The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage
Abstract
1. Introduction
2. Importance of Pharmacokinetic of Curcumin for Its Pharmaceutical Effects
3. Curcumin’s Effect on Adipogenic Differentiation
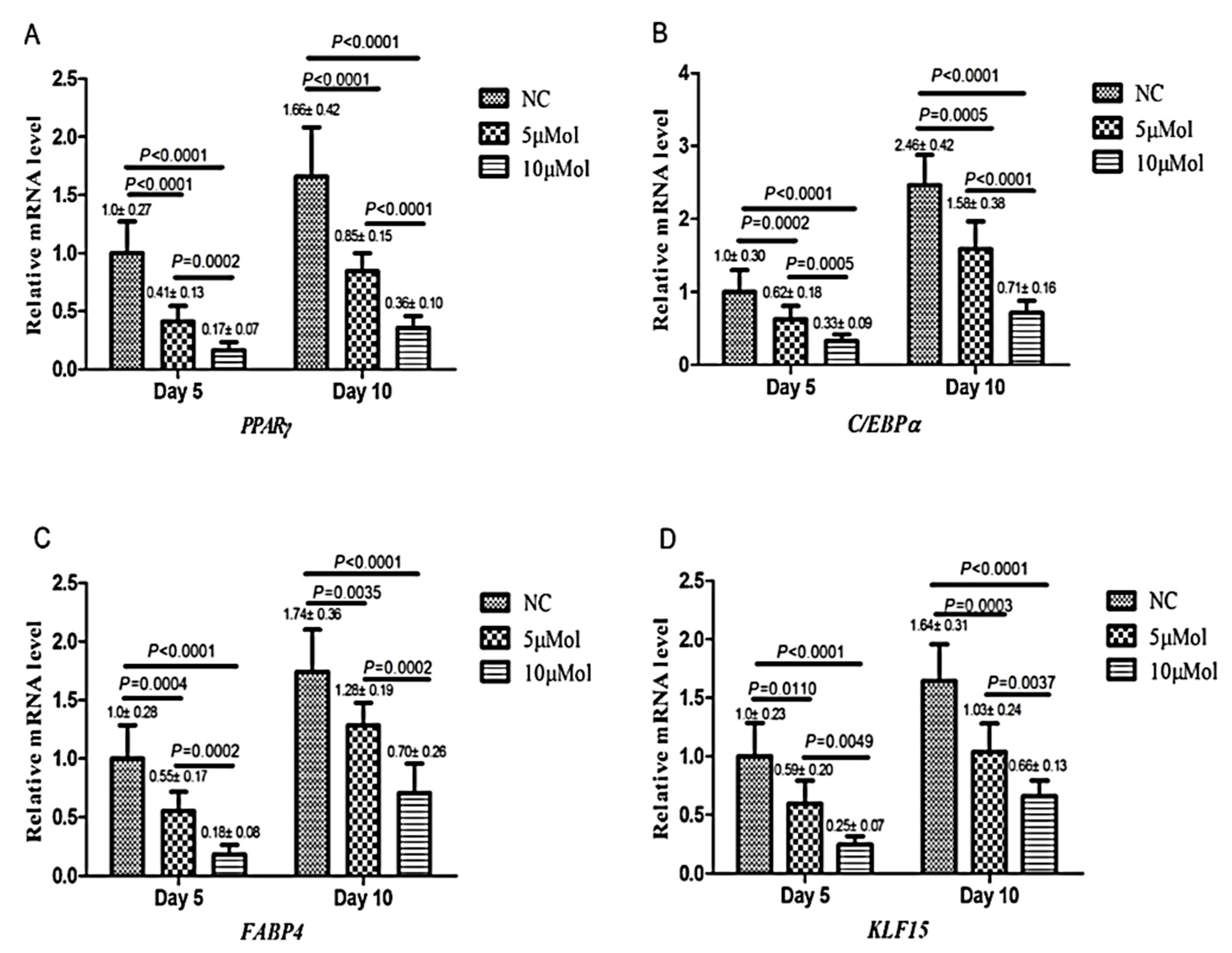
3.1. Inhibition of Adipogenic Differentiation
3.1.1. AMPK Modulation
3.1.2. Wnt Signaling Pathway Activation
3.1.3. Fatty Acid Synthase Inhibition
3.1.4. Interaction with PPARγ Receptors
3.1.5. Inhibition of Mitotic Clonal Expansion
4. Curcumin’s Effects on Osteogenic Differentiation
4.1. Induction of Osteogenic Differentiation
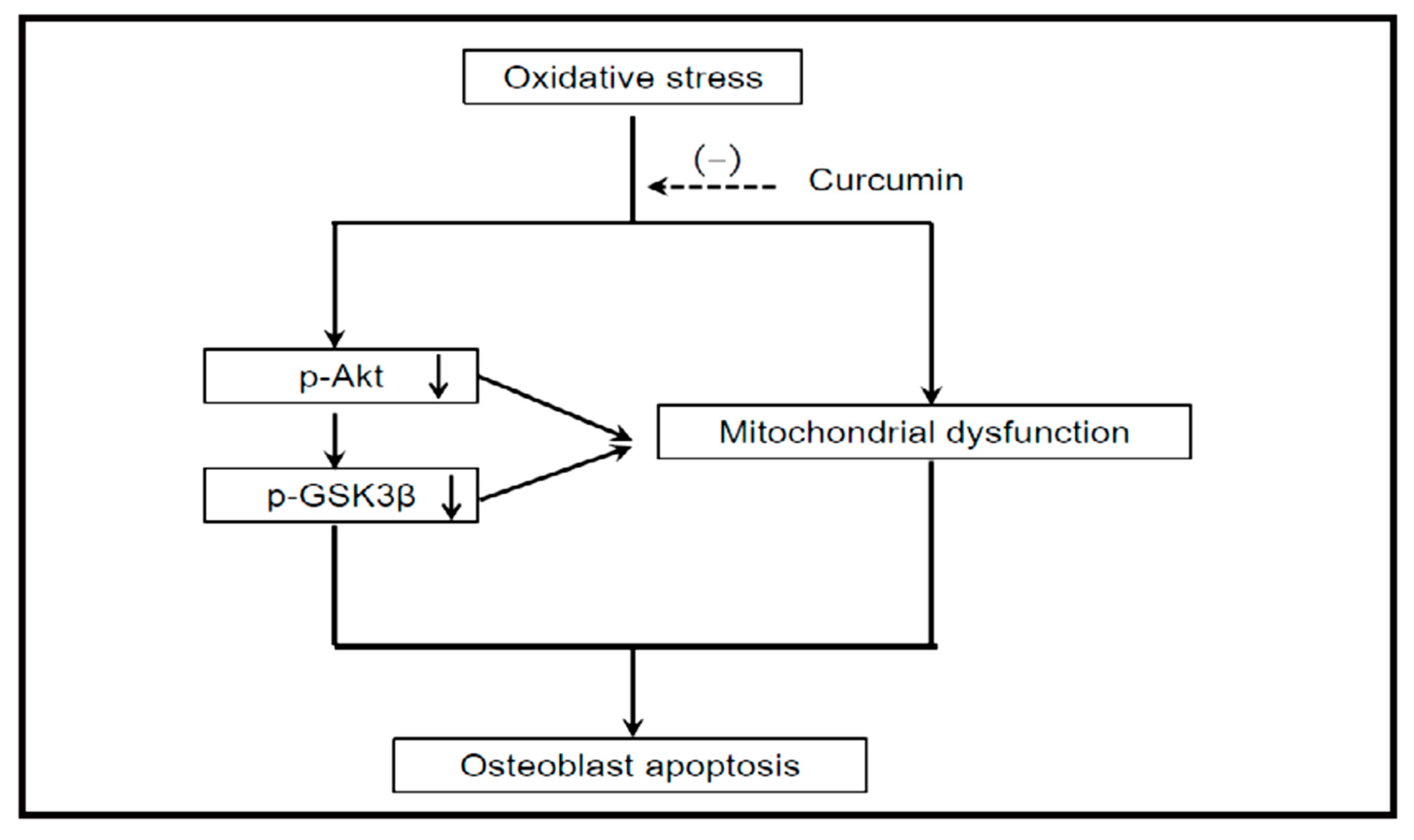
4.1.1. Akt/GSK3β Signaling Pathway Activation
4.1.2. Maintaining Wnt/β-Catenin and Wnt/TCF Pathways
4.1.3. Keap1/Nrf2/HO-1 Signaling Pathway Activation
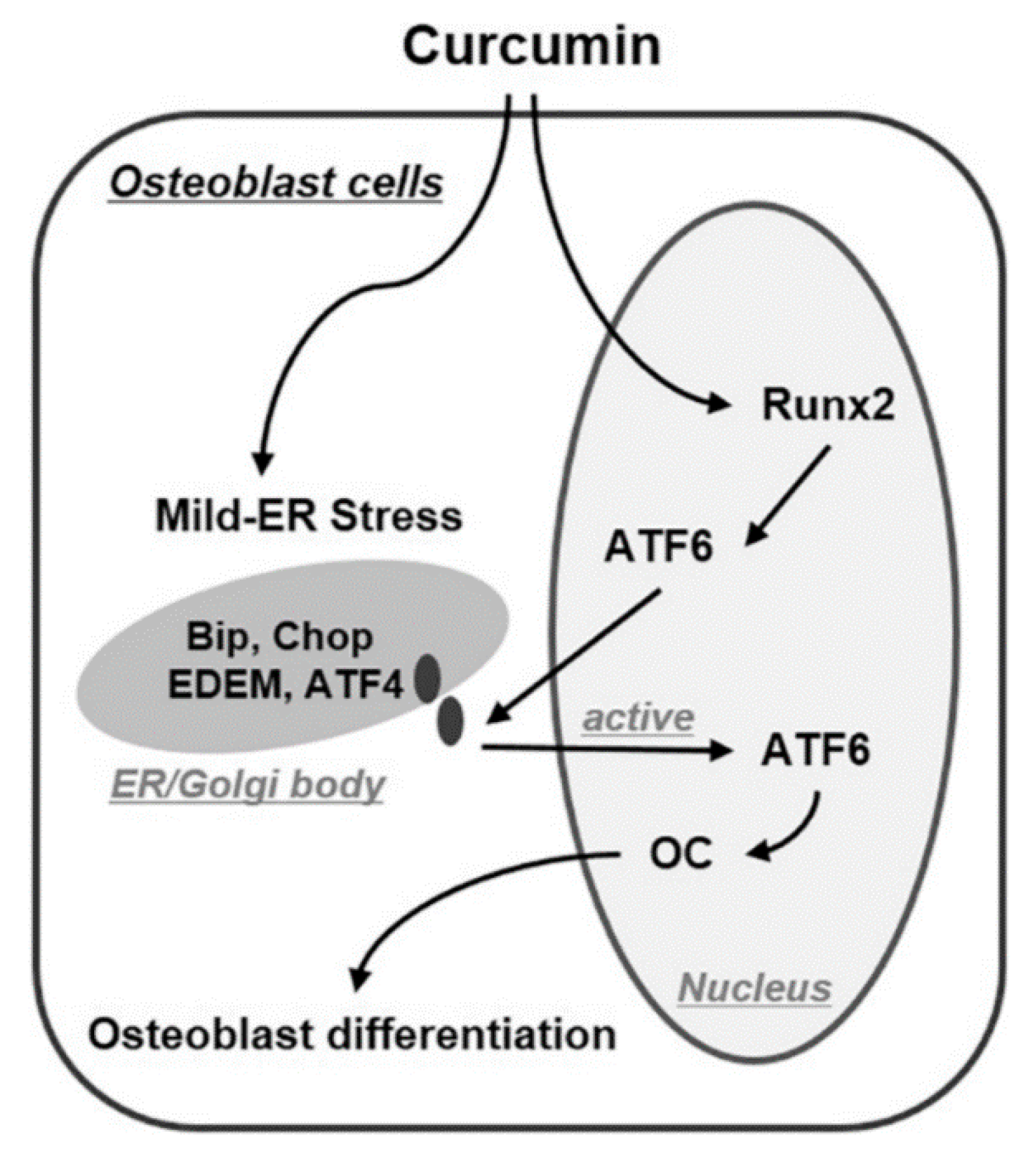
4.1.4. ER Stress Pathway Activation
4.2. Inhibition of Osteoblast Differentiation
JNK/Bax Signaling Suppression
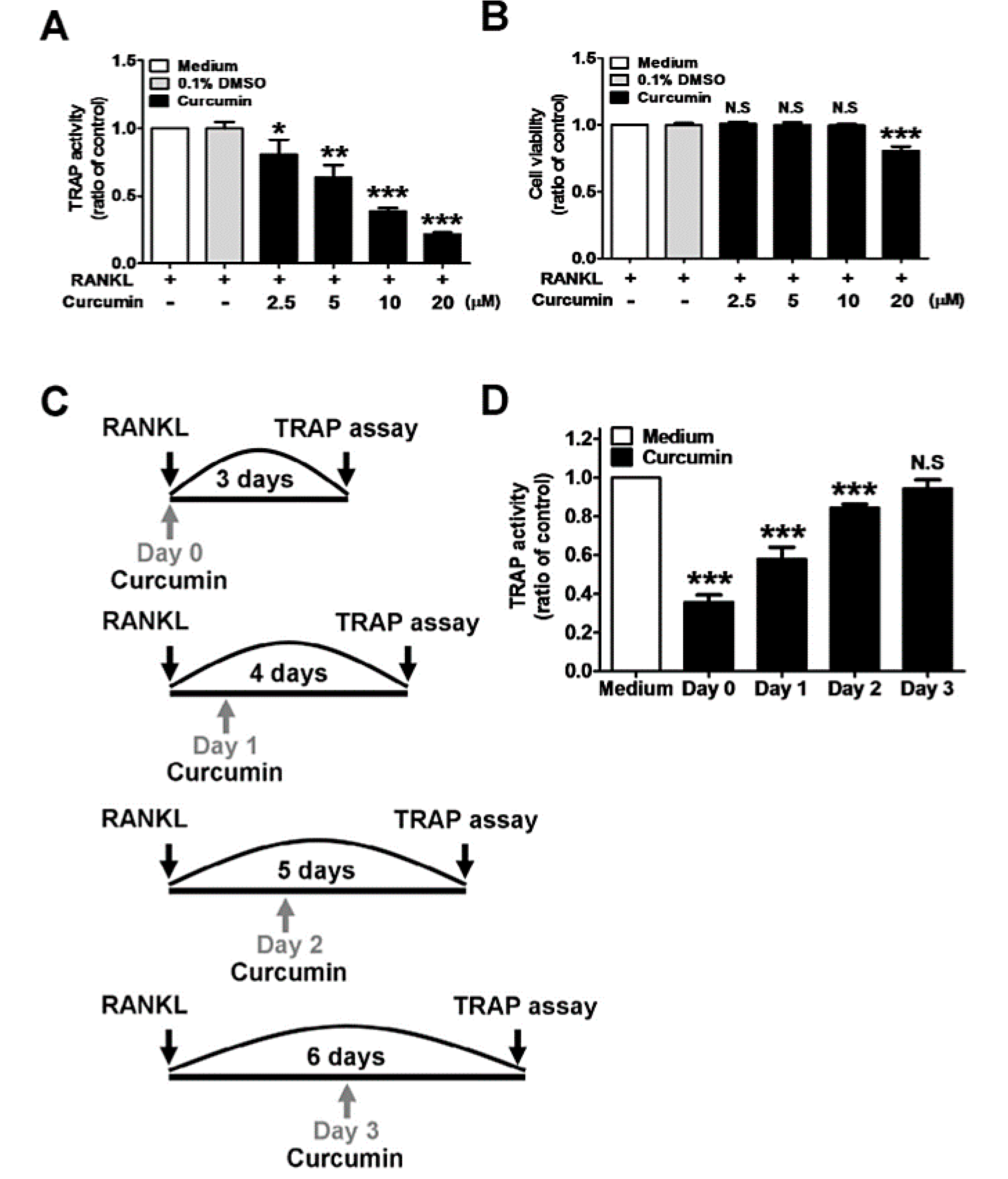
4.3. Inhibition of Osteoclast Differentiation
4.3.1. RANKL-Induced Signaling Pathway Inhibition
4.3.2. NF-κB Signaling Pathway Inhibition
4.3.3. RANKL/RANK Signaling Pathway Inhibition
4.3.4. Wnt/β-Catenin Signaling Pathway Activation
5. Curcumin Affects Chondrogenic Differentiation
5.1. Induction of Chondrogenic Differentiation
5.2. Inhibition of Chondrogenic Differentiation
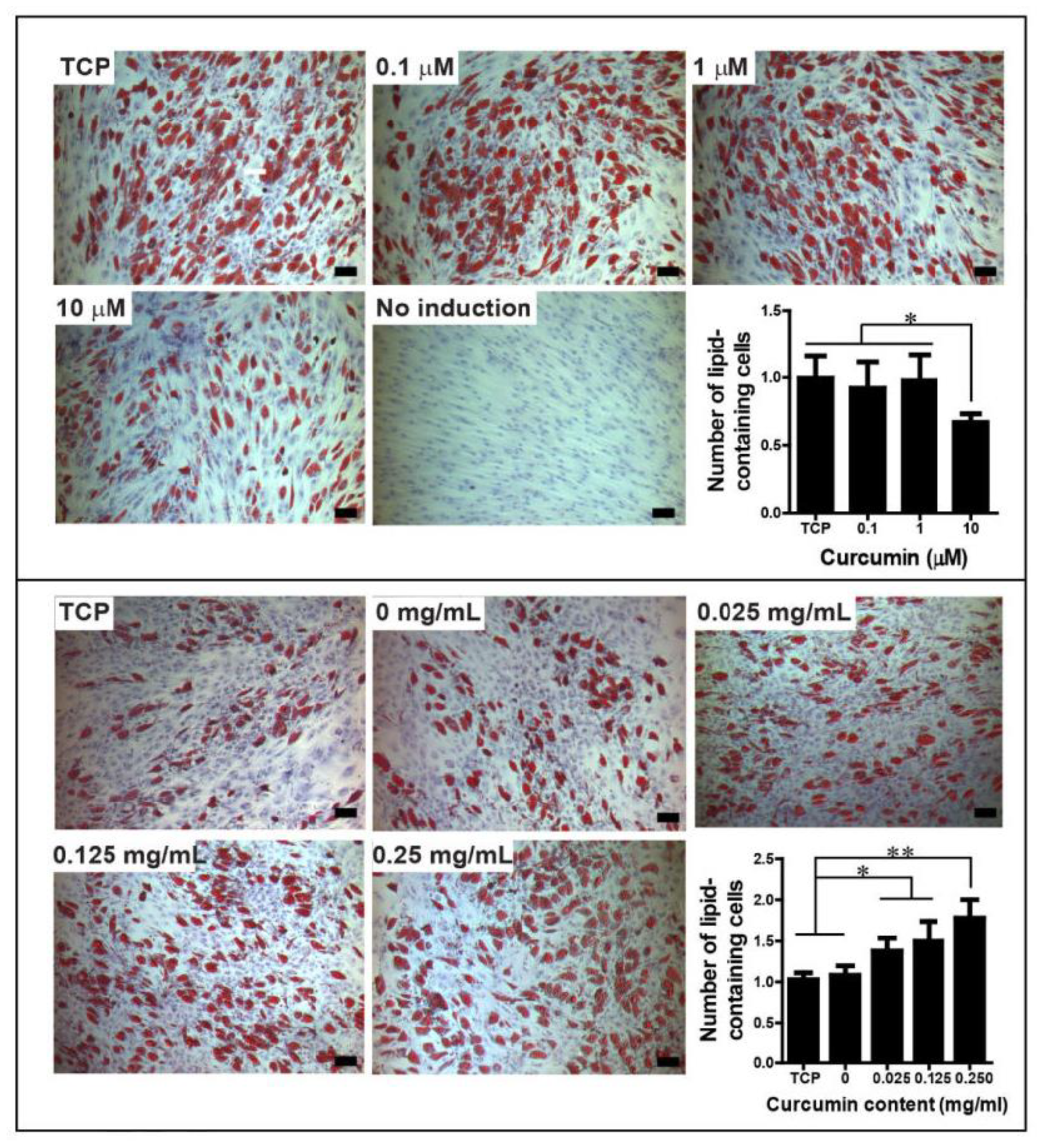
6. Effect of Curcumin Dose on Mesodermal Differentiation of Cells
7. Effect of Modified Forms of Curcumin on Mesodermal Differentiation
8. Summary and Clinical Applications
Funding
Conflicts of Interest
References
- Thaloor, D.; Singh, A.K.; Sidhu, G.S.; Prasad, P.V.; Kleinman, H.K.; Maheshwari, R.K. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ.-Publ. Am. Assoc. Cancer Res. 1998, 9, 305–312. [Google Scholar]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.S.; Pirro, M.; Majeed, M.; Sahebkar, A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017, 33, 55–63. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Rezaee, R.; Momtazi, A.A.; Monemi, A.; Sahebkar, A. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol. Res. 2017, 117, 218–227. [Google Scholar] [CrossRef]
- Sahebkar, A.; Cicero, A.F.G.; Simental-Mendía, L.E.; Aggarwal, B.B.; Gupta, S.C. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016, 107, 234–242. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. (Philadelphia, Pa.) 2011, 4, 1158–1171. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 279–312. [Google Scholar] [CrossRef]
- Deljoo, S.; Rabiee, N.; Rabiee, M. Curcumin-hybrid Nanoparticles in Drug Delivery System (Review). Asian J. Nanosci. Mater. 2019, 2, 66–91. [Google Scholar] [CrossRef]
- Karataş, D.; Tekin, A.; Bahadori, F.; Çelik, M.S. Interaction of curcumin in a drug delivery system including a composite with poly(lactic-co-glycolic acid) and montmorillonite: A density functional theory and molecular dynamics study. J. Mater. Chem. B 2017, 5, 8070–8082. [Google Scholar] [CrossRef]
- Peddada, K.V.; Peddada, K.V.; Shukla, S.K.; Mishra, A.; Verma, V. Role of Curcumin in Common Musculoskeletal Disorders: A Review of Current Laboratory, Translational, and Clinical Data. Orthop. Surg. 2015, 7, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, Y.; Li, B.; Cheng, M.; Wang, J.; Zhang, X. Curcumin Attenuation of Wear Particle-Induced Osteolysis via RANKL Signaling Pathway Suppression in Mouse Calvarial Model. Mediat. Inflamm. 2017, 2017, 5784374. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. (Lausanne) 2017, 4, 234. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; Deng, Y.; Verron, E. Therapeutic actions of curcumin in bone disorders. Bonekey Rep. 2016, 5, 793. [Google Scholar] [CrossRef]
- Maran, A.; Yaszemski, M.J.; Kohut, A.; Voronov, A. Curcumin and Osteosarcoma: Can Invertible Polymeric Micelles Help? Materials (Basel) 2016, 9, 520. [Google Scholar] [CrossRef]
- Liu, Y.-W.; An, S.-B.; Yang, T.; Xiao, Y.-J.; Wang, L.; Hu, Y.-H. Protection Effect of Curcumin for Macrophage-Involved Polyethylene Wear Particle-Induced Inflammatory Osteolysis by Increasing the Cholesterol Efflux. Med. Sci. Monit. 2019, 25, 10–20. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [CrossRef]
- Klickovic, U.; Doberer, D.; Gouya, G.; Aschauer, S.; Weisshaar, S.; Storka, A.; Bilban, M.; Wolzt, M. Human Pharmacokinetics of High Dose Oral Curcumin and Its Effect on Heme Oxygenase-1 Expression in Healthy Male Subjects. BioMed Res. Int. 2014, 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Prev. Biomark. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Alappat, L.; Awad, A.B. Curcumin and obesity: Evidence and mechanisms. Nutr. Rev. 2010, 68, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G. Curcumin and obesity. BioFactors (Oxford, England) 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2018, 8, e2559. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, W.S.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Curcumin Exerts Antidifferentiation Effect through AMPKα-PPAR-γ in 3T3-L1 Adipocytes and Antiproliferatory Effect through AMPKα-COX-2 in Cancer Cells. J. Agric. Food Chem. 2009, 57, 305–310. [Google Scholar] [CrossRef]
- Wang, T.; Yan, R.; Xu, X.; Li, X.; Cao, L.; Gao, L.; Liu, J.; Zhou, X.; Yu, H.; Wang, X.; et al. Curcumin represses adipogenic differentiation of human bone marrow mesenchymal stem cells via inhibiting kruppel-like factor 15 expression. Acta Histochem. 2019, 121, 253–259. [Google Scholar] [CrossRef]
- Soltani, A.; Salmaninejad, A.; Jalili-Nik, M.; Soleimani, A.; Javid, H.; Hashemy, S.I.; Sahebkar, A. 5’-Adenosine monophosphate-activated protein kinase: A potential target for disease prevention by curcumin. J. Cell Physiol. 2019, 234, 2241–2251. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/β-catenin signaling. Am. J. Physiol.-Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef]
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013, 5, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of Adipogenesis by Wnt Signaling. Science 2000, 289, 950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, X.B.; Ye, F.; Tian, W.X. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol. Cell. Biochem. 2011, 351, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin inhibits 3T3-L1 preadipocyte proliferation by mechanisms involving post-transcriptional p27 regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Migueliz, I.; Romo-Hualde, A.; Lopez-Yoldi, M.; Martinez, J.A.; Vizmanos, J.L.; Milagro, F.I.; Gonzalez-Navarro, C.J. Phenolic Compounds Inhibit 3T3-L1 Adipogenesis Depending on the Stage of Differentiation and Their Binding Affinity to PPARgamma. Molecules (Basel, Switzerland) 2019, 24, 1045. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdul Qadir, M.; Imtiaz Shafiq, M.; Muddassar, M.; Hameed, A.; Nadeem Arshad, M.; Asiri, A.M. Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase. Acta Pharm. (Zagreb, Croatia) 2017, 67, 385–395. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Chen, Q.; Siddiqui, I.; Sarva, K.; Shankar, S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1). Cell Cycle (Georgetown, Tex.) 2007, 6, 2953–2961. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.W.; Grønborg, M.; Urlaub, H.; Lane, M.D.; Tang, Q.-Q. Role of cdk2 in the sequential phosphorylation/activation of C/EBPβ during adipocyte differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 11597–11602. [Google Scholar] [CrossRef]
- Hatefi, M.; Ahmadi, M.R.H.; Rahmani, A.; Dastjerdi, M.M.; Asadollahi, K. Effects of Curcumin on Bone Loss and Biochemical Markers of Bone Turnover in Patients with Spinal Cord Injury. World Neurosurg. 2018, 114, e785–e791. [Google Scholar] [CrossRef]
- Riva, A.; Togni, S.; Giacomelli, L.; Franceschi, F.; Eggenhoffner, R.; Feragalli, B.; Belcaro, G.; Cacchio, M.; Shu, H.; Dugall, M. Effects of a curcumin-based supplementation in asymptomatic subjects with low bone density: A preliminary 24-week supplement study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1684–1689. [Google Scholar]
- Yang, M.W.; Wang, T.H.; Yan, P.P.; Chu, L.W.; Yu, J.; Gao, Z.D.; Li, Y.Z.; Guo, B.L. Curcumin improves bone microarchitecture and enhances mineral density in APP/PS1 transgenic mice. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-C.; Jung, H.-S.; Kim, K.-T.; Jeon, Y.; Sung, J.-K.; Hwang, J.-H. Therapeutic advantages of treatment of high-dose curcumin in the ovariectomized rat. J. Korean Neurosurg. Soc. 2013, 54, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch. Endocrinol. Metab. 2018, 62, 438–445. [Google Scholar] [CrossRef]
- Cirano, F.R.; Pimentel, S.P.; Casati, M.Z.; Correa, M.G.; Pino, D.S.; Messora, M.R.; Silva, P.H.F.; Ribeiro, F.V. Effect of curcumin on bone tissue in the diabetic rat: Repair of peri-implant and critical-sized defects. Int. J. Oral Maxillofac. Surg. 2018, 47, 1495–1503. [Google Scholar] [CrossRef]
- Son, H.E.; Kim, E.J.; Jang, W.G. Curcumin induces osteoblast differentiation through mild-endoplasmic reticulum stress-mediated such as BMP2 on osteoblast cells. Life Sci. 2018, 193, 34–39. [Google Scholar] [CrossRef]
- Ahmed, M.F.; El-Sayed, A.K.; Chen, H.; Zhao, R.; Yusuf, M.S.; Zuo, Q.; Zhang, Y.; Li, B. Comparison between curcumin and all-trans retinoic acid in the osteogenic differentiation of mouse bone marrow mesenchymal stem cells. Exp. Ther. Med. 2019, 17, 4154–4166. [Google Scholar] [CrossRef]
- Jain, S.; Krishna Meka, S.R.; Chatterjee, K. Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed. Mater. 2016, 11, 055007. [Google Scholar] [CrossRef]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar] [CrossRef]
- Borra, S.K.; Mahendra, J.; Gurumurthy, P.; Jayamathi; Iqbal, S.S.; Mahendra, L. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J. Clin. Diagn. Res. 2014, 8, CC01–CC05. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, N.T.; Cheng, H.S.; Yen, M.L. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J. Cell. Mol. Med. 2018, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxidative Med. Cell. Longev. 2016, 2016, 2989076. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, H.; Shi, Y. Upregulation of heme oxygenase-1 expression by curcumin conferring protection from hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblasts. Cell Biosci. 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ma, J.; Ni, Z.; et al. Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3β Signaling. Cell. Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Muller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, K.w.; He, J.; Niu, Y.; Lu, Y.; Zhang, L.; Wang, T. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J. Anim. Sci. 2018, 96, 867–879. [Google Scholar] [CrossRef]
- Yu, W.; Zha, W.; Ke, Z.; Min, Q.; Li, C.; Sun, H.; Liu, C. Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway. J. Diabetes Res. 2016, 2016, 11. [Google Scholar] [CrossRef]
- Krause, U.; Ryan, D.M.; Clough, B.H.; Gregory, C.A. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Amp Dis. 2014, 5, e1093. [Google Scholar] [CrossRef]
- Tornero-Esteban, P.; Peralta-Sastre, A.; Herranz, E.; Rodriguez-Rodriguez, L.; Mucientes, A.; Abasolo, L.; Marco, F.; Fernandez-Gutierrez, B.; Lamas, J.R. Altered Expression of Wnt Signaling Pathway Components in Osteogenesis of Mesenchymal Stem Cells in Osteoarthritis Patients. PLoS ONE 2015, 10, e0137170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.P.; Paulson, C.; Shao, J.Z.; Zhang, X.; Wu, M.; Chen, W. Wnt and the Wnt signaling pathway in bone development and disease. Front. Biosci. (Landmark edition) 2014, 19, 379–407. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, F.; Gao, Y.; Yin, P.; Pan, C.; Liu, W.; Zhou, Z.; Wang, J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J. Pharmacol. Sci. 2016, 132, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Paital, B.; Jena, S.; Swain, S.S.; Kumar, S.; Yadav, M.K.; Chainy, G.B.N.; Samanta, L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019, 9, 7408. [Google Scholar] [CrossRef] [PubMed]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase-1 in hepatocytes and is protective in simulated cold preservation and warm reperfusion injury. Transplantation 2006, 81, 623–626. [Google Scholar] [CrossRef]
- Li, y.; Zhang, Z.Z. Sustained curcumin release from PLGA microspheres improves bone formation under diabetic conditions by inhibiting the reactive oxygen species production. Drug Des. Dev. Ther. 2018, 12, 1453–1466. [Google Scholar] [CrossRef]
- Hou, M.; Song, Y.; Li, Z.; Luo, C.; Ou, J.S.; Yu, H.; Yan, J.; Lu, L. Curcumin attenuates osteogenic differentiation and calcification of rat vascular smooth muscle cells. Mol. Cell. Biochem. 2016, 420, 151–160. [Google Scholar] [CrossRef]
- Mau, L.P.; Cheng, W.C.; Chen, J.K.; Shieh, Y.S.; Cochran, D.L.; Huang, R.Y. Curcumin ameliorates alveolar bone destruction of experimental periodontitis by modulating osteoclast differentiation, Activation and function. J. Funct. Foods 2016, 22, 243–256. [Google Scholar] [CrossRef]
- Moon, H.J.; Ko, W.K.; Han, S.W.; Kim, D.S.; Hwang, Y.S.; Park, H.K.; Kwon, I.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem. Biophys. Res. Commun. 2012, 418, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.K.; Moon, H.J.; Kim, H.J.; Lee, S.J.; Lee, J.B.; Bae, M.S.; Yi, J.K.; Hwang, Y.S.; Bang, J.B.; et al. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano 2014, 8, 12049–12062. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Su, Y.H.; Lin, Y.J.; Chen, P.J.; Shi, C.S.; Chen, C.N.; Chang, H.I. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 2285–2300. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. (Seoul) 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of Transcription Factor NF-κB Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.; Hu, F.; Brown, A.; Sun, A.; Liotta, D.; Snyder, J.; Yoon, Y.; Shim, H.; Marcus, A.; et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2011, 12, 368–377. [Google Scholar] [CrossRef]
- Kohli, S.S.; Kohli, V.S. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–181. [Google Scholar] [CrossRef]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010, 12, R127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Le, T.T.; Chen, C.; Cheng, J.X.; Kim, K.H. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J. Nutr. Biochem. 2011, 22, 910–920. [Google Scholar] [CrossRef]
- Eom, Y.W.; Woo, H.B.; Ahn, C.M.; Lee, S. Synthesis of curcumin mimics library with α,β-Unsaturated carbonyl aromatic group and their inhibitory effect against adipocyte differentiation of 3T3-L1. Bull. Korean Chem. Soc. 2013, 34, 3923–3926. [Google Scholar] [CrossRef]
- Liu, Y.C.G.; Lerner, U.H.; Teng, Y.T.A. Cytokine responses against periodontal infection: Protective and destructive roles. Periodontology 2000 2010, 52, 163–206. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, V.K.; Kumar, D.; Yadav, P.; Kumar, S.; Beg, M.; Shankar, K.; Varshney, S.; Rajan, S.; Srivastava, A.; et al. Curcumin-3,4-Dichloro Phenyl Pyrazole (CDPP) overcomes curcumin’s low bioavailability, inhibits adipogenesis and ameliorates dyslipidemia by activating reverse cholesterol transport. Metab. Clin. Exp. 2017, 73, 109–124. [Google Scholar] [CrossRef]
- Curylofo-Zotti, F.A.; Elburki, M.S.; Oliveira, P.A.; Cerri, P.S.; Santos, L.A.; Lee, H.M.; Johnson, F.; Golub, L.M.; Rossa, C.J.; Guimaraes-Stabili, M.R. Differential effects of natural Curcumin and chemically modified curcumin on inflammation and bone resorption in model of experimental periodontitis. Arch. Oral Biol. 2018, 91, 42–50. [Google Scholar] [CrossRef]
| Transcription Factors | Enzymes | Protein Kinases | Apoptosis Related Genes and Proteins | Membrane or Soluble Receptors or Coreceptors | Genes | Other Proteins and Cytokines |
|---|---|---|---|---|---|---|
| β-catenin ATF-4 ATF-6 C/EBP Nrf2 NF-κB PPAR-γ Runx2 Osterix NFATc1 c-Fos Smad Tcf7l2 KLF15 Rex1 | COX-2 HO-1 MMP9 MMP3 CPT-1 GPAT-1 IRE-1 Skp2 Cathepsin K TRAP ALP ACC TERT teP1 MnSOD | ERK JNK p38 MAPK FAK PERK Akt GSK3β Cdk PI3K AMPK | Bax Caspase-3 Fas Cidea CHOP RANKL | LRP 5 LRP6 Fz2 Wnt10b OPG DC-STAMP OSCAR | NOX4 miR-17-5p C-myc TR | BMP-2 Cyclin D1 BiP EDEM OCN OPN COL1A1 p27 Ghrelin FABP-2 IL-1β |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage. Molecules 2019, 24, 4029. https://doi.org/10.3390/molecules24224029
Gorabi AM, Kiaie N, Hajighasemi S, Jamialahmadi T, Majeed M, Sahebkar A. The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage. Molecules. 2019; 24(22):4029. https://doi.org/10.3390/molecules24224029
Chicago/Turabian StyleGorabi, Armita Mahdavi, Nasim Kiaie, Saeideh Hajighasemi, Tannaz Jamialahmadi, Muhammed Majeed, and Amirhossein Sahebkar. 2019. "The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage" Molecules 24, no. 22: 4029. https://doi.org/10.3390/molecules24224029
APA StyleGorabi, A. M., Kiaie, N., Hajighasemi, S., Jamialahmadi, T., Majeed, M., & Sahebkar, A. (2019). The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage. Molecules, 24(22), 4029. https://doi.org/10.3390/molecules24224029






