2011, 25, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Zinjarde, S.; Bhargava, S.; Rajamohanan, P.; RaviKumar, A. Discovering Bisdemethoxycurcumin from Curcuma longa rhizome as a potent small molecule inhibitor of human pancreatic α-amylase, a target for type-2 diabetes. Food Chem. 2012, 135, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Bao, Y.D.; Liu, Z.; Qiao, W.; Ma, L.; Huang, Z.S.; Gu, L.Q.; Chan, A.S. Curcumin analogs as potent aldose reductase inhibitors. Archiv der Pharmazie 2006, 339, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-y.; Liu, R.-r.; Shao, W.-y.; Mao, X.-p.; Ma, L.; Gu, L.-q.; Huang, Z.-s.; Chan, A.S. α-Glucosidase inhibition of natural curcuminoids and curcumin analogs. Europ. J. Med. Chem. 2006, 41, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Halder, N.; Joshi, S.; Gupta, S. Lens aldose reductase inhibiting potential of some indigenous plants. J. Ethnopharmacol. 2003, 86, 113–116. [Google Scholar] [CrossRef]
- Madkor, H.R.; Mansour, S.W.; Ramadan, G. Modulatory effects of garlic, ginger, turmeric and their mixture on hyperglycaemia, dyslipidaemia and oxidative stress in streptozotocin–nicotinamide diabetic rats. British J. Nutri. 2011, 105, 1210–1217. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef]
- Rai, P.; Jaiswal, D.; Mehta, S.; Rai, D.; Sharma, B.; Watal, G. Effect of Curcuma longa freeze dried rhizome powder with milk in STZ induced diabetic rats. Ind. J. Clin. Biochem. 2010, 25, 175–181. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Takahashi, K.; Kawada, T.; Nakagawa, K.; Kitahara, M. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Bio. Pharm. Bullet. 2005, 28, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.S.; Srinivasan, K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 1997, 166, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Arun, N.; Nalini, N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Human Nutri. 2002, 57, 41–52. [Google Scholar] [CrossRef]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.G.; Ganai, B.A.; Akbar, S.; Dar, M.Y.; Masood, A. β-Cell protective efficacy, hypoglycemic and hypolipidemic effects of extracts of Achillea millifolium in diabetic rats. Chinese J. Nat. Med. 2012, 10, 185–189. [Google Scholar]
- Zolghadri, Y.; Fazeli, M.; Kooshki, M.; Shomali, T.; Karimaghayee, N.; Dehghani, M. Achillea millefolium L. hydro-alcoholic extract protects pancreatic cells by down regulating IL-1β and iNOS gene expression in diabetic rats. Int. J. Mol. Cell. Med. 2014, 3, 255–262. [Google Scholar]
- Aljamal, A. Effects of bay leaves on blood glucose and lipid profiles on the patients with type 1 diabetes. World Acad. Sci. Eng. Technol. 2010, 69, 211–214. [Google Scholar]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev. Diabetic Studies RDS 2014, 11, 258–266. [Google Scholar] [CrossRef]
- Andallu, B.; Ramya, V. Antihyperglycemic, cholesterol-lowering and HDL-raising effects of cumin (Cuminum cyminum) seeds in type-2 diabetes. J. Nat. Remed. 2007, 7, 142–149. [Google Scholar]
- Jafari, S.; Sattari, R.; Ghavamzadeh, S.i. Evaluation the effect of 50 and 100 mg doses of Cuminum cyminum essential oil on glycemic indices, insulin resistance and serum inflammatory factors on patients with diabetes type II: A double-blind randomized placebo-controlled clinical trial. J. Trad. Complemen. Med. 2017, 7, 332–338. [Google Scholar] [CrossRef]
- Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Bishak, Y.K.; Jafarabadi, M.A.; Mahluji, S. Anethum graveolens supplementation improves insulin sensitivity and lipid abnormality in type 2 diabetic patients. Pharm. Sci. 2014, 20, 40. [Google Scholar]
- Al Jamal, A. Effect of rosemary (Rosmarinus officinalis) on lipid profiles and blood glucose in human diabetic patients (type-2). African J. Biochem. Res. 2014, 8, 147–150. [Google Scholar]
- Milajerdi, A.; Jazayeri, S.; Hashemzadeh, N.; Shirzadi, E.; Derakhshan, Z.; Djazayeri, A.; Akhondzadeh, S. The effect of saffron (Crocus sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: A triple-blinded randomized clinical trial. J. Res. Med. Sci. 2018, 23, 1–6. [Google Scholar]
- Behradmanesh, S.; Derees, F.; Rafieian-kopaei, M. Effect of Salvia officinalis on diabetic patients. J. Renal Injury Prevention 2013, 2, 51–54. [Google Scholar]
- Kianbakht, S.; Dabaghian, F.H. Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L. leaf extract: A randomized placebo. Controlled clinical trial. Complement. Therapies Med. 2013, 21, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Van Poelje, P.D.; Dang, Q.; Erion, M.D. Fructose-1, 6-bisphosphatase as a therapeutic target for type 2 diabetes. Drug Discovery Today Therapeutic Strategies 2007, 4, 103–109. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Hayden, M.R.; Verchere, C.B. Cholesterol in β-cell dysfunction: The emerging connection between HDL cholesterol and Type 2 diabetes. Curr. Diabetes Rep. 2010, 10, 55–60. [Google Scholar] [CrossRef]
- Chui, P.C.; Guan, H.-P.; Lehrke, M.; Lazar, M.A. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Invest. 2005, 115, 2244–2256. [Google Scholar] [CrossRef]
- Senthil, S.L.; Raghu, C.; Arjun, H.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydrate Polymers 2019, 209, 350–355. [Google Scholar]
- Sánchez-Pérez, A.; Muñoz, A.; Peña-García, J.; den-Haan, H.; Bekas, N.; Katsikoudi, A.; Tzakos, A.G.; Péréz-Sánchez, H. DIA-DB: A Web-Accessible Database for the Prediction of Diabetes Drugs. In Proceedings of International Conference on Bioinformatics and Biomedical Engineering; Springer: New York, NY, USA, 2015; pp. 655–663. [Google Scholar]
- Oosterveer, M.H.; Mataki, C.; Yamamoto, H.; Harach, T.; Moullan, N.; van Dijk, T.H.; Ayuso, E.; Bosch, F.; Postic, C.; Groen, A.K. LRH-1–dependent glucose sensing determines intermediary metabolism in liver. J. Clin. Invest. 2012, 122, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, S.; Myers, R.W.; Trujillo, M.E.; Pachanski, M.J.; Malkani, S.; Chen, H.-s.; Chen, Z.; Campbell, B.; Eiermann, G.J. Discovery of orally active hepatoselective glucokinase activators for treatment of Type II Diabetes Mellitus. Bioorganic Med. Chem. Lett. 2017, 27, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, T.; Saramäki, A.; Malinen, M.; Rieck, M.; Väisänen, S.; Huotari, A.; Herzig, K.-H.; Müller, R.; Carlberg, C. Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor β/δ. J. Mol. Biol. 2007, 372, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Hieronimus, A.; Lamprinou, A.; Heni, M.; Hatziagelaki, E.; Ullrich, S.; Stefan, N.; Staiger, H.; Häring, H.-U.; Fritsche, A. Peroxisome proliferator-activated receptor gamma (PPARG) modulates free fatty acid receptor 1 (FFAR1) dependent insulin secretion in humans. Mol. Metab. 2014, 3, 676–680. [Google Scholar] [CrossRef]
- Kota, B.P.; Huang, T.H.-W.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectrum 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Wagner, R.; Kaiser, G.; Gerst, F.; Christiansen, E.; Due-Hansen, M.E.; Grundmann, M.; Machicao, F.; Peter, A.; Kostenis, E.; Ulven, T. Reevaluation of fatty acid receptor 1 as a drug target for the stimulation of insulin secretion in humans. Diabetes 2013, 62, 2106–2111. [Google Scholar] [CrossRef]
- Stulnig, T.; Waldhäusl, W. 11β-hydroxysteroid dehydrogenase type 1 in obesity and type 2 diabetes. Diabetologia 2004, 47, 1–11. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Herman, M.A.; Kahn, B.B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006, 116, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–34. [Google Scholar] [PubMed]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martinez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Therapeutic Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, N.H. Pyruvate dehydrogenase kinases: Therapeutic targets for diabetes and cancers. Diabetes Metab. J. 2015, 39, 188–197. [Google Scholar] [CrossRef]
- Martin, J.; Veluraja, K.; Ross, K.; Johnson, L.; Fleet, G.; Ramsden, N.; Bruce, I.; Orchard, M.; Oikonomakos, N. Glucose analog inhibitors of glycogen phosphorylase: The design of potential drugs for diabetes. Biochemistry 1991, 30, 10101–10116. [Google Scholar] [CrossRef]
- Mellado-Gil, J.M.; Cobo-Vuilleumier, N.; Gauthier, B.R. Islet B-cell mass preservation and regeneration in diabetes mellitus: Four factors with potential therapeutic interest. J. Transplantation 2012, 2012, 230870. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Med. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Csermely, P.; Agoston, V.; Pongor, S. The efficiency of multi-target drugs: The network approach might help drug design. Trends Pharmacol. Sci. 2005, 26, 178–182. [Google Scholar] [CrossRef]
- Peters, J.-U. Polypharmacology–foe or friend? J. Med. Chem. 2013, 56, 8955–8971. [Google Scholar] [CrossRef]
- Reddy, A.S.; Zhang, S. Polypharmacology: Drug discovery for the future. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. [Google Scholar] [CrossRef]
- Xu, J.; Han, J.; Epstein, P.N.; Liu, Y.Q. Regulation of PDK mRNA by high fatty acid and glucose in pancreatic islets. Biochem. Biophys. Res. Commun. 2006, 344, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Tso, S.-C.; Chuang, J.L.; Gui, W.-J.; Lou, M.; Sharma, G.; Khemtong, C.; Qi, X.; Wynn, R.M.; Chuang, D.T. Targeting hepatic pyruvate dehydrogenase kinases restores insulin signaling and mitigates ChREBP-mediated lipogenesis in diet-induced obese mice. Mol. Metab. 2018, 12, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Barish, G.D.; Wang, Y.-X. PPARs and the complex journey to obesity. Keio J. Med. 2004, 53, 53–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoon, S.-Y.; Lee, J.H.; Kwon, S.J.; Kang, H.J.; Chung, S.J. Ginkgolic acid as a dual-targeting inhibitor for protein tyrosine phosphatases relevant to insulin resistance. Bioorganic Chem. 2018, 81, 264–269. [Google Scholar] [CrossRef]
- Abbas, G.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorganic Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Zhu, Q.; Ge, F.; Dong, Y.; Sun, W.; Wang, Z.; Shan, Y.; Chen, R.; Sun, J.; Ge, R.-S. Comparison of flavonoids and isoflavonoids to inhibit rat and human 11β-hydroxysteroid dehydrogenase 1 and 2. Steroids 2018, 132, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tamori, Y.; Sakaue, H.; Kasuga, M. RBP4, an unexpected adipokine. Nat. Med. 2006, 12, 30–31. [Google Scholar] [CrossRef]
- Berry, D.C.; Noy, N. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 168–176. [Google Scholar] [CrossRef]
- Eleazu, C.; Charles, A.; Eleazu, K.; Achi, N. Free fatty acid receptor 1 as a novel therapeutic target for type 2 diabetes mellitus-current status. Chem. Biol. Interact. 2018, 289, 32–39. [Google Scholar] [CrossRef]
- Qiang, G.; Xue, S.; Yang, J.J.; Du, G.; Pang, X.; Li, X.; Goswami, D.; Griffin, P.R.; Ortlund, E.A.; Chan, C.B. Identification of a small molecular insulin receptor agonist with potent antidiabetes activity. Diabetes 2014, 63, 1394–1409. [Google Scholar] [CrossRef]
- Chen, L.; Lu, X.; El-Seedi, H.; Teng, H. Recent advances in the development of sesquiterpenoids in the treatment of type 2 diabetes. Trends Food Sci. Technol. 2019, 88, 46–56. [Google Scholar] [CrossRef]
- Brahmachari, G. Bio-flavonoids with promising antidiabetic potentials: A critical survey. Res. Signpost 2011, 661, 187–212. [Google Scholar]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Reis Bonorino Figueiredo, M.S.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Mena Barreto Silva, F.R. Flavonoids: Cellular and molecular mechanism of action in glucose homeostasis. Mini Rev. Med. Chem. 2008, 8, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, E.; Souard, F.; Faure, P.; Boumendjel, A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: Molecules of interest and structure-activity relationship. Curr. Med. Chem. 2011, 18, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Bonfigli, A.; Genovese, S.; De Nigris, V.; Ceriello, A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutri. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Google Scholar. Available online: https://scholar.google.com (accessed on 15 November 2018).
- ScienceDirect. Available online: https://www.sciencedirect-com.uplib.idm.oclc.org/ (accessed on 15 November 2018).
- FooDB. Available online: Foodb.ca (accessed on 15 November 2018).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. PubChem substance and compound databases. Nucl. Acids Res. 2015, 44, D1202–D1213. [Google Scholar] [CrossRef]
- ACD/Chemsketch, version 12.02; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2015.
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504, Cytoscape, version 3.4.0; Cytoscape Consortium: San Diego, CA, USA, 2016. [Google Scholar] [CrossRef]
- Schrödinger Canvas Suite, version 4.0.012; Schrödinger, LLC: New York, NY, USA, 2019.
Sample Availability: Not available. |

| Plant Name | Common Name | Part Evaluated | In Vitro Anti-Diabetic Effects | In Vivo Anti-Diabetic Effects | References |
|---|---|---|---|---|---|
| Pimenta Dioica | Allspice | Berries | Alpha-glucosidase and alpha-amylase inhibitory, increased insulin-stimulated glucose metabolism in adipocytes | Streptozotocin-induced diabetic rats-improves antioxidant status | [27,28,29] |
| Pimpinella anisum | Aniseed | Seeds | Alpha-glucosidase, alpha-amylase, HMGR and pancreatic lipase inhibitory activity | Diabetic patients-reduced hyperglycemia, reduced hyperlipidemia, improved antioxidant status | [30,31,32] |
| Ocimum basillicum | Basil | Leaves | Alpha-glucosidase, alpha-amylase, aldose reductase, pancreatic lipase inhibitory activity, increases insulin-stimulated glucose metabolism in adipocytes, increase GLUT4 translocation | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, improved antioxidant status, increased liver glycogen content, improved liver function | [27,33,34,35,36,37,38,39,40,41,42] |
| Laurus nobilis | Bay leaves | Leaves | Alpha-glucosidase inhibitory activity; increases insulin-stimulated glucose metabolism in adipocytes | Type 2 diabetic patients-reduced hyperglycemia, reduced hyperlipidemia | [27,43,44,45] |
| Piper nigrum | Black pepper | Fruit and leaves | Alpha-glucosidase, alpha-amylase and aldose reductase inhibitory activity, increased glucose consumption by adipocytes, induced transactivation of PPARA | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status, improved liver function | [40,42,46,47,48,49,50,51,52,53,54] |
| Carum carvi | Caraway | Fruit/seeds | Induced transactivation of PPARA | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status | [49,55,56,57,58,59] |
| Elettaria cardamomum | Cardamom | Seeds and leaves | No significant studies identified | Alloxan-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, decreased plasma insulin levels, improved liver function | [60,61,62,63] |
| Cinnamomum verum | Cinnamon | Bark | Alpha-glucosidase, alpha-amylase, aldose reductase inhibitory activity, increased insulin-stimulated glucose metabolism in adipocytes, increased expression and translocation of GLUT4 and GLUT1, induced transactivation of PPARA and PPARG | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased plasma insulin levels, improved liver function, increased GLP1 levels, increased pyruvate kinase activity, decreased PEPCK activity | [27,42,60,64,65,66,67,68,69] |
| Syzygium aromaticum | Clove | Flower buds | Alpha-glucosidase, alpha-amylase, PEPCK and G6Pase inhibitory activity, increased insulin-stimulated glucose metabolism in adipocytes, induced transactivation of PPARG | Streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, improved antioxidant status, improved liver function, reduced expression of GLUT2, SGLT1, alpha-amylase and alpha-glucosidase in rat small intestine, increased glycogen content of liver and muscles, increased activity of hexokinase in liver and muscle | [27,68,69,70,71,72,73,74,75,76,77,78,79] |
| Cuminum cyminum | Cumin | Seeds | Alpha-glucosidase, alpha-amylase, aldose reductase inhibitory activity, induced transactivation of PPARG, stimulated glucose uptake in myotubes | Streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, reduced/ increased serum insulin levels depending on model, improved antioxidant status, increased liver and skeletal muscle content | [42,70,80,81,82,83,84,85] |
| Anethum graveolens | Dill | Aerial parts and seeds | No significant studies identified | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, improved antioxidant status | [86,87,88,89] |
| Foeniculum vulgare | Fennel | Seeds and leaves | Alpha-glucosidase, alpha-amylase, aldose reductase inhibitory activity, increased glucose consumption by adipocytes | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status, improved liver function, increased liver glycogen content, increased liver and kidney hexokinase activity | [40,42,90,91,92,93,94,95] |
| Trigonella foenum-graecum | Fenugreek | Seeds | Alpha-glucosidase, alpha-amylase, aldose reductase, pancreatic lipase inhibitory activity, induced transactivation of PPARG, PPARD and PPARA | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status, improved liver function, increased liver, muscle and kidney glycogen content, reduced activity of intestinal maltase, sucrase and lactase, intestinal lipase, alpha-amylase, glycogen phosphorylase and G6Pase, increased activity of glycogen synthase, hexokinase, PPARG, PPARA and glucose-6-phosphate dehydrogenase | [42,69,96,97,98,99,100,101,102,103,104,105,106] |
| Zingiber officinale | Ginger | Root | Alpha-glucosidase, alpha-amylase, aldose reductase, pancreatic lipase inhibitory activity, increased GLUT4, increased glucose consumption by adipose tissues | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved liver function, increased activity of liver glucokinase, phosphofructokinase, and pyruvate kinase | [65,101,107,108,109,110,111,112] |
| Humulus lupulus | Hops | Cones and leaves | Alpha-glucosidase, alpha-amylase, aldose reductase, pancreatic lipase inhibitory activity, induced PPARG and PPARA transactivation; induced FXR activity | Streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased hepatic glycogen content, reduced expression of hepatic GLUT2 and hepatic acetyl-CoA carboxylase, increased hepatic FAS expression. Diabetic KK-Ay mice-reduced hyperglycemia, reduced hyperlipidemia, increased expression of acyl-CoA oxidase, fatty acid translocase, lipoprotein lipase and PPARA, reduced expression of SRE-BP1, FAS, AceCS, SCD-1, ACL, PEPCK, G6Pase, and FBP1. | [113,114,115,116,117,118,119,120] |
| Melissa officinalis | Lemon balm | Leaves | Alpha-glucosidase, alpha-amylase, pancreatic lipase inhibitory activity, induced activation of PPARA, PPARD, and PPARG, increased glucose consumption through adipocytes, increased expression of SREBP1, FABP4, fatty acid transport protein 4, CD36 molecule, PDK4, LXRA, lipogenic stearoyl CoA desaturase | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels | [40,101,121,122,123,124,125] |
| Cymbopogon citratus | Lemongrass | Leaves | Alpha-glucosidase, alpha-amylase, aldose reductase inhibitory activity | Poloxamer-47-induced type 2 diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, reduced serum insulin levels and insulin resistance, improved antioxidant status, increased GLP1 expression | [126,127,128,129,130] |
| Glycyrrhiza glabra | Liquorice | Root | Alpha-glucosidase, alpha-amylase, aldose reductase, PTP1B inhibitory activity, induced PPARG activation, increased insulin-stimulated glucose uptake by adipocytes, stimulated glucose-mediated insulin secretion from pancreatic islet cells, increased the expression of PDX-1 and GCK | Streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased/decreased serum insulin levels depending on model, improved antioxidant status, improved liver function, increased liver glycogen content, increased expression of PPARG and GLUT4 in muscles | [64,131,132,133,134,135,136,137,138,139,140,141,142,143] |
| Origanum marjorana | Marjoram | Leaves | Alpha-glucosidase, aldose reductase, DPP4, PTP1B inhibitory activity, induced activation of PPARA and PPARG; | Streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased/ decreased serum insulin levels depending on model, improved liver function, increased liver glycogen content, increased expression of adiponectin, lipoprotein lipase and PPARG in adipose tissue, decreased expression of leptin | [84,144,145,146,147,148,149] |
| Myristica fragrans | Nutmeg | Seed | Alpha-glucosidase, alpha-amylase, PTP1B inhibitory activity, induced PPARG and PPARA activation, increased expression of lipoprotein lipase, FAS, aP2, IRS2, CEBPA, GLUT4, CD36, CPT-1, PDK4, and acyl-CoA oxidase, stimulated phosphorylation of AMPK in myoblasts, stimulated the release of insulin from islet cells, increased phosphorylation of insulin receptor in myeloid cells | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, reduced serum insulin levels, increased expression of CD36, CPT-1, PDK4, acyl-CoA oxidase, lipoprotein lipase, glycerol kinase in adipose tissue, increased expression of CPT-1, LPL, ACO and CYP4A in the liver | [49,81,150,151,152,153,154,155,156] |
| Origanum vulgare | Oregano | Leaves | Alpha-glucosidase, alpha-amylase, aldose reductase, DPP4, PTP1B inhibitory activity, induced activation of PPARG and PPARD; stimulated insulin-dependent glucose uptake in adipocytes | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, increased liver and muscle glycogen content, reduced pancreatic alpha-amylase activity | [102,121,144,157,158,159,160,161] |
| Capsicum annuum | Paprika | Fruits | Alpha-glucosidase, alpha-amylase inhibitory activity | Alloxan-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia | [162,163,164] |
| Petroselinum crispum | Parsley | Leaves | No significant studies identified | Streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status, improved liver function, increased liver and muscle glycogen content, increased liver pyruvate kinase activity | [165,166,167,168,169] |
| Rosmarinus officinalis | Rosemary | Leaves | Alpha-glucosidase, alpha-amylase, pancreatic lipase, DPP4, PTP1B inhibitory activity, induced activation of PPARG, increased glucose consumption by adipocytes, increased AMPK phosphorylation in liver cells; decreased expression of G6Pase and acetyl-CoA carboxylase B, increased expression of low-density lipoprotein receptor, SIRT1 and PPARG-coactivator 1, promoted GLUT4 translocation | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status, improved liver function, reduced intestinal glucosidase activity, modulated activity of hexokinase, pyruvate kinase, G6Pase, FBP1, and glycogen metabolism | [40,64,68,101,144,161,170,171,172,173,174,175,176,177] |
| Crocus sativus | Saffron | Flower | Stimulated glucose uptake by skeletal muscle cells, increased phosphorylation of AMPK, increased GLUT4 translocation, induced activation of PPARA | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved antioxidant status improved liver, kidney and pancreatic B-cell function | [178,179,180,181,182,183,184] |
| Salvia officinalis | Sage | Leaves | Alpha-glucosidase, alpha-amylase inhibitory activity, induced activation of PPARG, stimulated insulin-dependent glucose uptake in adipocytes | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved liver and kidney function, increased GLUT4 expression | [62,102,121,185,186,187,188] |
| Illicium verum | Star anise | Fruits and seeds | Alpha-glucosidase inhibitory activity | Streptozotocin-induced diabetic rats-improved oral glucose tolerance test | [152] |
| Thymus vulgaris | Thyme | Aerial parts | Alpha-glucosidase inhibitory activity, induced activation of PPAR, stimulated glucose uptake by adipocytes and myotubes | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, improved antioxidant status, improved liver and kidney functions | [102,189,190,191,192,193] |
| Curcuma longa | Turmeric | Roots | Alpha-glucosidase, alpha-amylase, aldose reductase inhibitory activity, induced activation of PPARG, stimulated insulin secretion from pancreatic cells, stimulated glucose uptake in muscle tissue | Alloxan/streptozotocin-induced diabetic rats/ KK-Ay diabetic mice-reduced hyperglycemia, reduced hyperlipidemia, increased in serum insulin levels, improved antioxidant status, improved liver function, increased activity of cholesterol-7a-hydroxylase and hepatic HMGR | [67,80,194,195,196,197,198,199,200,201,202,203,204,205,206] |
| Achillea millefolium | Yarrow | Aerial parts | Alpha-glucosidase inhibitory activity, increased expression of PPARG and GLUT4, stimulated insulin secretion by pancreatic cells | Alloxan/streptozotocin-induced diabetic rats-reduced hyperglycemia, reduced hyperlipidemia, increased serum insulin levels, improved liver and pancreas function | [207,208,209] |
| Mode of Action | Protein Target | Function | PDB Code | Average Docking Score of Known Drugs (kcal/mol) | Docking Cutoff (kcal/mol) | Total Number of Potential Inhibitors |
|---|---|---|---|---|---|---|
| Regulation of insulin secretion and sensitivity | DPP4 | Degrades and inactivates glucagon-like peptide-1 that stimulates insulin secretion from pancreas [231] | 4A5S | −8.50 | −9.00 | 260 |
| FFAR1 | Binding of free fatty acids to receptor results in increased glucose-stimulated insulin secretion [232] | 4PHU | −10.00 | −10.50 | 6 | |
| HSD11B1 | Coverts inactive glucocorticoid precursors to active glucocorticoids; glucocorticoids counteract the effects of insulin [233] | 4K1L | −9.40 | −10.00 | 114 | |
| INSR | Regulates glucose uptake as well as glycogen, lipid, and protein synthesis [231] | 3EKN | −8.60 | −9.00 | 47 | |
| PTPN9 | Dephosphorylates the insulin receptor, thereby reducing insulin sensitivity [234] | 4GE6 | −7.80 | −8.00 | 246 | |
| RBP4 | Secreted as an adipokine that reduces insulin signaling and promotes gluconeogenesis [235] | 2WR6 | −7.40 | −8.00 | 412 | |
| Regulation of glucose metabolism | AKR1B1 | Catalyzes the reduction of glucose to sorbitol in the polyol pathway, plays a role in diabetic complications [236] | 3G5E | −9.95 | −10.50 | 96 |
| AMY2A | Hydrolyzes alpha-1,4-glycosidic bonds of starch during digestion of starch to glucose [237] | 4GQR | −7.60 | −8.00 | 429 | |
| FBP1 | Catalyzes the second last step in gluconeogenesis [220] | 2JJK | −5.40 | −6.00 | 210 | |
| GCK | Phosphorylates glucose to glucose-6-phosphate for glycolysis or glycogen synthesis [234] | 3IMX | −9.40 | −10.00 | 18 | |
| MGAM | Hydrolyzes 1,4-alpha bonds, the last step in the digestion of starch to glucose [237] | 3L4Y | −6.50 | −7.00 | 592 | |
| PDK2 | Responsible for inactivating the pyruvate dehydrogenase complex that is involved in glucose oxidation [238] | 4MPC | −7.90 | −8.00 | 190 | |
| PYGL | Catalyzes the first step of glycogenolysis by the phosphorolysis of glycogen to glucose-1-phosphate [239] | 3DDS | −8.10 | −8.50 | 113 | |
| Regulation of lipid metabolism | NR5A2 | Regulates the expression of genes involved in bile acid synthesis, cholesterol synthesis, and steroidogenesis [240] | 4DOR | −7.50 | −8.00 | 362 |
| PPARA | Regulates expression of genes involved in lipid metabolism, in particular, the oxidation of fatty acids as well as lipoprotein assembly and lipid transport [241] | 3FEI | −7.60 | −8.00 | 271 | |
| PPARD | Regulates expression of genes involved in fatty acid catabolism [241] | 3PEQ | −9.30 | −10.00 | 60 | |
| PPARG | Regulates expression of genes involved in adipogenesis and lipid metabolism particularly fatty acid transport, lipid droplet formation, triacyglycerol metabolism, as well as lipolysis of triglycerides [241] | 2FVJ | −9.70 | −10.00 | 75 | |
| RXRA | Heterodimerizes with PPARs, thereby initiating gene transcription [241] | 1FM9 | −9.95 | −10.00 | 24 |
| Plant Name | Total Number of Compounds Evaluated | Total Number of Potential Anti-Diabetic Compounds (% of Total) | Compounds with 3 or More Targets |
|---|---|---|---|
| Allspice | 84 | 41 (49%) | 13 |
| Aniseed | 125 | 50 (40%) | 23 |
| Basil | 214 | 58 (27%) | 15 |
| Bay leaves | 179 | 69 (39%) | 19 |
| Black Pepper | 183 | 84 (46%) | 31 |
| Caraway | 185 | 43 (23%) | 15 |
| Cardamom | 141 | 29 (21%) | 2 |
| Cinnamon | 74 | 26 (35%) | 18 |
| Clove | 147 | 59 (40%) | 21 |
| Cumin | 146 | 38 (26%) | 19 |
| Dill | 168 | 65 (39%) | 27 |
| Fennel | 123 | 66 (54%) | 42 |
| Fenugreek | 110 | 55 (50%) | 47 |
| Ginger | 326 | 80 (25%) | 8 |
| Hops | 98 | 60 (61%) | 32 |
| Lemon balm | 118 | 53 (45%) | 35 |
| Lemongrass | 132 | 55 (42%) | 28 |
| Liquorice | 215 | 157 (73%) | 135 |
| Marjoram | 103 | 31 (30%) | 18 |
| Nutmeg | 96 | 25 (26%) | 9 |
| Oregano | 177 | 71 (40%) | 34 |
| Paprika | 166 | 15 (9%) | 0 |
| Parsley | 78 | 28 (36%) | 12 |
| Rosemary | 158 | 85 (54%) | 43 |
| Saffron | 146 | 34 (23%) | 21 |
| Sage | 162 | 80 (49%) | 35 |
| Star anise | 69 | 27 (39%) | 10 |
| Thyme | 204 | 78 (38%) | 38 |
| Turmeric | 239 | 110 (46%) | 29 |
| Yarrow | 148 | 72 (49%) | 27 |
| Plant | Number of Clusters | Number of Compounds in Major Clusters | Representative Compounds (Cluster Centroids) | ||
|---|---|---|---|---|---|
| Allspice | 6 | 20 |  | ||
| Aniseed | 13 | 18 |  | ||
| Basil | 5 | 50 |  | ||
| Bay leaves | 5 | 41; 22 |  |  | |
| Black pepper | 9 | 36; 24 |  |  | |
| Clove | 6 | 21; 20 |  |  | |
| Cumin | 13 | 10 |  | ||
| Dill | 17 | 10; 10 |  |  | |
| Fennel | 6 | 52 |  | ||
| Fenugreek | 11 | 23; 15 |  |  | |
| Ginger | 8 | 51; 20 | 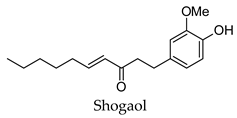 | 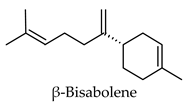 | |
| Hops | 18 | 19; 11 | 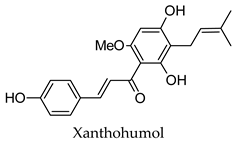 | 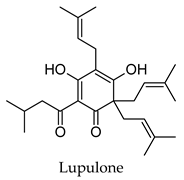 | |
| Lemon balm | 13 | 14; 12 | 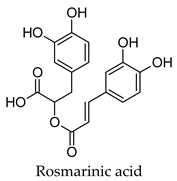 | 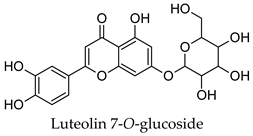 | |
| Lemongrass | 6 | 22; 19 | 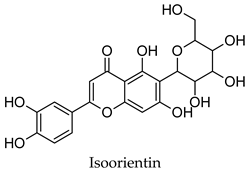 | 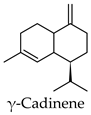 | |
| Liquorice | 12 | 53; 36; 30 | 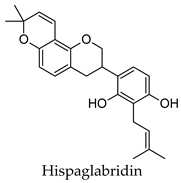 | 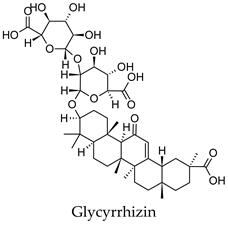 | 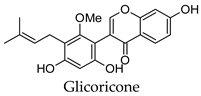 |
| Parsley | 7 | 11; 10 | 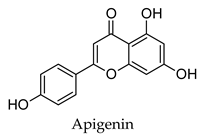 |  | |
| Rosemary | 20 | 28 |  | ||
| Saffron | 5 | 27 |  | ||
| Sage | 5 | 39; 21 |  |  | |
| Thyme | 13 | 22; 21 |  |  | |
| Turmeric | 25 | 25; 15 |  |  | |
| Yarrow | 10 | 16 |  | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.S.P.; Banegas-Luna, A.J.; Peña-García, J.; Pérez-Sánchez, H.; Apostolides, Z. Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules 2019, 24, 4030. https://doi.org/10.3390/molecules24224030
Pereira ASP, Banegas-Luna AJ, Peña-García J, Pérez-Sánchez H, Apostolides Z. Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules. 2019; 24(22):4030. https://doi.org/10.3390/molecules24224030
Chicago/Turabian StylePereira, Andreia S.P., Antonio J. Banegas-Luna, Jorge Peña-García, Horacio Pérez-Sánchez, and Zeno Apostolides. 2019. "Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening" Molecules 24, no. 22: 4030. https://doi.org/10.3390/molecules24224030
APA StylePereira, A. S. P., Banegas-Luna, A. J., Peña-García, J., Pérez-Sánchez, H., & Apostolides, Z. (2019). Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules, 24(22), 4030. https://doi.org/10.3390/molecules24224030