The Impact of Long- and Short-Term Strontium Treatment on Metabolites and Minerals in Glycine max
Abstract
1. Introduction
2. Results and Discussion
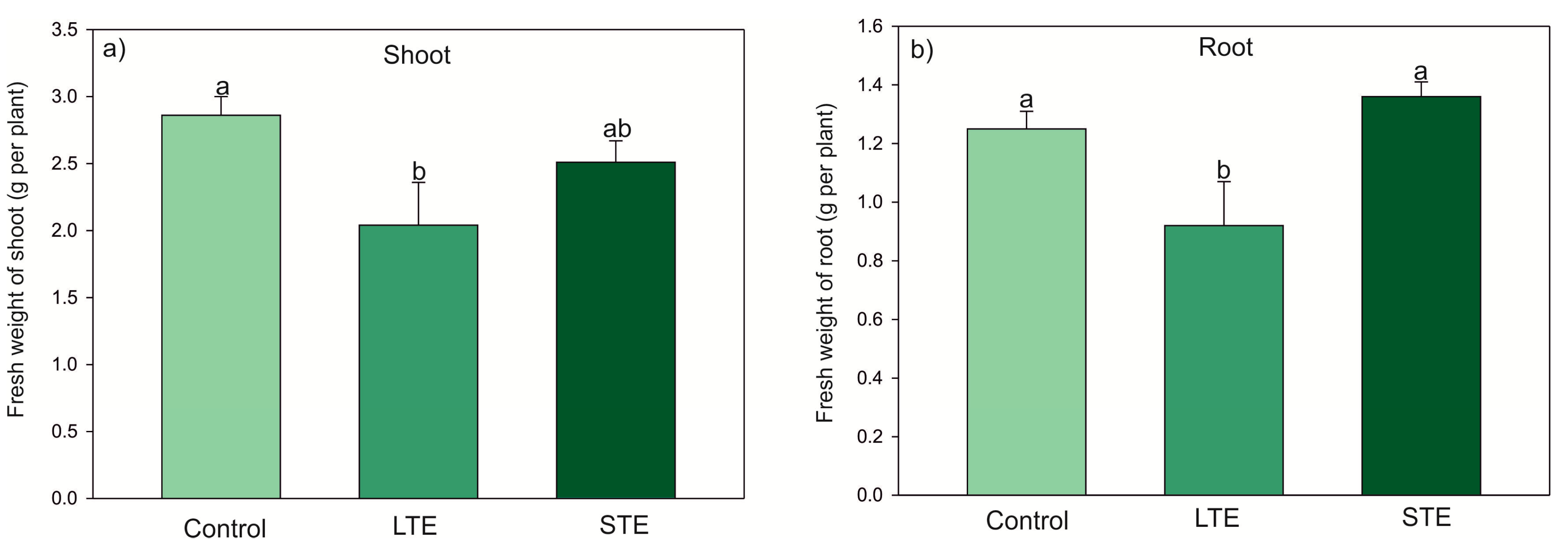
2.1. Leaf Visual Condition, Biomass, and Relative Chlorophyll Content
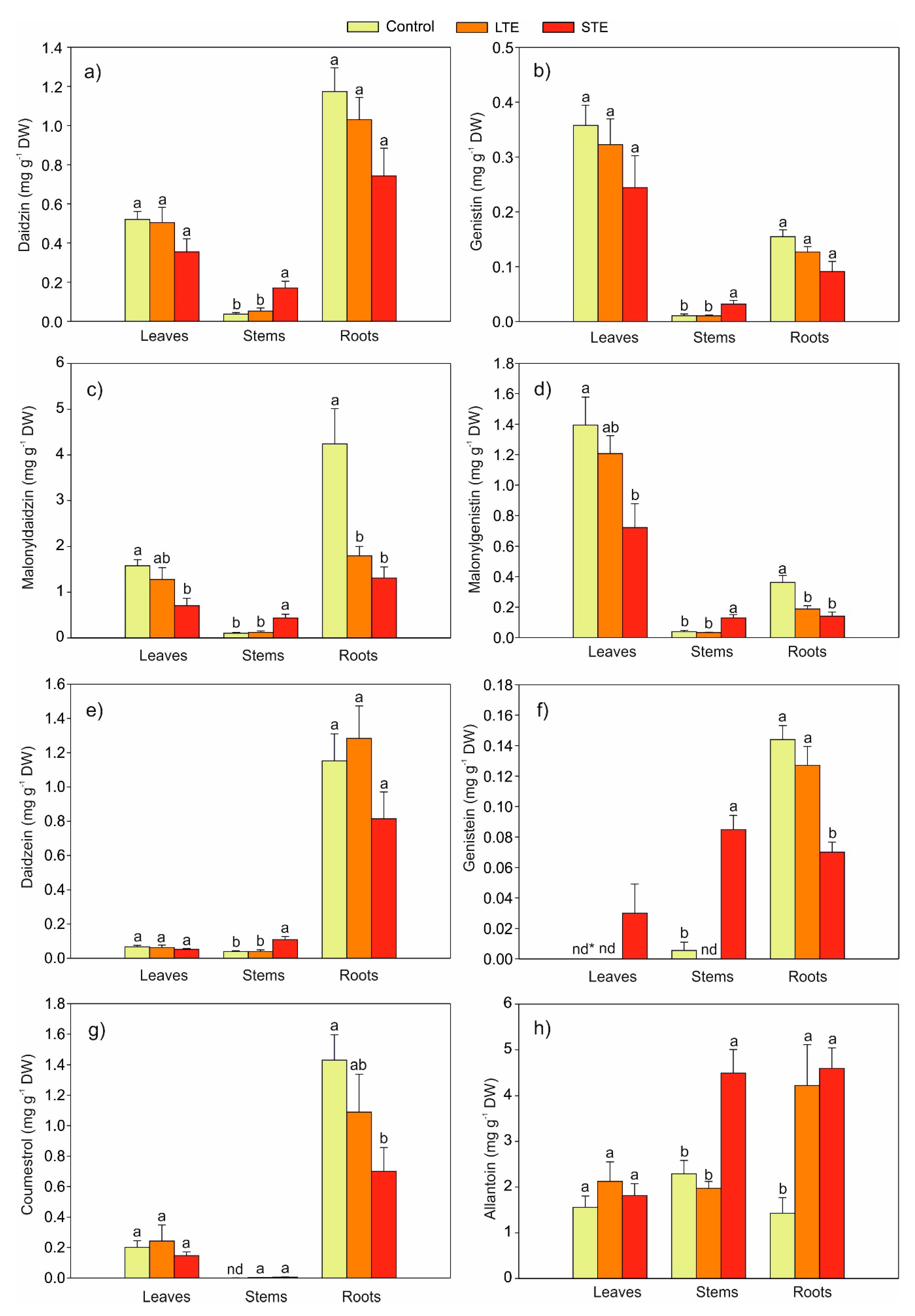
2.2. Effect of Exposure to Sr on the Content of Phytoestrogens in Soybeans
2.3. The Effect of Exposure to Sr on the Content of Allantoin in Soybean
2.4. The Influence of Sr on Its Accumulation and the Content of Selected Macroelements in Soybeans
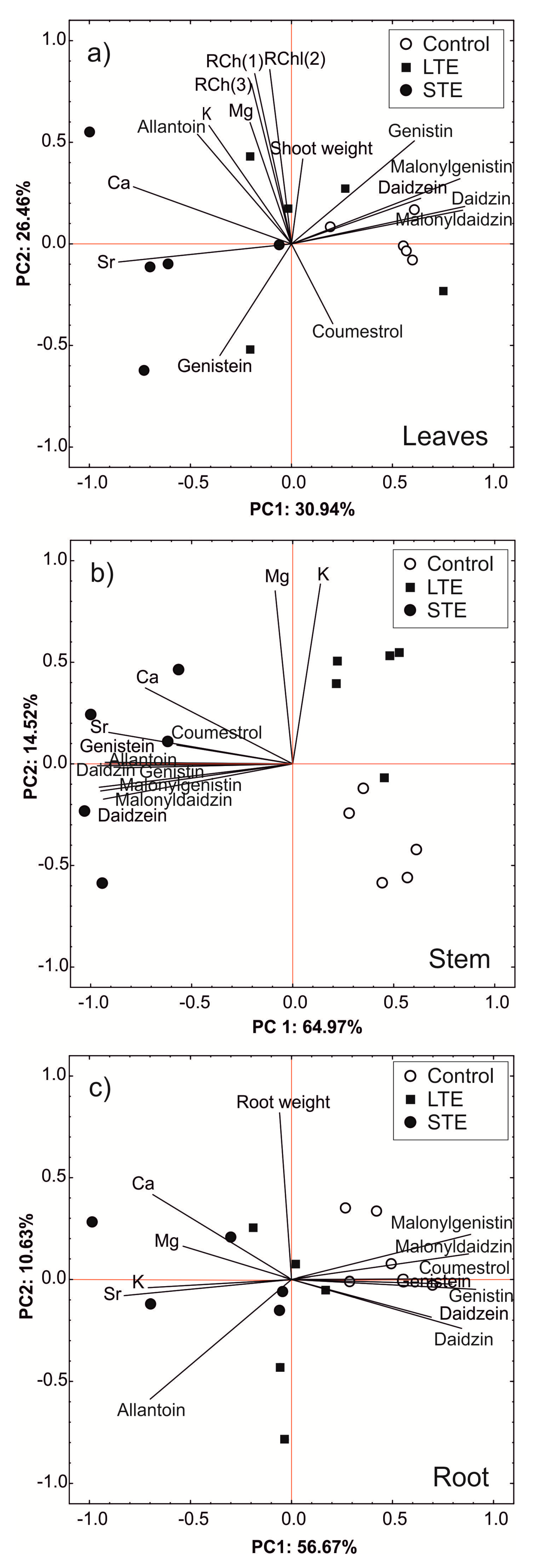
2.5. Principal Component Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Leaf Visual Condition, Biomass, and Relative Chlorophyll Content
3.3. Sample Preparation
3.4. Analysis of Phytoestrogens
3.5. Analysis of Allantoin
3.6. Analysis of Metals
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Lin, J.; Wang, C. Soybean saponins: Chemistry, analysis, and potential of health effects. In Soybeans as Functional Foods and Ingredients; Liu, K., Ed.; AOCS: Columbia, MO, USA, 2004. [Google Scholar]
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439S–450S. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Heinonen, S.-M.; Nurmi, T.; Deyama, T.; Nishibe, S.; Adlercreutz, H. Plant lignans in soy-based health supplements. J. Agric. Food Chem. 2004, 52, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef]
- Messina, M.; Messina, V. The role of soy in vegetarian diets. Nutrients 2010, 2, 855–888. [Google Scholar] [CrossRef] [PubMed]
- Villares, A.; Rostagno, M.A.; García-Lafuente, A.; Guillamón, E.; Martínez, J.A. Content and profile of isoflavones in soy-based foods as a function of the production process. Food Bioprocess. Tech. 2011, 4, 27–38. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Huang, L.-J.; Wang, J.-P.; Teng, C.-M.; Chen, S.-C.; Kuo, S.-C. Synthesis and anti-platelet, anti-inflammatory and anti-allergic activities of methoxyisoflavanquinone and related compounds. Chem. Pharm. Bull. 2000, 48, 964–973. [Google Scholar] [CrossRef][Green Version]
- Jackman, K.A.; Woodman, O.L.; Sobey, C.G. Isoflavones, equol and cardiovascular disease: Pharmacological and therapeutic insights. Curr. Med. Chem. 2007, 14, 2824–2830. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Davis, D.D.; Díaz-Cruz, E.S.; Landini, S.; Kim, Y.-W.; Brueggemeier, R.W. Evaluation of synthetic isoflavones on cell proliferation, estrogen receptor binding affinity, and apoptosis in human breast cancer cells. J. Steroid Biochem. 2008, 108, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J. Biol. Chem. 2006, 281, 37102–37110. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant. Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Coxam, V. Phyto-oestrogens and bone health. Proc. Nutr. Soc. 2008, 67, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Wójciak-Kosior, M.; Sowa, I.; Blicharski, T.; Strzemski, M.; Dresler, S.; Szymczak, G.; Wnorowski, A.; Kocjan, R.; Świeboda, R. The stimulatory effect of strontium ions on phytoestrogens content in Glycine max (L.) Merr. Molecules 2016, 21, 90. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Strzemski, M. Soy as a rich source of nutrient and bioactive components. In Recent Progress in Medicinal Plants. Nutraceuticals and Functional Foods; Manohar, P., Govil, J.N., Eds.; Studium Press: Houston, TX, USA, 2016; Volume 42, pp. 83–100. [Google Scholar]
- Wójciak-Kosior, M.; Dresler, S.; Sowa, I.; Łuć, K.; Staniak, M.; Latalski, M.; Zapała-Kiełbowicz, K.; Kocjan, R. Effect of various strontium concentrations on its uptake and the content of isoflavones in soybean sprouts. Acta Biol. Cracov. Ser. Bot. 2019, 61, 2, in press. [Google Scholar]
- Cianferotti, L.; D’Asta, F.; Brandi, M.L. A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther. Adv. Musculoskel. 2013, 5, 127–139. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: A novel mode of action optimizing bone formation and resorption. Osteoporos. Int. 2005, 16, S7–S10. [Google Scholar] [CrossRef]
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Dresler, S.; Szwerc, W.; Blicharski, T.; Szymczak, G.; Kocjan, R. Biofortification of soy (Glycine max (L.) Merr.) with strontium ions. J. Agric. Food Chem. 2014, 62, 5248–5252. [Google Scholar] [CrossRef]
- Höllriegl, V.; München, H.Z. Strontium in the environment and possible human health effects. Encycl. Environ. Health 2011, 268–275. [Google Scholar] [CrossRef]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Kováčik, J.; Blicharski, T. Effect of long-term strontium exposure on the content of phytoestrogens and allantoin in soybean. Int. J. Mol. Sci. 2018, 19, 3864. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Strontium transport, distribution, and toxic effects on maize seedling growth. Russ. J. Plant Physiol. 2004, 51, 215–221. [Google Scholar] [CrossRef]
- Watanabe, T.; Okada, K. Interactive effects of Al, Ca and other cations on root elongation of rice cultivars under low pH. Ann. Bot. 2005, 95, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xitao, X.; Renhao, X.; Zhili, L. Effects of strontium-induced stress on marine microalgae Platymonas subcordiformis (Chlorophyta: Volvocales). Chin. J. Oceanol. Limnol. 2006, 24, 154–160. [Google Scholar] [CrossRef]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanisławski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wójcik, M. Selected secondary metabolites in Echium vulgare L. populations from nonmetalliferous and metalliferous areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids—biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of some secondary metabolite content in the seventeen species of the boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef]
- Irani, S.; Lobo, J.M.; Gray, G.R.; Tood, C.D. Allantoin accumulation in response to increased growth irradiance in Arabidopsis thaliana. Biol. Plantarum 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Irani, S.; Todd, C.D. Exogenous allantoin increases Arabidopsis seedlings tolerance to NaCl stress and regulates expression of oxidative stress response genes. J. Plant Physiol. 2018, 221, 43–50. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Kováčik, J.; Pochwatka, M.; Hanaka, A.; Strzemski, M.; Sowa, I.; Wójciak-Kosior, M. Allantoin attenuates cadmium-induced toxicity in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 170, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nourimand, M.; Todd, C.D. Allantoin increases cadmium tolerance in Arabidopsis via activation of antioxidant mechanisms. Plant Cell Physiol. 2016, 57, 2485–2496. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Wang, X.; Chen, C.; Wang, J. Phytoremediation of strontium contaminated soil by Sorghum bicolor (L.) Moench and soil microbial community-level physiological profiles (CLPPs). Environ. Sci. Pollut. R. 2017, 24, 7668–7678. [Google Scholar] [CrossRef] [PubMed]
- Sasmaz, A.; Sasmaz, M. The phytoremediation potential for strontium of indigenous plants growing in a mining area. Environ. Exp. Bot. 2009, 67, 139–144. [Google Scholar] [CrossRef]
- Kamra, O.P. Somatic and genetic effects of incorporated strontium-90 and cesium-137 on Arabidopsis thaliana. Can. J. Bot. 1974, 52, 27–34. [Google Scholar] [CrossRef]
- Leung, J.K.C.; Shang, Z.R. Uptake of 137Cs and 90Sr in rice plants. Health Phys. 2003, 84, 170–179. [Google Scholar] [CrossRef]
- Bataitiene, I.P. 90Sr distribution in system "soil-Scots pine" (Pinus sylvestris L.). In Behaviour of Strontium in Plants and the Environment; Gupta, D.K., Walther, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 93–108. [Google Scholar] [CrossRef]
- Gupta, D.K.; Schulz, W.; Steinhauser, G.; Walther, C. Radiostrontium transport in plants and phytoremediation. Environ. Sci. Pollut. R. 2018, 25, 29996–29998. [Google Scholar] [CrossRef]
- Moyen, C.; Roblin, G. Uptake and translocation of strontium in hydroponically grown maize plants, and subsequent effects on tissue ion content, growth and chlorophyll a/b ratio: Comparison with Ca effects. Environ. Exp. Bot. 2010, 68, 247–257. [Google Scholar] [CrossRef]
- Kartosentono, S.; Nuraida, A.; Indrayanto, G.; Zaini, N.C. Phytoremediation of Sr2+ and its influence on the growth, Ca2+ and solasodine content of shoot cultures of Solanum laciniatum. Biotechnol. Lett. 2001, 23, 153–155. [Google Scholar] [CrossRef]
- Szymczak, G.; Wójciak-Kosior, M.; Sowa, I.; Zapała, K.; Strzemski, M.; Kocjan, R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. J. Food Compos. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of shikonin and rosmarinic acid in Echium vulgare L. and Echium russicum J.F. Gmel. by capillary electrophoresis. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 698–701. [Google Scholar] [CrossRef]



| Sr | Ca | Mg | K | |
|---|---|---|---|---|
| TFL | ||||
| Control | - | 3.37 ± 0.54 a | 0.29 ± 0.06 a | 1.50 ± 0.20 a |
| LTE | 0.89 ± 0.14 b | 3.38 ± 0.67 a | 0.24 ± 0.05 a | 1.20 ± 0.18 a |
| STE | 8.15 ± 1.16 a | 3.94 ± 0.47 a | 0.31 ± 0.06 a | 1.25 ± 0.18 a |
| TFS | ||||
| Control | - | 1.63 ± 0.24 b | 0.19 ± 0.04 a | 0.79 ± 0.10 a |
| LTE | 1.01 ± 0.27 b | 2.21 ± 0.17 a | 0.19 ± 0.03 a | 0.78 ± 0.15 a |
| STE | 4.42 ± 0.82 a | 1.94 ± 0.47 ab | 0.20 ± 0.05 a | 0.64 ± 0.11 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaka, A.; Dresler, S.; Wójciak-Kosior, M.; Strzemski, M.; Kováčik, J.; Latalski, M.; Zawiślak, G.; Sowa, I. The Impact of Long- and Short-Term Strontium Treatment on Metabolites and Minerals in Glycine max. Molecules 2019, 24, 3825. https://doi.org/10.3390/molecules24213825
Hanaka A, Dresler S, Wójciak-Kosior M, Strzemski M, Kováčik J, Latalski M, Zawiślak G, Sowa I. The Impact of Long- and Short-Term Strontium Treatment on Metabolites and Minerals in Glycine max. Molecules. 2019; 24(21):3825. https://doi.org/10.3390/molecules24213825
Chicago/Turabian StyleHanaka, Agnieszka, Sławomir Dresler, Magdalena Wójciak-Kosior, Maciej Strzemski, Jozef Kováčik, Michał Latalski, Grażyna Zawiślak, and Ireneusz Sowa. 2019. "The Impact of Long- and Short-Term Strontium Treatment on Metabolites and Minerals in Glycine max" Molecules 24, no. 21: 3825. https://doi.org/10.3390/molecules24213825
APA StyleHanaka, A., Dresler, S., Wójciak-Kosior, M., Strzemski, M., Kováčik, J., Latalski, M., Zawiślak, G., & Sowa, I. (2019). The Impact of Long- and Short-Term Strontium Treatment on Metabolites and Minerals in Glycine max. Molecules, 24(21), 3825. https://doi.org/10.3390/molecules24213825










