2. Results and Discussion
Commercially available papaverine hydrochloride (
1a) [
25] was chosen as a model compound for our initial foray into C–H functionalization studies utilizing the sulfinate chemistry that has been described by Baran and other research groups [
2,
9,
14]. Fluoroalkyl substituents have become increasingly valuable in modern drug discovery due to their resistance toward oxidation by cytochrome P450 oxidases. Also, incorporation of halogen atoms on hit/lead compounds has been performed in order to exploit their steric effects through the ability of these atoms to occupy the active site of molecular targets [
26,
27,
28], and establish intermolecular bonds in a manner that resembles H-bonding [
29,
30,
31].
Baran et al. have published a number of sulfinate reaction conditions, where the Diversinate
TM reagents are compatible with different organic solvents (e.g., DMSO, CH
2Cl
2, ClCH
2CH
2Cl, perfluorotoluene, perfluorohexane, and anisole). Furthermore, it has been determined that fluoroalkyl zinc sulfinate reagents react best in halogenated solvents, such as CH
2Cl
2, alkyl zinc sulfinate salts react more favorably in DMSO [
2], and stoichiometric conditions for the peroxide and Diversinate
TM reagents, as well as reaction temperatures and times, vary greatly in the literature. Before synthesizing the targeted papaverine library, we initially conducted several experiments that tested the effect of solvents (e.g., DMSO/CH
2Cl
2/H
2O), reagent stoichiometry, and additives [e.g., trifluoroacetic acid (TFA)] with papaverine HCl (
1a) and the commonly used Diversinate
TM, zinc trifluoromethanesulfinate [(CF
3SO
2)
2Zn] (
Table S71). From this data, it was clear that the best yield for the major mono-CF
3 analogue (
2) was obtained using 6 mol eq. of (CF
3SO
2)
2Zn and 6 mol eq. of
tert-butyl hydroperoxide (TBHP) in CH
2Cl
2 for 16 h. In order to ascertain whether the presence of HCl was affecting the yields (the chloride ion could be competitively oxidized by TBHP), we generated the free base of papaverine (
1b) and repeated the test reactions. Surprisingly, the reaction on the free base gave a lower yield of
2 (15%) compared to the papaverine HCl reaction (compound
2, 24%). Subsequently, the effect of the addition of TFA was investigated using both the free base and HCl salt of papaverine and a mixture of products was produced, with the free base (
1b) affording the best yield (10%) with the solvent conditions CH
2Cl
2/TFA/H
2O (1 mol eq. TFA). Based on these data (
Table S71), all subsequent reactions were performed using the HCl salt of papaverine and 6 mol eq. of the Diversinate
TM reagent in CH
2Cl
2/H
2O (2.5:1) for the fluorinated Diversinates
TM, while DMSO/H
2O (2.5:1) was chosen for use with the non-fluorinated Diversinates
TM.
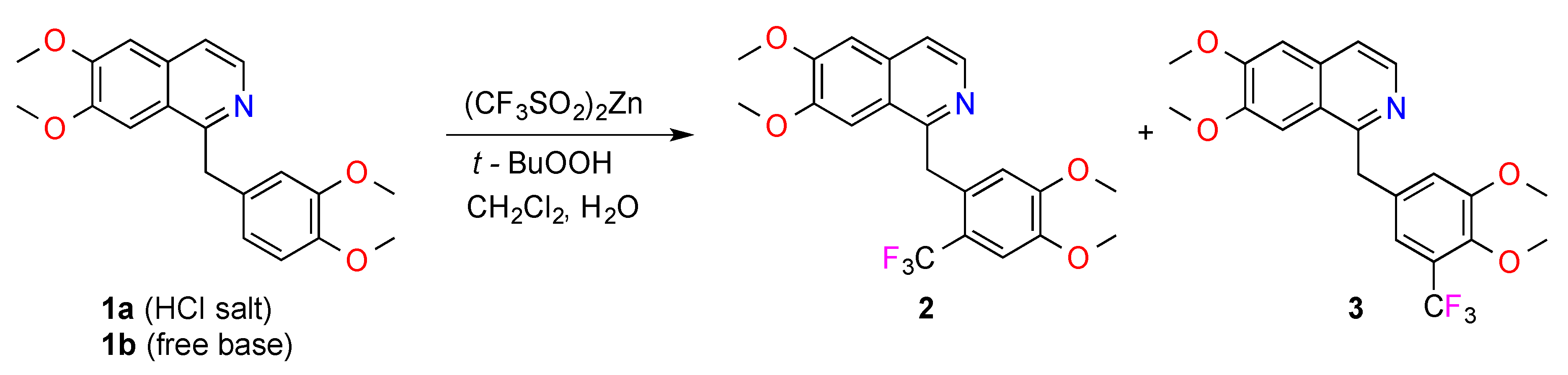
For example, scaffold
1a (0.1 mmol of the HCl salt) was treated with (CF
3SO
2)
2Zn (0.6 mmol) in a mixture of CH
2Cl
2 (100 μL) and H
2O (40 μL) at 0 °C. The mixture was stirred at 0 °C and TBHP (0.6 mmol) was slowly added, followed by stirring for 20 min. The mixture was then allowed to warm to room temperature over 16 h [
2], before evaporation under a stream of nitrogen and chromatography (HPLC on NH
2-bonded silica) was undertaken in order to give the major products, substituted papaverines
2 (24%) and
3 (4%), and the recovered starting material (7%) (
Scheme 1). HPLC showed a multitude of UV-active peaks. This, and the recovery of only 7% of the starting material, suggested that the reaction of trifluoromethyl radicals with papaverine was very unselective and/or the products were unstable under the reaction conditions. The preferred attack on the electron-rich 3,4-dimethoxybenzyl ring was not surprising as the trifluoromethyl radical is electrophilic.
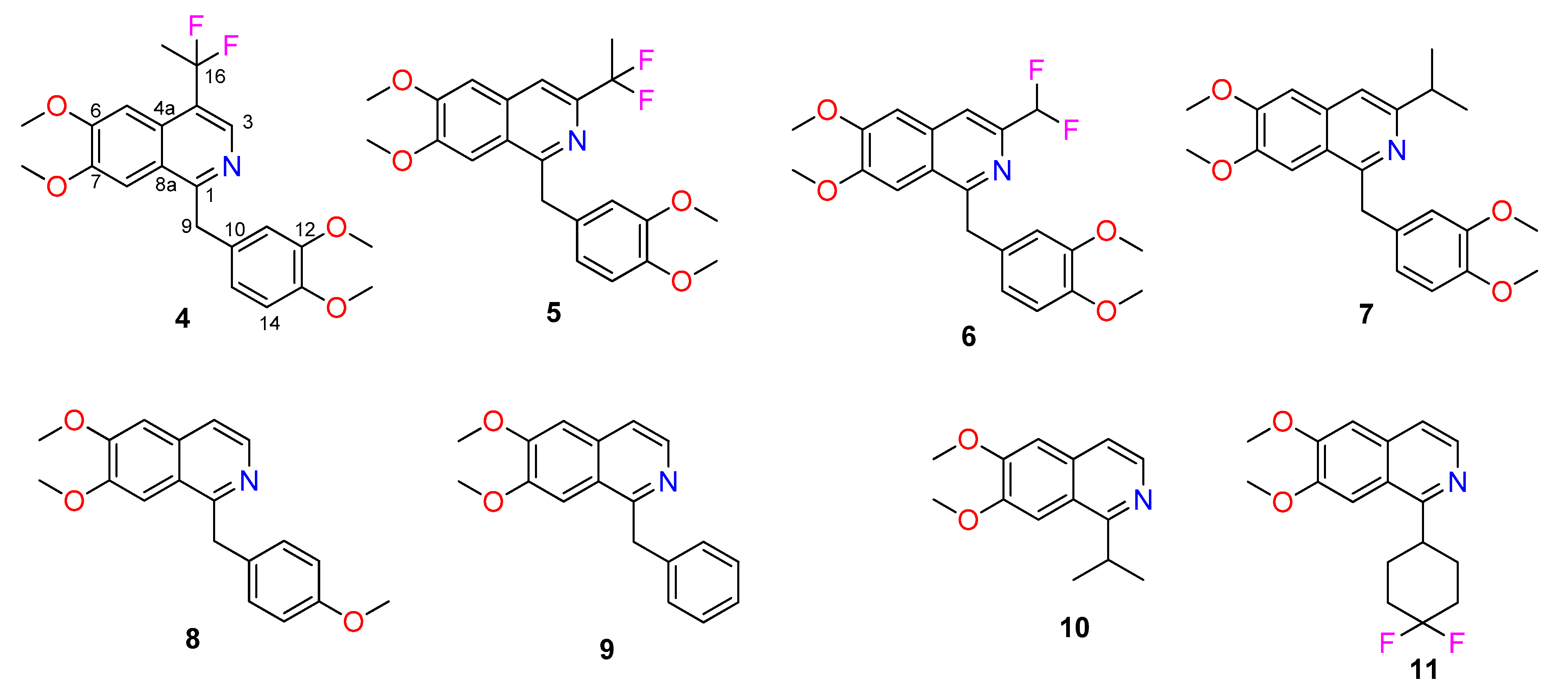
Interestingly, when the reaction was repeated with the less electrophilic reagent sodium 1,1-difluoroethanesulfinate, the products obtained were those involving substitution on the isoquinoline ring, rather than substitution on the dimethoxybenzyl ring. The products obtained were
4 (7%) and
5 (3%), as well as the recovered starting material (8%) (
Figure 1). In all of these reactions, a significant amount (generally between 7 and 14%) of starting material was recovered even though a six-fold excess of reagents was employed.
The closely related zinc difluoromethanesulfinate gave a low yield of
6 (3%) analogous to
5, but only when the reaction was carried out in the presence of TFA (0.1 mmol). No products were detected in the absence of TFA. Although the reaction employed the HCl salt of papaverine under the reaction conditions of excess (CF
2HSO
2)
2Zn and
t-BuOOH, the HCl would be neutralized by the zinc hydroxide formed. Presumably, the addition of TFA ensured at least partial protonation of the isoquinoline nitrogen, rendering the papaverine more electrophilic and therefore more reactive toward radicals that had some nucleophilic character. Baran et al. found that the difluoromethyl radical had nucleophilic properties, preferring to attack
N-heterocyclic compounds at electron-deficient centers [
12].
Use of the nucleophilic radical precursor zinc isopropylsulfinate gave the papaverine derivative
7 (4%), analogous to
5 and
6. Once again, TFA was required for the formation of
7. Surprisingly, the major product was
10 (19%), formed by an apparent radical substitution reaction. Analogous reactions were observed with the nucleophilic radical precursors zinc 4-methoxybenzylsulfinate, zinc benzylsulfinate, and 4,4-difluorocyclohexylsulfinate to give
8 (4%),
9 (14%), and
11 (4%), respectively. A suggested mechanism for these radical substitution reactions is outlined in
Scheme 2.
The isolated yields in these reactions were generally low. This was partly due to the large number of minor products formed with this particular scaffold (as evidenced by the HPLC data) and partly due to losses during HPLC purification. Some of these minor products may be due to the attack by trifluoromethyl radicals on the dimethoxyisoquinoline ring and/or bis-trifluoromethylation (which could yield 21 different compounds). In addition, the papaverine scaffold contains four methoxyl groups and a benzylic methylene group, all of which could undergo hydrogen atom abstraction by
tert-butoxyl radicals leading to a plethora of minor products. Interestingly, Kuttruff et al. [
32] have recently reported rather low yields (3–30%) and sometimes extensive decomposition with these reactions. They employed a range of scaffolds incorporating benzene, pyridine, pyrimidine, imidazole, pyrazole and thiazole rings. They also explored different reaction conditions and were able to achieve modest increases in yields in some cases via the addition of Fe(acac)
3. However, in other cases, the presence of Fe(acac)
3 led to rapid decomposition. Baran et al. [
9] have also noted that one of the limitations of the method is that some substrates deliver only moderate amounts of product.
We suggest that the main reason for the poor reactivity of
1a toward difluoromethyl radicals is the presence of the dimethoxybenzyl group at position 1. Difluoromethyl radicals react readily with isoquinoline and with 3-methylisoquinoline at position 1 (the most electrophilic position), but they do not react with 1-methylisoquinoline [
33]. It is interesting to note that the more nucleophilic isopropyl radicals attack at position 1 of the papaverine despite the significant steric hindrance to attack at this position. This is followed by loss of the (more stable) 3,4-dimethoxybenzyl radical to give
10 in moderate (19%) yield.
Compounds
2,
8, and
9 have previously been synthesized via multi-step syntheses;
2 was generated using the copper-mediated trifluoromethylation-allylation of arynes [
34];
8 was produced from a reaction of Raney nickel with N-benzylsulfonamide [
35], and via the desulfonylation of N-sulfonyl tetrahydroisoquinolines with KF/Al
2O
3 [
36]; and
9 was synthesized using a ruthenium-mediated dual catalytic reaction, and oxidative cross-dehydrogenative coupling with methyl arenes [
37,
38]. However, this is the first report of the synthesis of
2,
8, and
9 using sulfinate chemistry. Furthermore, these three compounds were only partially characterized and none of them had their
1H and
13C chemical shifts assigned to specific positions within the alkaloidal skeleton. We report here the first synthesis of several other papaverine analogues as their free base; full characterization using 1D/2D NMR, UV, IR, and MS data was performed during these studies.
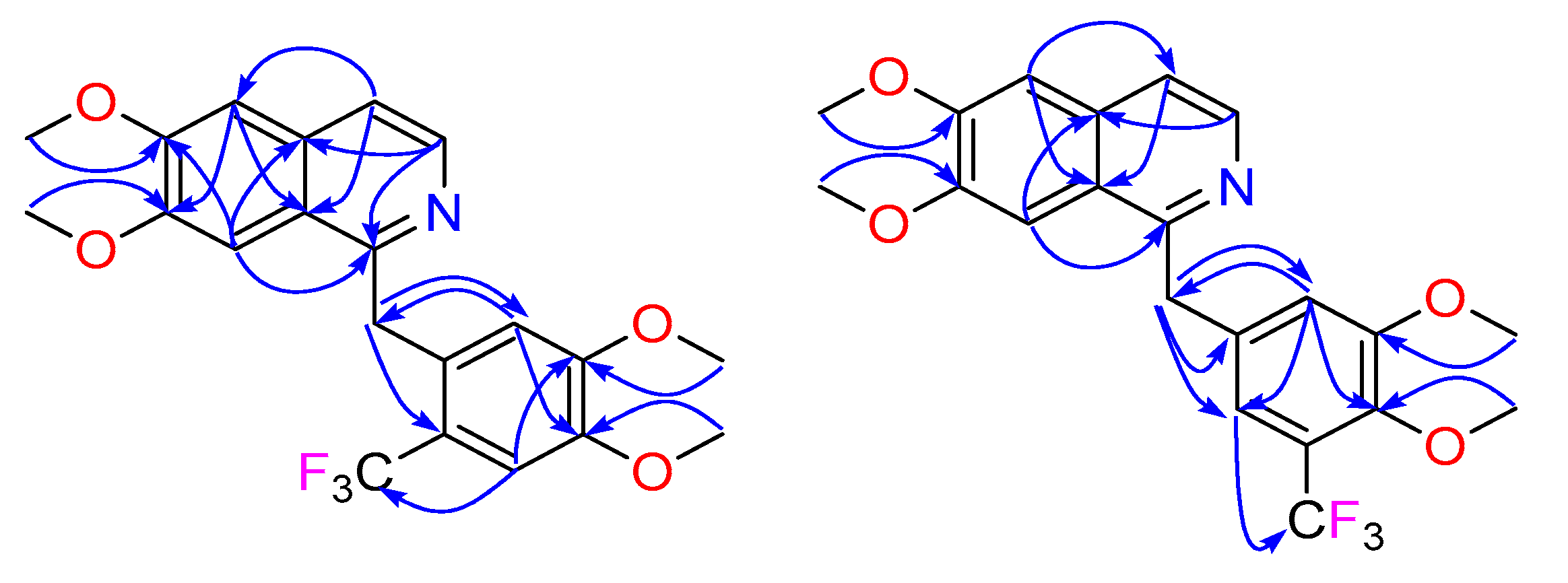
The first products to be fully characterized were compounds
2 and
3. The
1H NMR spectra (
Table 1) of these two mono-CF
3 derivatives enabled the definitive positioning of the CF
3. Careful comparison of the natural product scaffold (
1b) NMR data in CD
3OD with the fluorinated analogue
2 indicated that this molecule had a CF
3 group attached to C-15, since the
1H NMR chemical shifts and multiplicities associated with the pendant benzyl moiety of the starting material had changed from a classic 1,3,4-trisubstituted benzene system (δ
H 7.03, d,
J = 2.1 Hz, H-11, 6.92, d,
J = 8.4 Hz, H-14 and 6.82, dd,
J = 8.4, 2.1 Hz, H-15) to a 1,3,4,6-tetrasubstituted benzene system (δ
H 6.41, s, H-11 and 7.26, s, H-14) (
Table 1). Furthermore, a heteronuclear multiple bond correlation (HMBC) (
Figure 2) from H-14 (
δH 7.26) to CF
3 (
δC 126.4) provided further proof of the structural assignment. In a similar fashion to
2, NMR data analysis of
3 also showed that the CF
3 was attached to the pendant aromatic ring of papaverine; however, in this case, the fluorinated moiety was attached to C-14, as indicated once again by the
1H NMR chemical shifts and multiplicities associated with the pendant benzyl moiety of papaverine that had changed from the 1,3,4-trisubstituted benzene system to a 1,3,4,5-tetrasubstituted benzene system (δ
H 7.22, d,
J = 2.2 Hz, H-11 and 7.11, d,
J = 2.2 Hz, H-15) (
Table 1). The HMBC spectrum analysis of
3 was also critical in confirming the CF
3 positioning; key HMBC correlations for
3 are shown in
Figure 2.
Detailed 2D NMR data analyses were also performed on all other analogues generated during these studies (see
Supplementary Materials).
Surprisingly, the reactions that used other commercially available DiversinatesTM, such as zinc chloromethanesulfinate, zinc chloroethanesulfinate, sodium (2,4-dichlorophenyl)methanesulfinate, sodium 1-(trifluoromethyl)cyclopropanesulfinate, and sodium tert-butylsulfinate were not successful using our methodology (with or without TFA), as determined by LCMS analysis of the crude reaction mixtures after 16 h. The lack of reaction with sodium tert-butylsulfinate and sodium 1-(trifluoromethyl)cyclopropanesulfinate was possibly due to steric hindrance, but it is unclear why the other DiversinateTM reactions were unsuccessful.
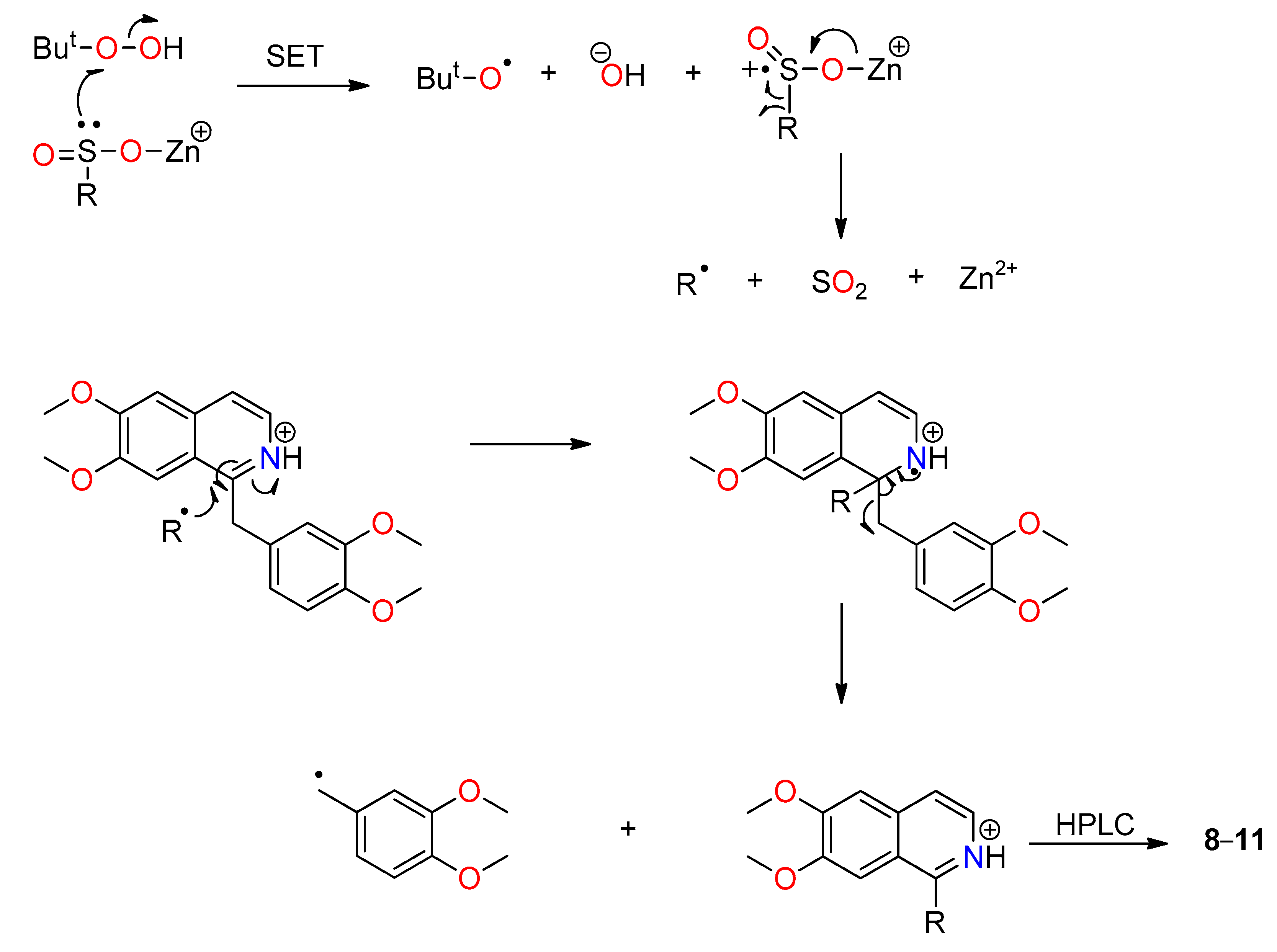
In
Scheme 2, we propose that the zinc bis(alkanesulfinate) underwent oxidation by TBHP via a single-electron transfer (SET) process to give a
tert-butoxyl radical, hydroxide ion, and the radical cation of the zinc sulfinate, which then underwent fragmentation to give the alkyl radical and sulfur dioxide. The nucleophilic alkyl radical then underwent addition at position 1 of papaverine followed by elimination of the dimethoxybenzyl radical. This addition–elimination mechanism is analogous to that proposed for the reaction of carbon-centered radicals with β-bromostyrene [
39]. The
tert-butoxyl radical generated in the first step could oxidize a second molecule of sulfinate to give the
tert-butoxide anion and the radical cation of the zinc sulfinate. A similar mechanism has been proposed by Baran et al. [
9] where the presence of a trace metal initiates
tert-butoxyl radical formation, compared to our proposed mechanism that involves SET. The dimethoxybenzyl radical could also be oxidized by TBHP to give the corresponding dimethoxybenzyl alcohol and a
tert-butoxyl radical.
Again, it is interesting that the more nucleophilic radicals attack position 1 of the isoquinoline ring, while the 1,1-difluoroethyl and difluoromethyl radicals, which appear to have both nucleophilic and electrophilic properties, prefer to attack at the 3- or 4-position.
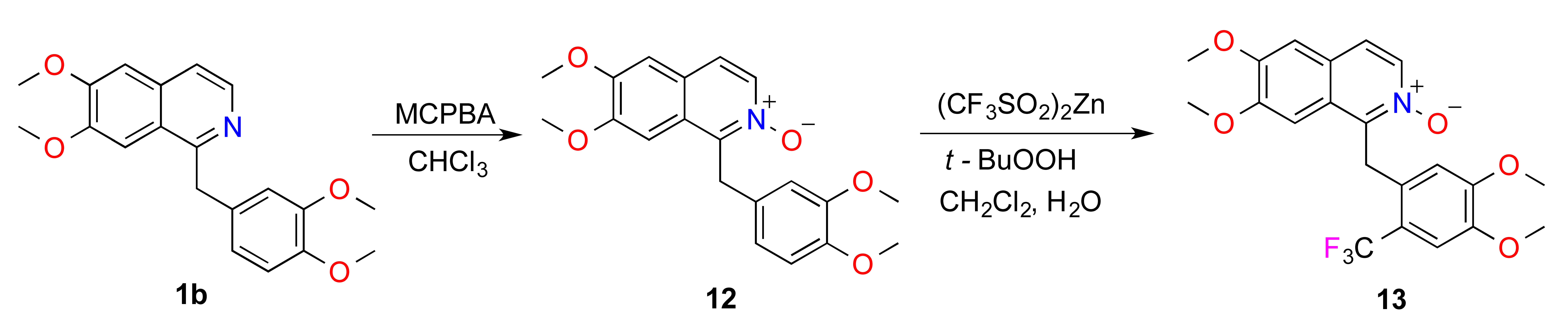
In a final attempt to improve the yields and/or the selectivity of these reactions, we decided to modify the reactivity of the papaverine scaffold. It was envisaged that converting papaverine to its
N-oxide might increase reactivity, particularly toward electrophilic trifluoromethyl radicals. The free base of papaverine was readily converted to the corresponding
N-oxide derivative
12 in moderate yield (58%) using
meta-chloroperbenzoic acid (MCPBA) in CHCl
3 without the need for chromatography, using a method previously described by Bremner et al. [
40] (
Scheme 3). Unfortunately, treatment of
12 with (CF
3SO
2)
2Zn and TBHP under the same conditions as that used for
1a did not result in an improvement in the yield of the desired Diversinate
TM product. After work-up and C
18 HPLC purification of the reaction mixture,
1H NMR and LCMS analysis indicated that the major product formed was compound
13, albeit with a low yield (<12%) and purity (<80%). Significant amounts of starting material (
12, 26%) were isolated, indicating that papaverine
N-oxide was less reactive than papaverine toward zinc trifluoromethanesulfinate. As the yield of the desired product had not improved, the reaction of papaverine
N-oxide with other Diversinate
TM reagents was not investigated.
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra were recorded at 25 °C on a Bruker AVANCE HDX 800 MHz spectrometer (Fällanden, Zürich, Switzerland) equipped with a TCI cryoprobe. The 1H and 13C NMR chemical shifts were referenced to the solvent peak for CD3OD (δH 3.31 and δC 49.00) and CDCl3 (δH 7.26 and δC 77.16). UV spectra were recorded using a JASCO V-650 UV/vis spectrophotometer (Tokyo, Japan). IR data were acquired using an attached Universal Attenuated Total Reflectance (UATR) Two module on a PerkinElmer spectrophotometer (Waltham, MA, USA). LRESIMS data were recorded on a Thermo Fisher MSQ Plus single quadrupole mass spectrometer (Waltham, MA, USA). HRESIMS data were recorded on a Bruker maXis II ETD ESI-qTOF (Bremen, Germany). A Thermo Scientific Dionex Ultimate 3000 HPLC (Waltham, MA, USA) was used for semi-preparative HPLC separations. An Alltech stainless steel guard cartridge (10 mm × 30 mm) (Sydney, NSW, Australia) was used for loading pre-adsorbed synthetic reaction products onto the semi-preparative HPLC columns. Alltech Davisil NH2-bonded silica (35–75 µm, 150 Å) (Sydney, NSW, Australia) and C18-bonded silica (35–75 µm, 150 Å) (Sydney, NSW, Australia) were used for pre-adsorption work before HPLC separations. TLC was carried out on Merck gel F254s pre-coated NH2 glass plates (Darmstadt, Germany) and was observed using UV light. A YMC NH2-bonded silica column (5 µm, 120 Å, 20 mm × 150 mm) (Kyoto, Japan) and a ThermoElectron Betasil C18-bonded silica column (5 µm, 143 Å, 21.2 × 150 mm) (Waltham, MA, USA) were used for semi-preparative HPLC separations. All solvents used for chromatography, UV and MS were Honeywell Burdick & Jackson HPLC grade (Muskegon, MI, USA), and the H2O was Sartorius arium proVF (Göttingen, Germany) filtered. All synthetic reagents (including papaverine HCl) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. NMR spectra were processed using MestReNova version 11.0 (Santiago de Compostela, Spain).
3.2. Generation of the Papaverine Library (TFA-Free Method): Compounds 2–5, 9, and 11
Papaverine hydrochloride (1a, 37.5 mg, 0.1 mmol) was dissolved in CH2Cl2/H2O or DMSO/H2O (2.5:1, 100 μL:40 μL) before the addition of the sulfinate reagent (85.6–198.9 mg, 0.6 mmol) at room temperature. The mixture was cooled to 0 °C before TBHP (40 μL, 0.6 mmol) was slowly added, after which time the stirred mixture slowly warmed to room temperature over 16 h. Crude reaction products were dried under N2 and pre-adsorbed to NH2-bonded silica (≈1 g) overnight with the dry material packed into a stainless steel guard cartridge, which was subsequently attached to a NH2 semi-preparative HPLC column. Isocratic conditions of 100% n-hexane for 10 min, followed by a linear gradient to 20% i-PrOH/n-hexane over 50 min, then isocratic conditions of 20% i-PrOH/n-hexane for 10 min all at a flow rate of 9 mL/min was used for each HPLC separation. Sixty fractions (60 × 1 min) were collected from the start of the HPLC run. Fractions containing UV-active material from each HPLC run were analyzed using 1H NMR spectroscopy and LCMS, and relevant fractions with a purity >90% were combined to produce the desired products. Compounds 2–5 and 11 were synthesized using the CH2Cl2/H2O solvent system, while compound 9 was generated using the DMSO/H2O mixture.
3.2.1. Compound 2
Colorless gum (6.0 mg, 15%); UV (MeOH) λ
max (log ε) 241 (4.20), 279 (3.30), 327 (3.19) nm; IR (UATR) ν
max 3440, 2981, 1747, 1709, 1279, 1199, 1167, 1078, 1026, 976, 712 cm
−1; For NMR data see
Table 1; (+)-LRESIMS
m/
z (rel. int.) 408 (100) [M + H]
+; (+)-HRESIMS
m/
z 408.1425 [M + H]
+ (calcd for C
21H
21F
3NO
4, 408.1417).
3.2.2. Compound 3
Colorless gum (1.9 mg, 4%); UV (MeOH) λ
max (log ε) 240 (4.24), 283 (3.47), 324 (3.20) nm; IR (UATR) ν
max 3440, 2982, 1746, 1709, 1278, 1199, 1168, 1078, 1026, 975, 712 cm
−1; For NMR data see
Table 1; (+)-LRESIMS
m/
z (rel. int.) 408 (100) [M + H]
+; (+)-HRESIMS
m/
z 408.1422 [M + H]
+ (calcd for C
21H
21F
3NO
4, 408.1417).
3.2.3. Compound 4
Colorless gum (2.7 mg, 7%); UV (MeOH) λmax (log ε) 240 (4.08), 276 (3.35), 327 (3.17) nm; IR (UATR) νmax 3400, 2981, 1747, 1705, 1278, 1200, 1164, 1078, 975, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 2.17 (3H, t, J = 18.8 Hz, H-17), 3.74 (3H, s, 12-OMe), 3.75 (3H, s, 13-OMe), 3.87 (3H, s, 7-OMe), 3.97 (3H, s, 6-OMe), 4.56 (2H, s, H-9), 6.77 (1H, dd, J = 8.2, 2.0 Hz, H-15), 6.83 (1H, d, J = 8.2 Hz, H-14), 6.92 (1H, d, J = 2.0 Hz, H-11), 7.51 (1H, s, H-5), 7.53 (1H, s, H-8), 8.42 (1H, s, H-3); 13C NMR (200 MHz, CD3OD) δC 25.5 (t, 2JCF = 28.6 Hz, C-17), 42.4 (C-9), 56.36 (12-OMe), 56.38 (13-OMe), 56.41 (7-OMe), 56.5 (6-OMe), 104.4 (C-5), 106.4 (C-8), 113.2 (C-14), 113.6 (C-11), 122.0 (C-15), 124.0 (t, 1JCF = 238.3 Hz, C-16), 124.6 (C-8a), 126.8 (t, 2JCF = 25.3 Hz, C-4), 131.2 (C-4a), 133.1 (C-10), 138.1 (t, 3JCF = 9.3 Hz, C-3), 149.3 (C-13), 150.6 (C-12), 151.6 (C-7), 154.8 (C-6), 162.1 (C-1); (+)-LRESIMS m/z (rel. int.) 404 (100) [M + H]+; (+)-HRESIMS m/z 426.1481 [M + Na]+ (calcd for C22H23F2NO4Na, 426.1487).
3.2.4. Compound 5
Colorless gum (1.3 mg, 3%); UV (MeOH) λmax (log ε) 243 (3.95), 283 (3.19), 328 (3.08) nm; IR (UATR) νmax 3400, 2972, 1747, 1705, 1279, 1199, 1165, 1078, 976, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 2.08 (3H, t, J = 18.4 Hz, H-17), 3.73 (3H, s, 12-OMe), 3.75 (3H, s, 13-OMe), 3.88 (3H, s, 7-OMe), 3.97 (3H, s, 6-OMe), 4.56 (2H, s, H-9), 6.80 (1H, dd, J = 8.3, 2.0 Hz, H-15), 6.83 (1H, d, J = 8.3 Hz, H-14), 6.95 (1H, d, J = 2.0 Hz, H-11), 7.34 (1H, s, H-5), 7.46 (1H, s, H-8), 7.86 (1H, s, H-4); 13C NMR (200 MHz, CD3OD) δC 24.4 (t, 2JCF = 28.7 Hz, C-17), 41.9 (C-9), 56.3 (12-OMe), 56.39 (13-OMe), 56.41 (7-OMe), 56.5 (6-OMe), 107.4 (C-5), 105.5 (C-8), 113.0 (C-14), 113.4 (C-11), 115.7 (t, 3JCF = 5.3 Hz, C-4), 121.8 (C-15), 122.5 (t, 1JCF = 237.4 Hz, C-16), 124.3 (C-8a), 133.5 (C-10), 135.0 (C-4a), 147.6 (t, 2JCF = 28.3 Hz, C-3), 149.0 (C-13), 150.4 (C-12), 152.3 (C-7), 154.7 (C-6), 159.8 (C-1); (+)-LRESIMS m/z (rel. int.) 404 (100) [M + H]+; (+)-HRESIMS m/z 426.1478 [M + Na]+ (calcd for C22H23F2NO4Na, 426.1487).
3.2.5. Compound 9
Colorless gum (4.0 mg, 14%); UV (MeOH) λmax (log ε) 241 (3.90), 326 (2.94) nm; IR (UATR) νmax 3458, 2981, 1747, 1705, 1278, 1199, 1165, 1078, 1026, 975, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 3.83 (3H, s, 7-OMe), 3.97 (3H, s, 6-OMe), 4.60 (2H, s, H-9), 7.16 (1H, m, H-13), 7.24 (2H, m, H-11, H-15), 7.27 (1H, s, H-5), 7.38 (1H, s, H-8), 7.40 (2H, m, H-12, H-14), 7.61 (1H, d, J = 5.7 Hz, H-4), 8.22 (1H, d, J = 5.7 Hz, H-3); 13C NMR (200 MHz, CD3OD) δC 42.5 (C-9), 56.3 (7-OMe), 56.5 (6-OMe), 105.5 (C-8), 106.5 (C-5), 120.6 (C-4), 124.3 (C-8a), 127.4 (C-13), 129.6 (2C, C-11, C-15), 129.5 (2C, C-12, C-14), 135.5 (C-4a), 140.5 (C-10), 140.8 (C-3), 151.7 (C-7), 154.6 (C-6), 158.9 (C-1); (+)-LRESIMS m/z (rel. int.) 280 (100) [M + H]+; (+)-HRESIMS m/z 280.1327 [M + H]+ (calcd for C18H18NO2, 280.1332).
3.2.6. Compound 11
Colorless gum (1.2 mg, 4%); UV (MeOH) λmax (log ε) 237 (3.94), 327 (2.96) nm; IR (UATR) νmax 3436, 2986, 1747, 1705, 1280, 1199, 1166, 1078, 1026, 975, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 2.00–2.11 (4H, m, H-10, H-14), 2.12–2.21 (4H, m, H-11, H-13), 3.71 (1H, m, H-9), 3.99 (3H, s, 6-OMe), 4.03 (3H, s, 7-OMe), 7.28 (1H, s, H-5), 7.52 (1H, d, J = 5.7 Hz, H-4), 7.54 (1H, s, H-8), 8.19 (1H, d, J = 5.7 Hz, H-3); 13C NMR (200 MHz, CD3OD) δC 29.6 (2C, d, 3JCF = 10.3 Hz, C-10, C-14), 34.8 (2C, dd, 2JCF = 25.5, 22.6 Hz, C-11, C-13), 40.2 (C-9), 56.46 (6-OMe), 56.54 (7-OMe), 104.2 (C-8), 106.9 (C-5), 119.9 (C-4), 123.3 (C-8a), 124.5 (dd, 1JCF = 242.0, 239.2 Hz, C-12), 135.2 (C-4a), 140.9 (C-3), 152.0 (C-7), 154.5 (C-6), 162.3 (C-1); (+)-LRESIMS m/z (rel. int.) 308 (100) [M + H]+; (+)-HRESIMS m/z 308.1454 [M + H]+ (calcd for C17H20F2NO2, 308.1457).
3.3. Generation of the Papaverine Library (TFA-Addition Method): Compounds 6–8 and 10
Papaverine hydrochloride (1a, 37.5 mg, 0.1 mmol) was dissolved in CH2Cl2/H2O or DMSO/H2O (2.5:1, 100 μL:40 μL) before the addition of the sulfinate reagent (85.6–198.9 mg, 0.6 mmol) and TFA (7.7 μL, 0.1 mmol) at room temperature. The mixture was cooled to 0 °C before TBHP (40 μL, 0.6 mmol) was slowly added, after which time, the stirred mixture slowly warmed to room temperature over 16 h. Crude reaction products was dried under N2 and pre-adsorbed to NH2-bonded silica (≈1 g) overnight before being subjected to identical NH2 semi-preparative HPLC conditions, which are described above. This method generated compound 6 using the CH2Cl2/H2O solvent system, while compounds 7, 8, and 10 were generated using the DMSO/H2O mixture.
3.3.1. Compound 6
Colorless gum (1.0 mg, 3%); UV (MeOH) λmax (log ε) 240 (4.15), 273 (3.36), 327 (3.08) nm; IR (UATR) νmax 3458, 2981, 1747, 1705, 1278, 1199, 1165, 1078, 1026, 975, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 3.72 (3H, s, 12-OMe), 3.76 (3H, s, 13-OMe), 3.87 (3H, s, 7-OMe), 3.98 (3H, s, 6-OMe), 4.57 (2H, s, H-9), 6.78 (1H, dd, J = 8.3, 2.0 Hz, H-15), 6.83 (1H, d, J = 8.3 Hz, H-14), 6.84 (1H, t, J = 55.7 Hz, H-16), 6.93 (1H, d, J = 2.0 Hz, H-11), 7.37 (1H, s, H-5), 7.48 (1H, s, H-8), 7.88 (1H, s, H-4); 13C NMR (200 MHz, CD3OD) δC 42.0 (C-9), 56.4 (12-OMe), 56.45 (13-OMe), 56.49 (7-OMe), 56.6 (6-OMe), 107.4 (C-5), 105.8 (C-8), 113.2 (C-14), 113.6 (C-11), 115.6 (t, 1JCF = 238.0 Hz, C-16), 117.4 (t, 3JCF = 4.8 Hz, C-4), 121.9 (C-15), 125.0 (C-8a), 133.4 (C-10), 135.0 (C-4a), 144.9 (t, 2JCF = 24.3 Hz, C-3), 149.2 (C-13), 150.6 (C-12), 152.6 (C-7), 154.9 (C-6), 160.1 (C-1); (+)-LRESIMS m/z (rel. int.) 390 (100) [M + H]+; (+)-HRESIMS m/z 390.1506 [M + H]+ (calcd for C21H22F2NO4, 390.1511).
3.3.2. Compound 7
Colorless gum (1.5 mg, 4%); UV (MeOH) λmax (log ε) 239 (3.72), 276 (3.00), 328 (2.83) nm; IR (UATR) νmax 3413, 2986, 1756, 1718, 1279, 1198, 1170, 1078, 1025, 973, 712 cm−1; δH 1.40 (6H, d, J = 6.9 Hz, H-17), 3.22 (1H, sept, J = 6.9 Hz, H-16), 3.72 (3H, s, 12-OMe), 3.76 (3H, s, 13-OMe), 3.84 (1H, s, 7-OMe), 3.96 (3H, s, 6-OMe), 4.53 (1H, s, H-9), 6.78 (1H, dd, J = 8.5, 2.2 Hz, H-15), 6.83 (1H, d, J = 8.5 Hz, H-14), 6.91 (1H, d, J = 2.2 Hz, H-11), 7.21 (1H, s, H-5), 7.44 (1H, s, H-4), 7.37 (1H, s, H-8); 13C NMR (200 MHz, CD3OD) δC 23.2 (2C, C-17), 36.6 (C-16), 41.7 (C-9), 56.31 (6-OMe), 56.33 (7-OMe), 56.4 (12-OMe), 56.5 (13-OMe), 105.4 (C-8), 106.5 (C-5), 113.1 (C-14), 113.4 (C-11), 115.3 (C-4), 121.8 (C-15), 123.0 (C-8a), 133.7 (C-10), 136.2 (C-4a), 149.0 (C-13), 159.3 (C-3), 151.0 (C-12), 151.8 (C-7), 154.4 (C-6), 158.7 (C-1); (+)-LRESIMS m/z (rel. int.) 382 (100) [M + H]+; (+)-HRESIMS m/z 382.2024 [M + H]+ (calcd for C23H28NO4, 382.2013).
3.3.3. Compound 8
Colorless gum (1.3 mg, 4%); UV (MeOH) λmax (log ε) 241 (3.90), 326 (2.94) nm; IR (UATR) νmax 3456, 2981, 1745, 1705, 1279, 1199, 1167, 1079, 1026, 978, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 3.72 (3H, s, 13-OMe), 3.84 (3H, s, 7-OMe), 3.96 (3H, s, 6-OMe), 4.52 (2H, s, H-9), 7.15 (2H, m, H-11, H-15), 7.26 (1H, s, H-5), 7.39 (1H, s, H-8), 6.81 (2H, m, H-12, H-14), 7.58 (1H, d, J = 5.7 Hz, H-4), 8.20 (1H, d, J = 5.7 Hz, H-3); 13C NMR (200 MHz, CD3OD) δC 41.6 (C-9), 55.6 (13-OMe), 56.3 (7-OMe), 56.5 (6-OMe), 105.6 (C-8), 106.6 (C-5), 120.5 (C-4), 124.2 (C-8a), 130.4 (2C, C-11, C-15), 115.0 (2C, C-12, C-14), 135.5 (C-4a), 132.7 (C-10), 140.5 (C-3), 151.7 (C-7), 154.6 (C-6), 159.7 (C-1), 159.3 (C-13); (+)-LRESIMS m/z (rel. int.) 310 (100) [M + H]+; (+)-HRESIMS m/z 310.1436 [M + H]+ (calcd for C19H20NO3, 310.1438).
3.3.4. Compound 10
Colorless gum (4.5 mg, 19%); UV (MeOH) λmax (log ε) 237 (3.91), 324 (2.83) nm; IR (UATR) νmax 3413, 2986, 1747, 1709, 1281, 1198, 1169, 1078, 1026, 973, 712 cm−1; 1H NMR (800 MHz, CD3OD) δH 1.40 (6H, d, J = 6.8 Hz, H-10), 3.91 (1H, sept, J = 6.8 Hz, H-9), 3.97 (1H, s, 6-OMe), 3.98 (3H, s, 7-OMe), 7.23 (1H, s, H-5), 7.47 (1H, d, J = 5.6 Hz, H-4), 7.51 (1H, s, H-8), 8.17 (1H, d, J = 5.6 Hz, H-3); 13C NMR (200 MHz, CD3OD) δC 22.4 (2C, C-10, C-11), 31.9 (C-9), 56.4 (2C, 6-OMe, 7-OMe), 104.4 (C-8), 106.8 (C-5), 119.6 (C-4), 123.3 (C-8a), 135.1 (C-4a), 140.8 (C-3), 151.8 (C-7), 154.4 (C-6), 165.1 (C-1); (+)-LRESIMS m/z (rel. int.) 232 (100) [M + H]+; (+)-HRESIMS m/z 232.1333 (calcd for C14H18NO2, 232.1332).
3.4. Synthesis of Papaverine N-oxide (12)
Using a method previously reported by Bremner et al. [
40], the free base of papaverine (
1b, 0.50 g, 1.5 mmol) was dissolved in CHCl
3 (10 mL) and treated portion wise with MCPBA (0.35 g, 2.4 mmol) over 5 min. After completion of the addition, the solution was stirred at room temperature for 19 h. The colorless precipitate that formed was filtered and the filtrate was extracted with 5% NaOH (3 × 25 mL). The CHCl
3-soluble material was dried down then recrystallized from acetone to yield pure papaverine
N-oxide (
12, 310 mg, 58%).
Comparison of the
1H NMR and LRMS data of product
12 with literature values confirmed the compound [
40], although the
1H NMR chemical shifts for two methoxyl signals had been incorrectly assigned to δ
H 6.14 and 6.15; this being a typographical error. The
1H NMR data for papaverine
N-oxide was initially reported in CDCl
3 at 60 MHz; no
13C NMR data was reported in the original article [
40], hence we recorded 1D and 2D NMR data for
12 in both CDCl
3 and CD
3OD, and report the full NMR assignments here for completeness.
Compound 12
White amorphous solid (310 mg, 58%); 1H NMR (800 MHz, CDCl3) δH 3.800a (3H, s, 12-OMe), 3.803a (3H, s, 13-OMe), 3.94 (3H, s, 7-OMe), 3.99 (3H, s, 6-OMe), 4.73 (2H, s, H-9), 6.73 (1H, d, J = 8.3 Hz, H-14), 6.80 (1H, dd, J = 8.3, 2.1 Hz, H-15), 7.01 (1H, d, J = 2.1 Hz, H-11), 7.03 (1H, s, H-5), 7.21 (1H, s, H-8), 7.42 (1H, d, J = 7.0 Hz, H-4), 8.16 (1H, d, J = 7.0 Hz, H-3); 13C NMR (200 MHz, CDCl3) δC 31.8 (C-9), 56.00b (12-OMe), 56.01b (13-OMe), 56.2 (7-OMe), 56.3 (6-OMe), 103.1 (C-8), 106.1 (C-5), 111.4 (C-14), 112.3 (C-11), 120.4 (C-15), 121.1 (C-4), 124.9 (C-8a), 125.7 (C-4a), 130.1 (C-10), 135.3 (C-3), 145.8 (C-1), 147.9 (C-13), 149.3 (C-12), 151.3 (C-6), 151.8 (C-7); 1H NMR (800 MHz, CD3OD) δH 3.747b (3H, s, 12-OMe), 3.748b (3H, s, 13-OMe), 3.91 (3H, s, 6-OMe), 3.96 (3H, s, 7-OMe), 4.79 (2H, s, H-9), 6.79 (1H, dd, J = 8.3, 1.8 Hz, H-15), 6.82 (1H, d, J = 8.3 Hz, H-14), 7.01 (1H, d, J = 1.8 Hz, H-11), 7.33 (1H, s, H-5), 7.38 (1H, s, H-8), 7.72 (1H, d, J = 7.0 Hz, H-4), 8.14 (1H, d, J = 7.0 Hz, H-3); 13C NMR (200 MHz, CD3OD) δC 32.2 (C-9), 56.44c (12-OMe), 56.47c (13-OMe), 56.63d (7-OMe), 56.66d (6-OMe), 104.8 (C-8), 107.3 (C-5), 113.2 (C-14), 113.8 (C-11), 121.9 (C-15), 123.2 (C-4), 125.6 (C-8a), 129.1 (C-4a), 131.3 (C-10), 134.8 (C-3), 148.4 (C-1), 149.4 (C-13), 150.7 (C-12), 153.6 (C-7), 154.1 (C-6); (+)-LRESIMS m/z (rel. int.) 356 (100) [M + H]+. a,b,c,d Interchangeable signals.
3.5. Synthesis of Papaverine N-oxide DiversinateTM Analogue (13)
Papaverine N-oxide (12, 35.5 mg, 0.1 mmol) was dissolved in CH2Cl2/H2O (2.5:1, 100 μL:40 μL) before the addition of (CF3SO2)2Zn (198.9 mg, 0.6 mmol) at room temperature. The mixture was cooled to 0 °C before TBHP (40 μL, 0.6 mmol) was slowly added, after which time, the stirred mixture slowly warmed to room temperature over 16 h. The crude reaction product was dried under N2 and pre-adsorbed to C18-bonded silica (≈1 g) overnight with the dry material packed into a stainless-steel guard cartridge, which was subsequently attached to a C18 semi-preparative HPLC column. Isocratic conditions of H2O/MeOH/TFA (90:10:0.1) were run for the first 10 min, followed by a linear gradient of MeOH/TFA (100:0.1) over 40 min, then isocratic conditions of MeOH/TFA (100:0.1) for a further 10 min, all at a flow rate of 9 mL/min. Sixty fractions (60 × 1 min) were collected from the start of the HPLC run. Fractions containing UV-active material were analyzed using 1H NMR spectroscopy and LCMS in order to identify products of interest. The starting material (12, 9.3 mg, 26%) eluted in fraction 39, while a mixture (<80% pure) containing predominantly the mono-CF3 papaverine N-oxide analogue (13, 5.1 mg, <12%) eluted in fraction 43. While 1H NMR and LCMS analysis of the fraction 43 mixture indicated product 13 had been made, the paucity of material and low yield, meant no further purification or characterization was undertaken.
3.6. Generation of the Free Base of Papaverine (1b)
Papaverine hydrochloride (1a, 200 mg) was dissolved in a mixture of ammoniated H2O (9:1, H2O:28% aq. NH3, 20 mL) and then extracted with CH2Cl2 (3 × 20 mL). The free base of papaverine (1b) was soluble in the CH2Cl2 layer, and following evaporation, yielded the desired product with a high purity and yield (174 mg, 93%).
3.7. Generation of Compounds 2 and 3 Using the Free Base of Papaverine (1b)
The free base of papaverine (
1b, 33.9 mg, 0.1 mmol) was dissolved in CH
2Cl
2/H
2O or DMSO/H
2O (2.5:1, 100 μL:40 μL) before the addition of the (CF
3SO
2)
2Zn (198.9 mg, 0.6 mmol) at room temperature. Where TFA was used, 7.7 μL (0.1 mmol) was added to the reaction while stirring. The mixture was cooled to 0 °C before TBHP (40 μL, 0.6 mmol) was added, after which time, the stirred mixture was slowly warmed to room temperature over 16 h. The crude reaction product was dried under N
2 and pre-adsorbed to NH
2-bonded silica (≈1 g) overnight before being subjected to identical NH
2 semi-preparative HPLC conditions, which are described above. Reaction conditions and yields for these free base reactions can be found in
Table S71.
 ) correlations for 2 and 3.
) correlations for 2 and 3.