Synthesis, Antiproliferative, and Antioxidant Evaluation of 2-Pentylquinazolin-4(3H)-one(thione) Derivatives with DFT Study
Abstract
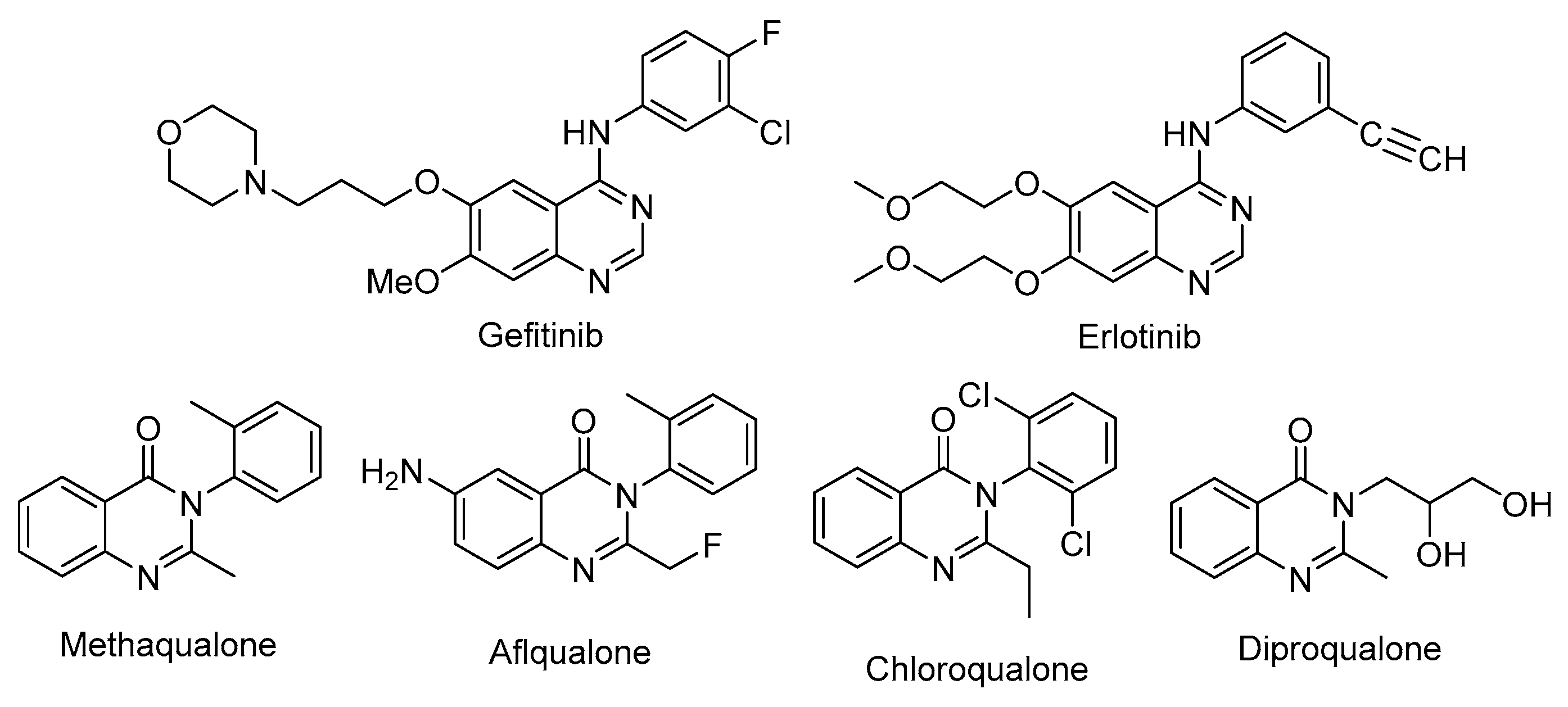
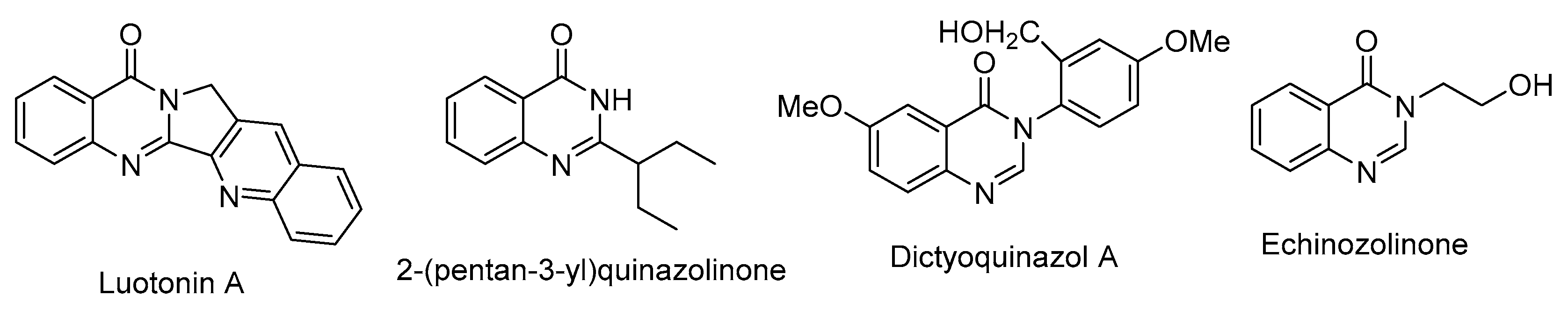
1. Introduction
2. Results and Discussion
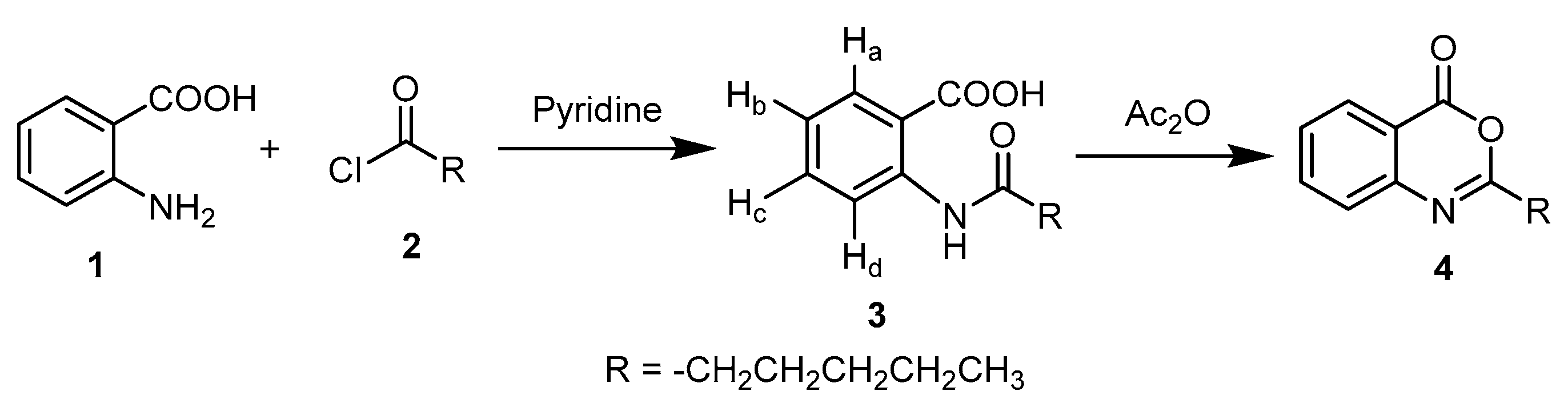
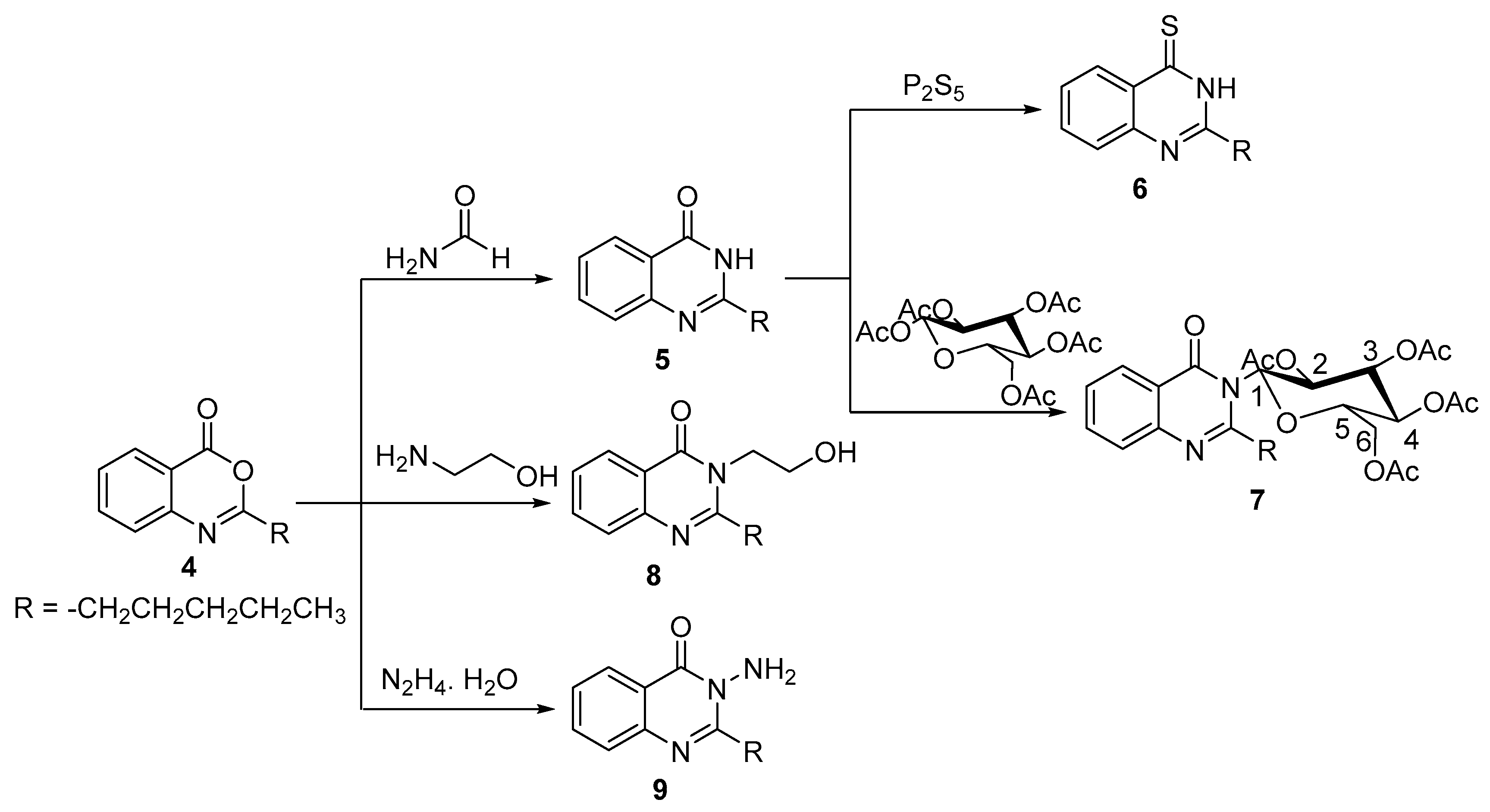
2.1. Chemistry
2.2. Biological Evaluation
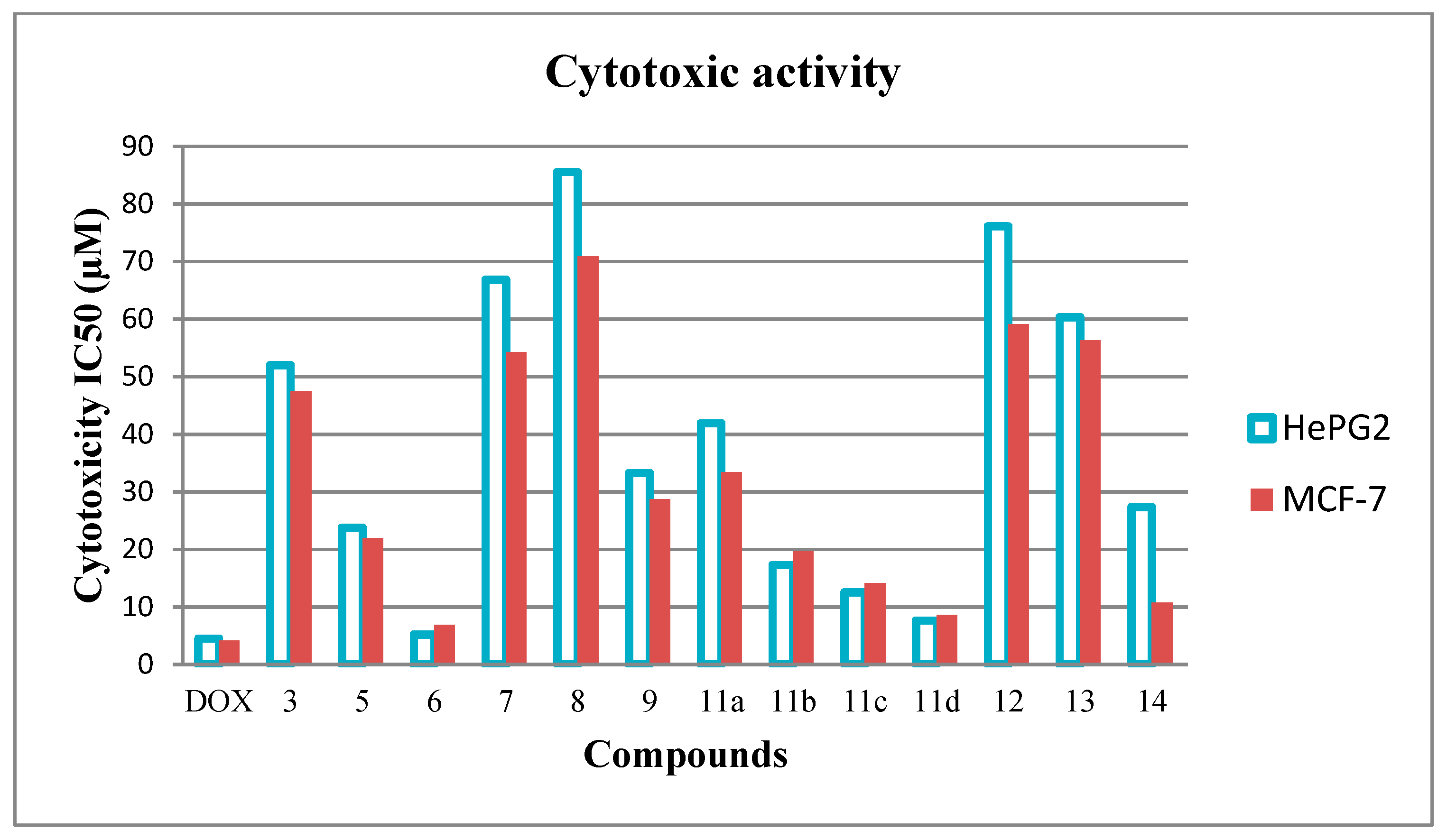
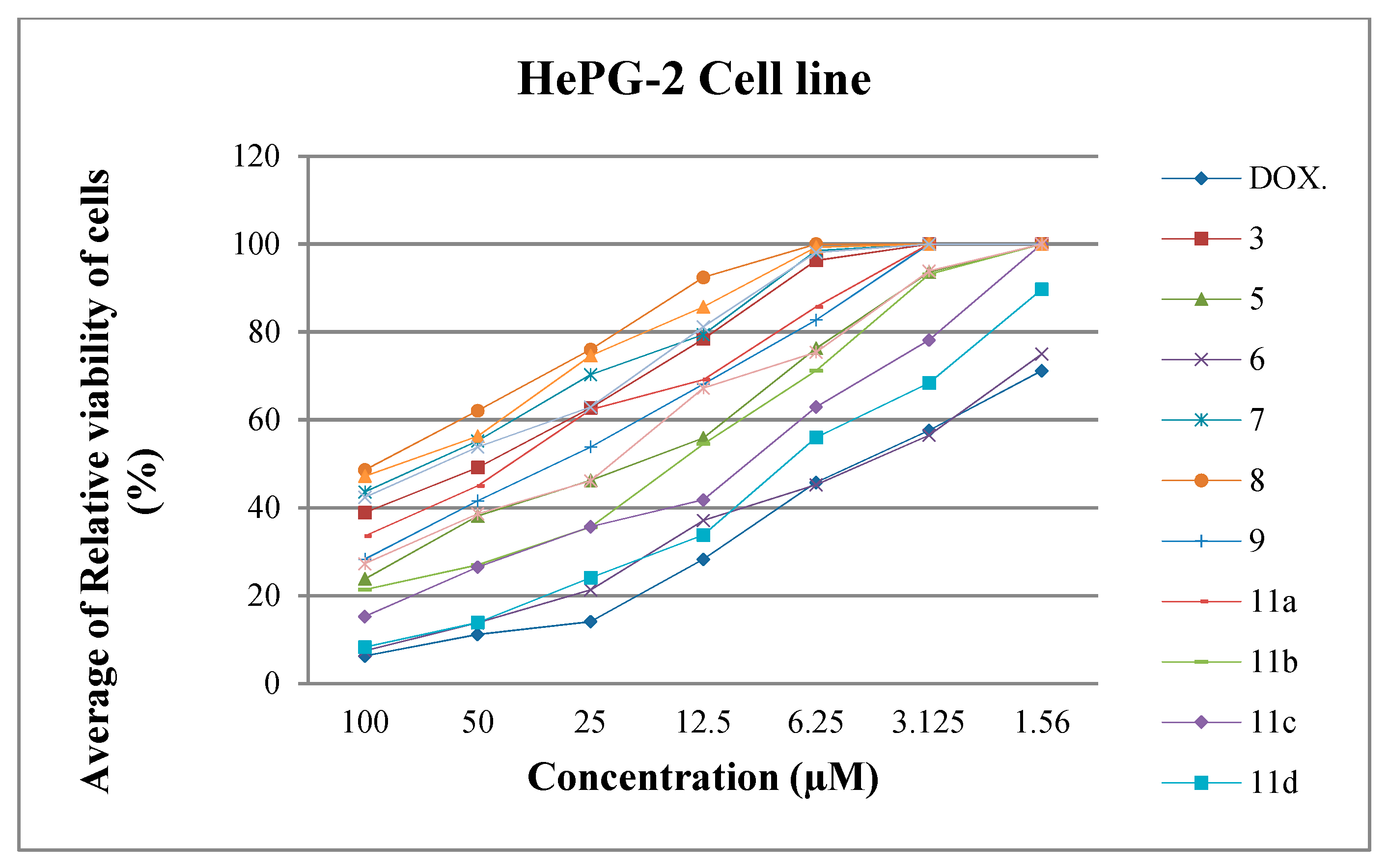
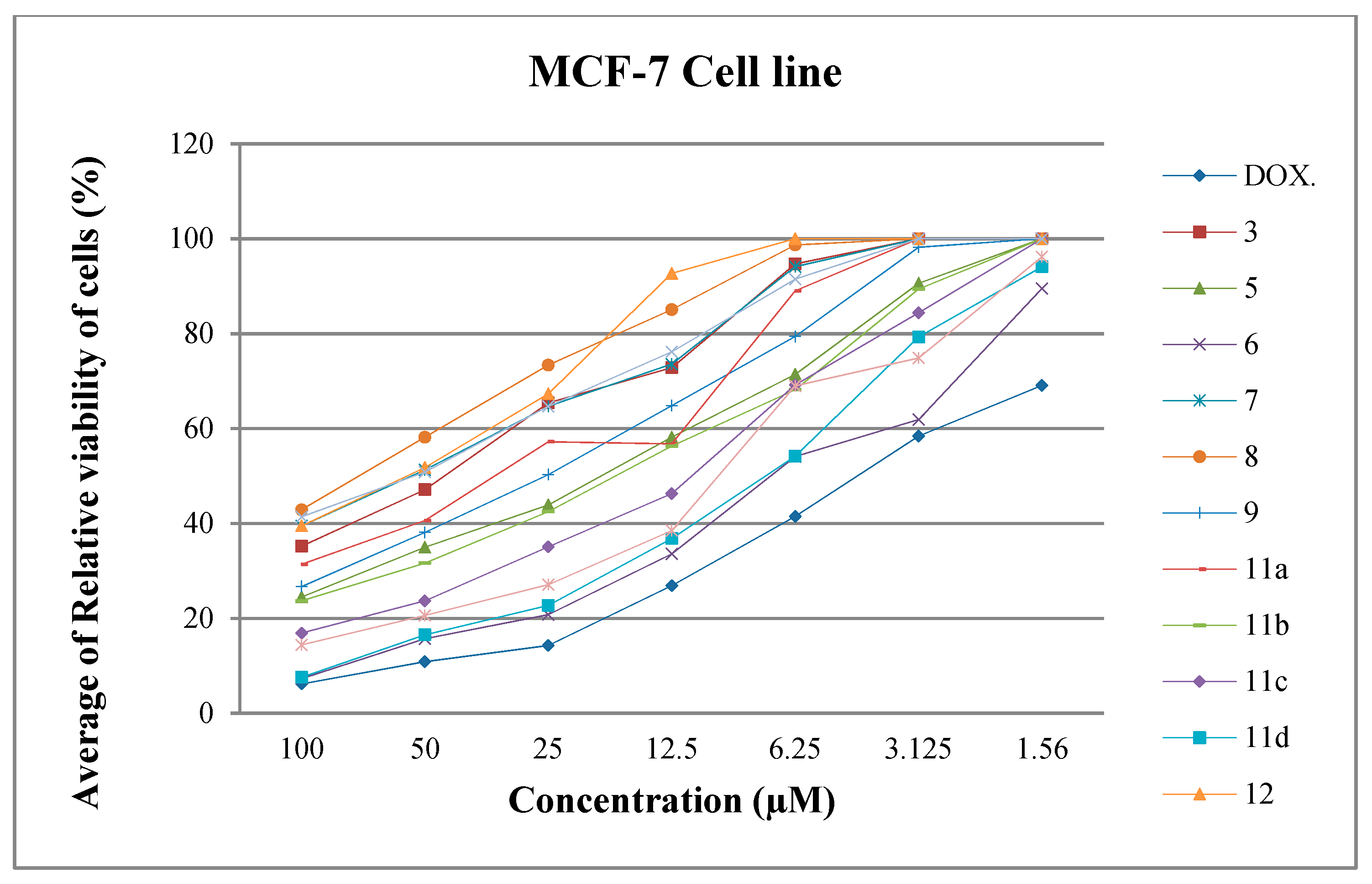
2.2.1. Antiproliferative Screening
Structure Activity Relationship (SAR)
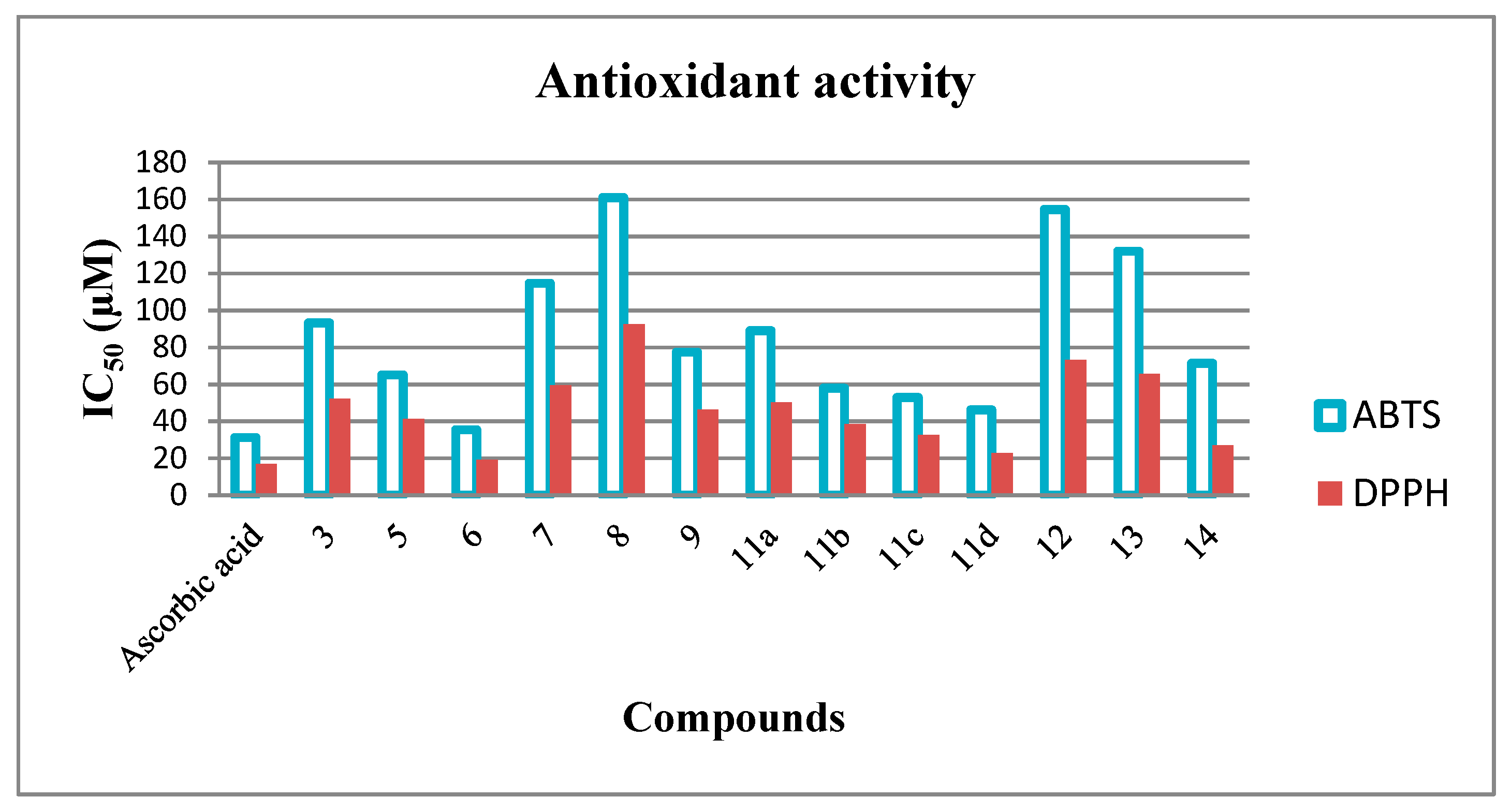
2.2.2. Antioxidant Activity Screening
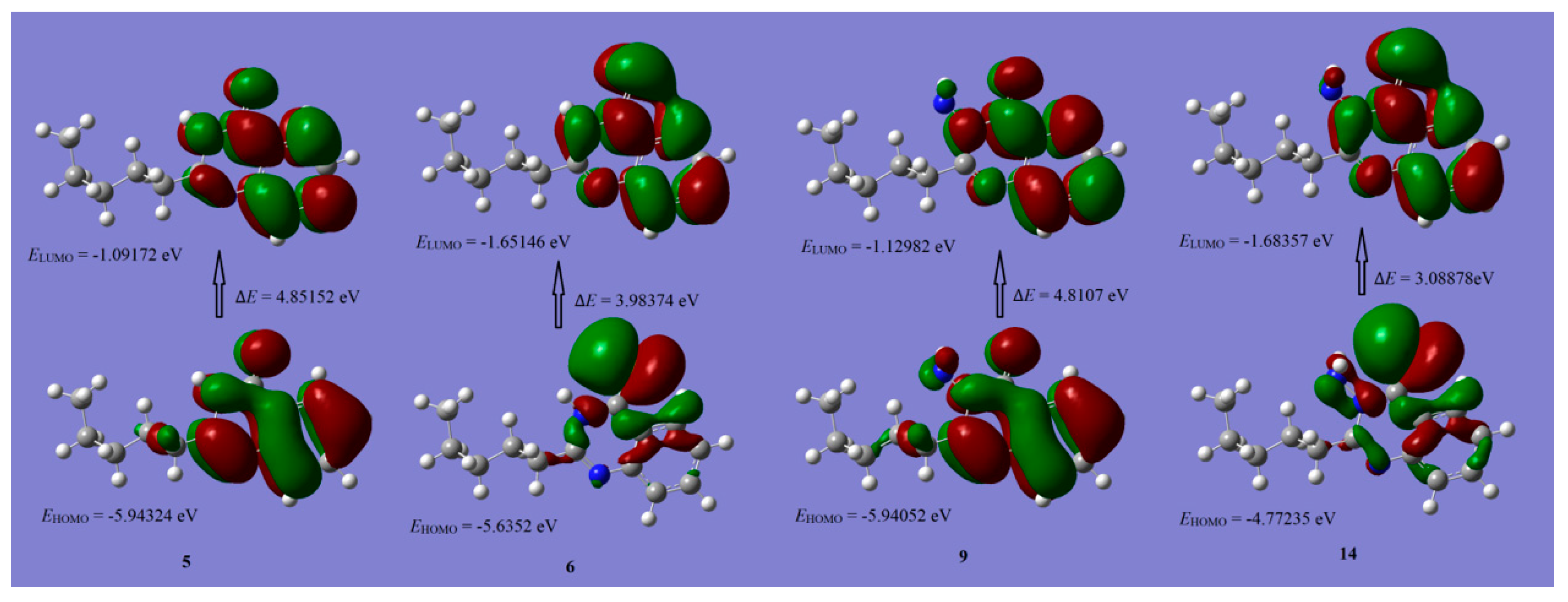
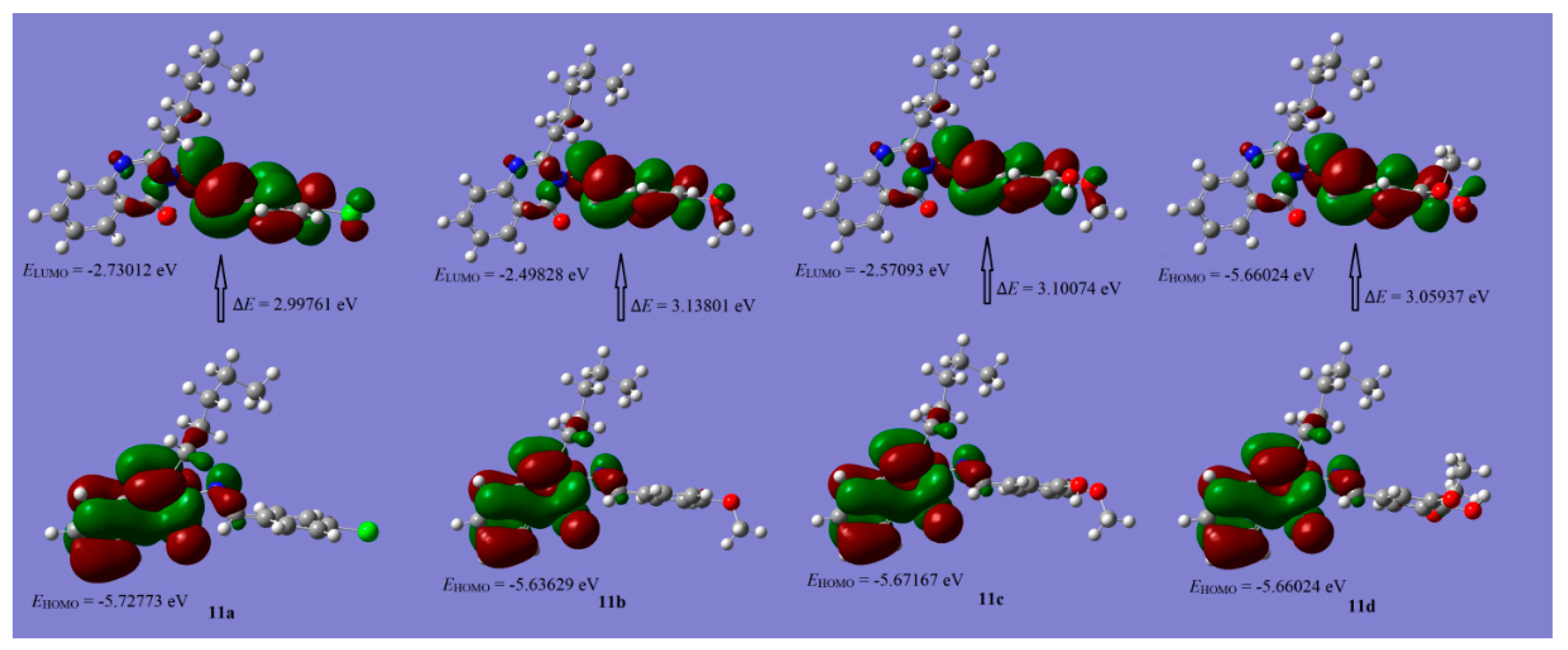
2.3. Density Functional Theory
3. Materials and Methods
3.1. Chemistry
3.1.1. 2-Hexanamidobenzoic Acid 3
3.1.2. 2-Pentyl-4H-benzo[d][1,3]oxazin-4-one 4
3.1.3. 2-Pentylquinazolin-4(3H)-one 5
3.1.4. 2-Pentylquinazoline-4(3H)-thione 6
3.1.5. N-(β-d-Glucopyranosyl-2,3,4,6-tetraacetate)-2-pentylquinazolin-4(3H)-one 7
3.1.6. 3-(2-Hydroxyethyl)-2-pentylquinazolin-4(3H)-one 8
3.1.7. 3-Amino-2-pentylquinazolin-4(3H)-one 9
3.1.8. General Procedure for Synthesis of 11a–d
3-((4-Chlorobenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11a
3-((4-Methoxybenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11b
3-((3-Hydroxy-4-methoxybenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11c
3-((4-Hydroxy-3,5-dimethoxybenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11d
3.1.9. 2-(4-Chlorophenyl)-3-(4-oxo-2-pentylquinazolin-3(4H)-yl)thiazolidin-4-one 12
3.1.10. 4,5,6,7-Tetrachloro-2-(4-oxo-2-pentylquinazolin-3(4H)-yl)isoindoline-1,3-dione 13
3.1.11. 3-Amino-2-pentylquinazoline-4(3H)-thione 14
3.2. Cytotoxicity and Antiproliferative Evaluation
MTT Assay
3.3. Antioxidant Assay
3.3.1. Antioxidant Activity Screening Assay
ABTS Method
DPPH Method
3.4. Computational Procedures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stewart, B.W.; Wild, C.P. (Eds.) World Cancer Report; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Diana, P.; Cirrincione, G.; Castagliuolo, I.; Brun, P.; Issinger, O.G.; Tisi, S.; et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur. J. Med. Chem. 2014, 74, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Sordella, R.; Bell, D.W.; Haber, D.A.; Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004, 305, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Faivre, S.; Armand, J.P. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs 2000, 60, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Wang, Y.H.; Liou, K.T.; Chen, C.M.; Chen, C.H.; Wang, W.Y.; Chang, S.; Hou, Y.C.; Chen, K.T.; Chen, C.F.; et al. Antiinflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur. J. Pharmacol. 2007, 555, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Eltahir, K.E. Synthesis and anticonvulsant evaluation of some new 2,3,8-trisubstituted-4(3H)-quinazoline derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- Shcherbakova, I.; Balandrin, M.F.; Fox, J.; Ghatak, A.; Heaton, W.L.; Conklin, R.L. 3H-Quinazolin-4-ones as a new calcilytic template for the potential treatment of osteoporosis. Bioorg. Med. Chem. Lett. 2005, 15, 1557–1560. [Google Scholar] [CrossRef]
- Reddy, A.G.; Babu, V.H.; Rao, Y.J.P. A review on quinazolines as anticancer agents. J. Chem. Pharm.Sci. 2017, 10, 1492–1504. [Google Scholar]
- Mohamed, M.A.; Ayyad, R.R.; Shawer, T.Z.; Abdel-Aziz, A.A.-M.; El-Azab, A.S. Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur. J. Med. Chem. 2016, 112, 106–113. [Google Scholar] [CrossRef]
- Chinigo, G.M.; Paige, M.; Grindrod, S.; Hamel, E.; Dakshanamurthy, S.; Chruszcz, M.; Minor, W.; Brown, M.L. Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 2008, 51, 4620–4631. [Google Scholar] [CrossRef]
- Kubo, K.; Shimizu, T.; Ohyama, S.; Murooka, H.; Iwai, A.; Nakamura, K.; Hasegawa, K.; Kobayashi, Y.; Takahashi, N.; Takahashi, K.; et al. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: synthesis, structure-activity relationships, and antitumor activities of N-phenyl-N’-{4-(4-quinolyloxy)phenyl}ureas. J. Med. Chem. 2005, 48, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Hour, M.-J.; Huang, L.-J.; Kuo, S.-C.; Xia, Y.; Bastow, K.; Nakanishi, Y.; Hamel, E.; Lee, K.-H. 6-Alkylamino- and 2,3-Dihydro-3‘-methoxy-2-phenyl-4-quinazolinones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 2000, 43, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Takase, Y.; Saeki, T.; Watanabe, N.; Adachi, H.; Souda, S.; Saito, I. Cyclic GMP Phosphodiesterase inhibitors. 2. requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity. J. Med. Chem. 1994, 37, 2106−2111. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, G.; Jafari, E.; Hakimelahi, G.; Abedi, D.; Rahmani, K.M.; Hassanzadeh, F. Synthesis of some new quinazolinone derivatives and evaluation of their antimicrobial activities. Iran, J. Pharm. Res. 2012, 11, 789–797. [Google Scholar]
- Venkatesh, R.; Kasaboina, S.; Jain, N.; Janardhan, S.; Holagunda, U.D.; Nagarapu, L. Design and synthesis of novel sulphamide tethered quinazolinone hybrids as potential antitumor agents. J. Mol. Struct. 2019, 1181, 403–411. [Google Scholar] [CrossRef]
- Patel, M.B.; Kumar, S.P.; Valand, N.N.; Jasrai, Y.T.; Menon, S.K. Synthesis and biological evaluation of cationic fullerene quinazolinone conjugates and their binding mode with modeled Mycobacterium tuberculosis hypoxanthine-guanine phosphoribosyltransferase enzyme. J. Mol. Model. 2013, 19, 3201–3217. [Google Scholar] [CrossRef]
- Mhaske, S.B.; Argade, N.P. the chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 2006, 62, 9787–9826. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Yu, K.; Chen, C.; Guo, H.; Yang, N.; Gao, H.; Liu, X.; Liu, M.; Tong, Y.; et al. Quinazolin-4-one coupled with pyrrolidin-2-iminium alkaloids from marine-derived fungus Penicillium aurantiogriseum. Mar Drugs. 2012, 10, 1297–1306. [Google Scholar] [CrossRef]
- Obafemi, C.A.; Fadare, O.A.; Jasinski, J.P.; Millikan, S.P.; Obuotor, E.M.; Iwalewa, E.O.; Famuyiwa, S.O.; Sanusi, K.; Yilmaz, Y.; Ceylan, Ü. Microwave-assisted synthesis, structural characterization, DFT studies, antibacterial and antioxidant activity of 2-methyl-4-oxo-1,2,3,4- tetrahydroquinazoline-2-carboxylic acid. J. Mol. Struct. 2018, 1155, 610–622. [Google Scholar] [CrossRef]
- Patel, N.B.; Patel, V.N. New 2,3-disubstituted quinazolin-4 (3H)-ones as antimicrobial agents. Ind. J. Heterocycl. Chem. 2007, 16, 247–250. [Google Scholar]
- Peet, N.P.; Baugh, L.E.; Sunder, S.; Lewis, J.E.; Matthews, E.H.; Olberding, E.L.; Shah, D.N. 3-(1H-Tetrazol-5-yl)-4(3H)-quinazolinone sodium salt (MDL 427): A new antiallergic agent. J. Med. Chem. 1986, 29, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Barge, M.; Rashinkar, G.; Salunkhe, R. Aqueous hydrotrope: An efficient and reusable medium for a green one-pot, diversity-oriented synthesis of quinazolinone derivatives. Mol. Divers. 2015, 19, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.N.; Haiba, N.S.; Asal, A.M.; Bekhit, A.A.; Guemei, A.A.; Amer, A.; El-Faham, A. Study of antileishmanial activity of 2-aminobenzoyl amino acid hydrazides and their quinazoline derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Haghighijoo, Z.; Firuzi, O.; Hemmateenejad, B.; Emami, S.; Edraki, N.; Miri, R. Synthesis and biological evaluation of quinazolinone-based hydrazones with potential use in Alzheimer’s disease. Bioorg. Chem. 2017, 74, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, S.B.; Argade, N.P. Regioselective quinazolinone-directed ortho lithiation of quinazolinoylquinoline: practical synthesis of naturally occurring human DNA topoisomerase I poison luotonin a and luotonins B and E. J. Organomet. Chem. 2004, 69, 4563–4566. [Google Scholar] [CrossRef]
- Houck, D.R.; Ondeyka, J.; Zink, D.L.; Inamine, E.; Goetz, M.A.; Hensens, O.D. On the biosynthesis of asperlicin and the directed biosynthesis of analogs in Aspergillus alliaceus. J. Antibiot. (Tokyo) 1988, 41, 882–891. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X. Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Chaudhuri, P.K. Echinozolinone, an alkaloid from Echinops echinatus. Phytochemistry 1987, 26, 587–589. [Google Scholar] [CrossRef]
- Ramanathan, M.; Hsu, M.T.; Liu, S.T. Preparation of 4(3H)-quinazolinones from aryldiazonium salt, nitriles and 2-aminobenzoate via a cascade annulation. Tetrahedron 2019, 75, 791–796. [Google Scholar] [CrossRef]
- Sales, Z.S.; Mani, N.S.; Allison, B.D. The synthesis of 2-amino-4(3H)-quinazolinones and related heterocycles via a mild electrocyclization of aryl guanidines. Tetrahedron Lett. 2018, 59, 1623–1626. [Google Scholar] [CrossRef]
- Ismail, M.F.; El-sayed, G.A. Dodecanoyl isothiocyanate and N’-(2-cyanoacetyl)dodecane- hydrazide as precursors for the synthesis of different heterocyclic compounds with interesting antioxidant and antitumor activity. Synth. Commun. 2018, 48, 892–905. [Google Scholar] [CrossRef]
- Ajani, O.O.; Audu, O.Y.; Aderohunmu, D.V.; Owolabi, F.E.; Olomieja, A.O. Review Article Undeniable Pharmacological Potentials of Quinazoline Motifs in Therapeutic Medicine. Am. J. Drug Discov. Dev. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Abbas, S.Y.; El-Bayouki, K.A.M.; Basyouni, W.M. Utilization of isatoic anhydride in the syntheses of various types of quinazoline and quinazolinone derivatives. Synth. Commun. 2016, 46, 993–1035. [Google Scholar] [CrossRef]
- Maiden, T.M.M.; Harrity, J.P.A. Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 2016, 14, 8014–8025. [Google Scholar] [CrossRef]
- Rohokale, R.S.; Kshirsagar, U.A. Advanced Synthetic Strategies for Constructing Quinazolinone Scaffolds. Synthesis 2016, 48, 1253–1268. [Google Scholar] [CrossRef]
- Youssef, Y.M.; El-Sayed, A.A.; Azab, M.E. Utility of Benzoxazin-4-one and 3-amino- quinazolin-4-one Derivatives as Precursors for Construction of Potent Insecticidal Heterocycles. J. Heterocyclic Chem. 2019. [Google Scholar] [CrossRef]
- Park, W.J.; Ma, E. Inhibition of PCAF histone acetyltransferase and cytotoxic effect of N-acylanthranilic acids. Pharmacal. Res. 2012, 35, 1379–1386. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.-F. Palladium-catalyzed carbonylative synthesis of benzoxazinones from N-(o-Bromoaryl)amides using paraformaldehyde as the carbonyl source. J. Org. Chem. 2014, 79, 10410–10416. [Google Scholar] [CrossRef]
- Mahindroo, N.; Ahmed, Z.; Bhagat, A.; Bedi, K.L.; Khajuria, R.K.; Kapoor, V.K.; Dhar, K.L. Synthesis and structure-activity relationships of vasicine analogues as bronchodilatory agents. Med. Chem. Res. 2006, 14, 347–368. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, C.; Liu, Y.; Tang, Y.; Li, F. Auto-tandem catalysis with ruthenium: From o-aminobenzamides and allylic alcohols to quinazolinones via redox isomerization/acceptorless dehydrogenation. Adv. Synth. Catal. 2018, 360, 3751–3759. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, V.; Balachandran, V. FT-IR, FT-Raman spectra, NBO, HOMO-LUMO and thermodynamic functions of 4-chloro-3-nitrobenzaldehyde based on ab initio HF and DFT calculations. Spectrochim. Acta A 2012, 98, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ayyamperumal, N.; Balachandran, V.; Thangavel, K. Molecular structure, vibrational spectra, first hyperpolarizability and HOMO-LUMO analysis of p-acetylbenzonitrile using quantum chemical calculation. J. Mol. Struct. 2013, 1038, 134–144. [Google Scholar] [CrossRef]
- Prashanth, J.; Ramesh, G.; Naik, J.L.; Ojha, J.K.; Reddy, B.V. Molecular geometry, NBO analysis, Hyperpolarizability and HOMO-LUMO energies of 2-azido-1-phenylethanone using Quantum chemical calculations. Mater. Today Proc. 2016, 3, 3761–3769. [Google Scholar] [CrossRef]
- Al-Omary, F.A.M.; Mary, Y.S.; Panicker, C.Y.; El-Emam, A.A.; Al-Swaidan, I.A.; Al-Saadi, A.A.; Alsenoy, C.V. Spectroscopic investigations, NBO, HOMO-LUMO, NLO analysis molecular docking of 5-(adamantan-1-yl)-3anilinomethyl-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential bioactive agent. J. Mol. Struct. 2015, 1096, 1–14. [Google Scholar] [CrossRef]
- Koparir, M.; Orek, C.; Koparir, P.; Sarac, K. Synthesis, experimental, theoretical characterization and biological activities of4-ethyl-5-(2-hydroxyphenyl)-2H-1,2,4-triazole- 3(4H)-thione. Spectrochim. Acta, Part A 2013, 105, 522–531. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Şahin, Z.S.; Kantar, G.K.; Şaşmaz, S.; Büyükgüngör, O. Synthesis, molecular structure, spectroscopic analysis, thermodynamic parameters and molecular modeling studies of (2-methoxyphenyl)oxalate. J. Mol. Struct. 2015, 1087, 104–112. [Google Scholar] [CrossRef]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA. 1986, 83, 8440–8441. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; El-sayed, A.A. Synthesis and in-vitro antioxidant and antitumor evaluation of novel pyrazole-based heterocycles. J. Iran. Chem. Soc. 2019, 16, 921–937. [Google Scholar] [CrossRef]
- Lissi, E.A.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Compounds | In Vitro Cytotoxicity IC50 (µM) | |
|---|---|---|
| HePG2 | MCF-7 | |
| DOX | 4.50 ± 0.3 | 4.17 ± 0.2 |
| 3 | 52.01 ± 3.3 | 47.53 ± 2.9 |
| 5 | 23.75 ± 1.9 | 21.98 ± 1.8 |
| 6 | 5.20 ± 0.5 | 6.88 ± 0.4 |
| 7 | 66.84 ± 4.0 | 54.23 ± 3.2 |
| 8 | 85.55 ± 4.5 | 70.86 ± 4.1 |
| 9 | 33.27 ± 2.5 | 28.69 ± 2.2 |
| 11a | 41.92 ± 2.8 | 33.39 ± 2.5 |
| 11b | 17.27 ± 1.4 | 19.68 ± 1.6 |
| 11c | 12.54 ± 1.1 | 14.10 ± 1.3 |
| 11d | 7.63 ± 0.6 | 8.60 ± 0.7 |
| 12 | 76.14 ± 4.3 | 59.15 ± 3.8 |
| 13 | 60.34 ± 3.8 | 56.28 ± 3.5 |
| 14 | 27.39 ± 2.3 | 10.78 ± 0.9 |
| Compounds | ABTS IC50 (µM) | DPPH IC50 (µM) |
|---|---|---|
| Ascorbic acid | 31.26 ± 0.20 | 16.83 ± 0.15 |
| 3 | 93.24 ± 0.70 | 52.14 ± 0.65 |
| 5 | 65.13 ± 0.46 | 41.16 ± 0.42 |
| 6 | 35.48 ± 0.23 | 19.25 ± 0.18 |
| 7 | 114.63 ± 0.73 | 59.43 ± 0.68 |
| 8 | 160.92 ± 0.94 | 92.36 ± 0.76 |
| 9 | 77.38 ± 0.56 | 46.23 ± 0.48 |
| 11a | 89.10 ± 0.64 | 50.12 ± 0.54 |
| 11b | 58.03 ± 0.41 | 38.47 ± 0.37 |
| 11c | 52.87 ± 0.36 | 32.69 ± 0.29 |
| 11d | 46.25 ± 0.28 | 22.91 ± 0.21 |
| 12 | 154.51 ± 0.85 | 73.03 ± 0.72 |
| 13 | 131.95 ± 0.77 | 65.58 ± 0.66 |
| 14 | 71.42 ± 0.52 | 26.87 ± 0.23 |
| Comp. No. | 5 | 6 | 9 | 14 | 11a | 11b | 11c | 11d | |
|---|---|---|---|---|---|---|---|---|---|
| E HOMO (eV) | −5.9432 | −5.6352 | −5.9405 | −4.7724 | −5.7277 | −5.6363 | −5.6717 | −5.6602 | |
| E LUMO (eV) | −1.0917 | −1.6515 | −1.1298 | −1.6836 | −2.7301 | −2.4983 | −2.5709 | −2.6009 | |
| (∆E) Energy gap (eV) | 4.8515 | 3.9837 | 4.8107 | 3.0888 | 2.9976 | 3.1380 | 3.1008 | 3.0593 | |
| (I) Ionization energy (eV) | 5.9432 | 5.6352 | 5.9405 | 4.7724 | 5.7277 | 5.6363 | 5.6717 | 5.6602 | |
| (A) Electron affinity (eV) | 1.0917 | 1.6515 | 1.1298 | 1.6836 | 2.7301 | 2.4983 | 2.5709 | 2.6009 | |
| (ƞ) Chemical hardness (eV) | 2.4258 | 1.9919 | 2.4054 | 1.5444 | 1.49881 | 1.56901 | 1.5504 | 1.52969 | |
| (S) Chemical softness (eV−1) | 0.4122 | 0.5020 | 0.4157 | 0.6475 | 0.6672 | 0.6374 | 0.6450 | 0.6537 | |
| (ET) Binding energy (a.u) | −689.79 | −1012.73 | −745.11 | −1068.05 | −1473.81 | −1128.73 | −1203.94 | −1318.44 | |
| (µ) Dipole moment (D) | 3.2867 | 3.4641 | 1.764 | 1.852 | 3.2722 | 4.4472 | 4.1944 | 6.232 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, A.A.; Ismail, M.F.; Amr, A.E.-G.E.; Naglah, A.M. Synthesis, Antiproliferative, and Antioxidant Evaluation of 2-Pentylquinazolin-4(3H)-one(thione) Derivatives with DFT Study. Molecules 2019, 24, 3787. https://doi.org/10.3390/molecules24203787
El-Sayed AA, Ismail MF, Amr AE-GE, Naglah AM. Synthesis, Antiproliferative, and Antioxidant Evaluation of 2-Pentylquinazolin-4(3H)-one(thione) Derivatives with DFT Study. Molecules. 2019; 24(20):3787. https://doi.org/10.3390/molecules24203787
Chicago/Turabian StyleEl-Sayed, Amira A., Mahmoud F. Ismail, Abd El-Galil E. Amr, and Ahmed M. Naglah. 2019. "Synthesis, Antiproliferative, and Antioxidant Evaluation of 2-Pentylquinazolin-4(3H)-one(thione) Derivatives with DFT Study" Molecules 24, no. 20: 3787. https://doi.org/10.3390/molecules24203787
APA StyleEl-Sayed, A. A., Ismail, M. F., Amr, A. E.-G. E., & Naglah, A. M. (2019). Synthesis, Antiproliferative, and Antioxidant Evaluation of 2-Pentylquinazolin-4(3H)-one(thione) Derivatives with DFT Study. Molecules, 24(20), 3787. https://doi.org/10.3390/molecules24203787







