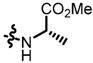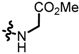Abstract
Twenty-seven L-shaped ortho-quinone analogs were designed and synthesized using a one pot double-radical synthetic strategy followed by removing methyl at C-3 of the furan ring and introducing a diverse side chain at C-2 of the furan ring. The synthetic derivatives were investigated for their cytotoxicity activities against human leukemia cells K562, prostate cancer cells PC3, and melanoma cells WM9. Compounds TB1, TB3, TB4, TB6, TC1, TC3, TC5, TC9, TC11, TC12, TC14, TC15, TC16, and TC17 exhibited a better broad-spectrum cytotoxicity on three cancer cells. TB7 and TC7 selectively displayed potent inhibitory activities on leukemia cells K562 and prostate cancer cells PC3, respectively. Further studies indicated that TB3, TC1, TC3, TC7, and TC17 could significantly induce the apoptosis of PC3 cells. TC1 and TC17 significantly induced apoptosis of K562 cells. TC1, TC11, and TC14 induced significant apoptosis of WM9 cells. The structure-activity relationships evaluation showed that removing methyl at C-3 of the furan ring and introducing diverse side chains at C-2 of the furan ring is an effective strategy for improving the anticancer activity of L-shaped ortho-quinone analogs.
1. Introduction
Over several decades, cancer continues to be the most awful disease due to its uncontrolled cell growth and the fact that it is a dominate killer of human beings worldwide [1]. Especially in China, millions of deaths have been caused by tumor. The common cancer types in Chinese male, in 2018, were lung, stomach, colorectum, liver, and esophageal cancer. Additionally, breast, lung, colorectum, thyroid, and stomach cancer were the common types in Chinese female [2]. The incidence of colorectal cancer in males and females has increased, however, the incidence of esophageal, stomach, and liver cancer has decreased between 2000 and 2011 [3]. Meanwhile, the incidence and mortality of prostate cancer and bladder cancer in males, together with obesity and hormonal exposure-related cancers, namely thyroid, breast, and ovarian cancer in females have shown a rising trend [3]. However, the standardized treatments of cancer, including surgery, chemotherapy, and radiation therapy, show many limitations, such as severe adverse effects, recurrence, and increasing drug resistance [4].
Currently, phytochemicals have become a valuable source of anticancer drugs. Actually, over 75% of nonbiological anticancer drugs approved are either plant-derived natural products or developed based on these products [5]. Therefore, natural products have continued to be a hot research topic for the development of new antitumor drugs [6,7,8,9].
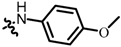
Tanshinone IIA is a natural ortho-quinone isolated from the rhizome of Salvia miltiorrhiza Bunge with antineoplastic activity, such as gastric cancer, breast cancer, osteosarcoma, etc. [10,11,12,13]. These various properties demonstrate that tanshinone IIA is a potential antitumor drug candidate. Furthermore, beta-lapachone is another natural ortho-quinone which has been reported to selectively kill many human cancer cells [14], however, the pyran ring of beta-lapachone has been found to be unstable during metabolism in the human body, and may led to side effects on normal tissues [15,16]. Recently, studies have revealed that some tanshinone analogs show similar or stronger antitumor activity when the ring-A is removed but the furan ring is retained [17,18]. You et al. [19,20] discovered that the binding site for quinone oxidoreductase-1 (NQO1) substrates was an L-shaped pocket (Figure 1B) which binds well with tanshinone analogs, and showed higher antitumor activities than the planar compound 1 and beta-lapachone. Therefore, we surmise that removing methyl at C-3 of the furan ring is more suitable for the binding site, and we anticipate that a novel L-shaped molecule without methyl at C-3 of the furan ring could provide better antitumor activities. Considering that some nitrogen, oxygen-substituted, and amino acid substrates can improve aqueous solubility and antitumor activities [21,22,23,24,25], we have attempted to introduce a great diversity of oxygen-substituted, nitrogen-containing groups and amino acids. Thus, in this work, we developed quinone-directed agents by removing methyl at C-3 of the furan ring and introducing a diverse side chain at C-2 of the furan ring, culminating in the discovery of a promising scaffold. The inhibitory activity was assessed in vitro using three cell lines including K562, PC3, and WM9.
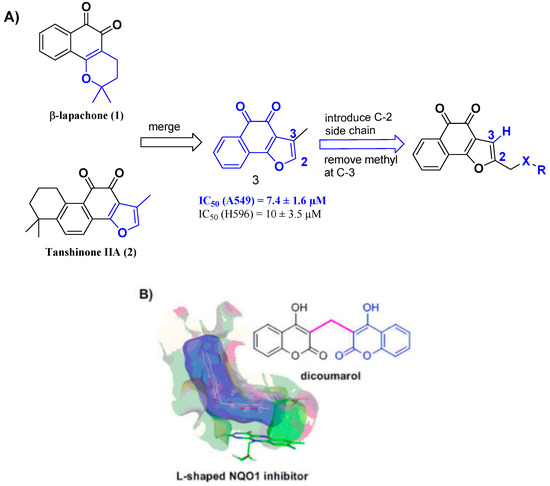
Figure 1.
(A) Structural design strategy and (B) l-shaped pocket. The figure is available in reference [19].
2. Discussion and Results
2.1. Chemistry
The synthesis of two substituted naphtho[1,2-b]furan-4,5-diones is outlined in Scheme 1. Briefly, treatment of lawsone 6 with allyl bromide followed by subsequent Claisen rearrangement afforded 7, which was then cyclized to get the ortho-quinone 8 by using Lewis acid NbCl5 at room temperature [26].
Scheme 1.
Synthesis of L-shaped ortho-quinone analogs.
Initially, dealing 8 with N-bromosuccinimide (NBS) and 2,2′-azobis(2-methylpropionitrile) (AIBN) afforded only trace amounts of 8a. Another intermediate, 8b, was obtained by Nelson’s method [27] as shown in Scheme 1. Then, compound 9 was obtained from 8b through a second radical reaction. Considering the same reaction condition, we successfully got 9 from 8 through a bis-radical reaction. The brominated intermediate 9 was reacted with substituted phenol or amine to provide ortho-quinone derivatives TB1–TB9 and TC1–TC18, respectively. All the structures of ortho-quinone derivatives were identified through 1H, 13C, and HRMS.
In summary, we successfully established an effective synthetic strategy, which removed the methyl at C-3 of the furan ring and introduced diverse side chains at C-2 of the furan ring. In addition, we replaced the bromide of 9 with a variety of oxygen-substituted, nitrogen-containing group, and amino acid by a nucleophilic substitution.
2.2. In Vitro Cytotoxicity Assay
The cytotoxic activities of 5 µmol/L of the synthesized l-shaped ortho-quinone analogs were determined by using three cancer cell lines (Table 1). The results revealed that compounds TB1, TB3, TB4, TB6, TC1, TC3, TC5, TC9, TC11, TC12, TC15, TC16, and TC17 showed a broad-spectrum potent inhibitory activity on the proliferation of the cancer cell lines, with a more than 70% inhibition rate, and TB7 showed better inhibitory activity on K562 cells as compared with other cells. Moreover, we observed that TC7 inhibited the growth of PC3 cells more efficiently than other cells.

Table 1.
The structures and inhibitory rates after treating cancer cell lines with 5 µmol/L of target compounds, respectively. Data was presented as the mean ± SD of three independent experiments.
The concentration inhibition curves (Figure 2) were analyzed to calculate the IC50 values of the selected active compounds. The results indicated there was a dose-dependent trend of the inhibitory response of all active compounds on three cancer cells for treating 48 h. The IC50 values were summarized in Table 2 and show that the cytotoxicity of compounds TB3, TC1, TC3, TC7, TC9, and TC17 on PC3 were better (P < 0.05) than that of the positive control (tanshinone IIA and paclitaxel), and another active compound exhibited similar activity to that of the positive control. The inhibition activity of TC1 against the growth of K562 was better than that of the positive control, paclitaxel, and tanshinone IIA. Compounds TB6, TC1, TC11, TC14, and TC15 inhibited the growth of WM9 better (P < 0.05) than that of tanshinone IIA and paclitaxel. In summary, most of novel L-shaped ortho-quinone analogs exhibited relatively better cytotoxicity activity as compared with the two positive controls, which indicated that the analogs containing L-shaped ortho-quinone as the core structure, possessed stronger anticancer activity. This result provided a preliminary biological activity basis for the investigation of anticancer candidate agents.
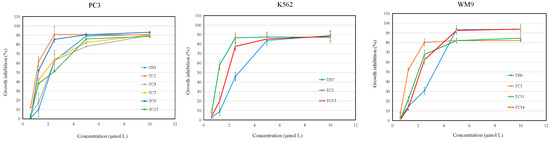
Figure 2.
Growth inhibition induced by the active L-shaped ortho-quinone analogs on PC3, K562, and WM9 cells by MTT assay. The IC50 values (µM) of the compounds were determined according to these curves at different incubation times. The 100 µL tested compounds were added to 96-well microculture plates and 100 µL cells (a final concentration of 5 × 104/well) were incubated for 48 h at 37 °C. Cell survival was evaluated by MTT assay. The inhibition ratio (%) was calculated as described in the Methods section. Data was presented as mean ± SD of three independent experiments.

Table 2.
IC50 values of selected compounds in vitro.
2.3. Structure-Activity Relationships Study
To obtain two series of analogs, we successfully built an effective synthetic strategy by removing the methyl at C-3 of the furan ring and introducing diverse side chains at C-2 of the furan ring. On the basis of the cytotoxicity results (Table 1 and Table 2), a preliminary structure-activity relationships could be established. The TB series molecules bearing electron-withdrawing groups or multi-substituted groups such as compounds TB3, TB4, and TB6 showed a better inhibitory effect on PC3 cell lines, K562 cell lines, and WM9 cell lines, whereas the TB series molecules bearing alkane groups at the 2-position showed decreased cytotoxicity in PC3 cell lines, K562 cell lines, and WM9 cell lines, such as compounds TB9. The TC series molecules with electron-withdrawing groups, saturate six-membered rings, or multi-substituted groups emerged greater inhibitory effects on three cancer cell lines, such as TC11, TC12, TC15, and TC16, whereas the TC series molecules bearing donating groups or alkane groups at the 2-position showed reduced cytotoxicity in three cancer cell lines. The structure-activity relationships evaluation also showed that removing methyl at C-3 of the furan ring and introducing diverse side chains at C-2 of the furan ring were good strategies for improving the anticancer activity of L-shaped ortho-quinone analogs.
2.4. Effects of Active Compounds on Cell Apoptosis
According to the above IC50 values of all active compounds, we selected six active compounds (TB3, TC1, TC3, TC7, TC9, and TC17) for PC3, three active compounds (TB6, TC1, and TC17) for K562, and four active compounds (TB6, TC1, TC11, and TC14) for WM9, based on their higher activities than that of the positive control and better selectivity and, then, studied their effects on cell apoptosis by microscope observation (Figure 3) and flow cytometry (Figure 4). The microscopic observations (Figure 3A) showed that the number of PC3 cells was significantly reduced by treatments with 2.5 µmol/L of TB3, TC1, TC3, and TC7; the apoptotic bodies and cell fragments were significantly observed as compared with the control group. The PC3 cells treated with TC9 showed that the number of cells were significantly reduced, while fewer cells died and there were no significant apoptotic bodies. The PC3 cells treated with TC7 showed a significant decrease in the number of cells, meanwhile, some cells died, apoptotic bodies appeared obviously, and the morphology of some cells became an irregular shape of spindle length. Above all, the inhibitory activity of TB3, TC1, TC3, and TC7 may be through inducing apoptosis, another two compounds may be through different types.
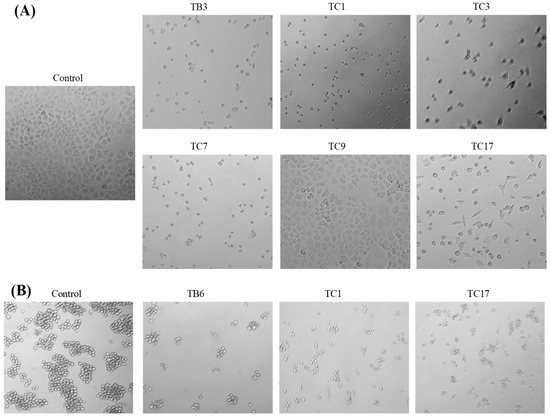
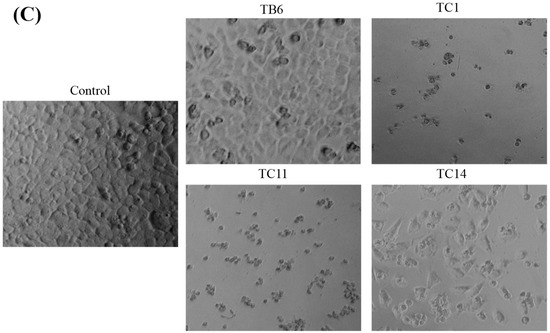
Figure 3.
Effects of the active compounds on PC3 (A), K562 (B), and WM9 (C) cell growth and apoptosis. Cell number and morphological appearance of the two types of cell lines treated with 2.5 µmol/L of active compounds, then, it was observed by a fluorescent inverted microscope after 24 h. Scale bar = 100 µM in all images. All experiments were performed in triplicate.
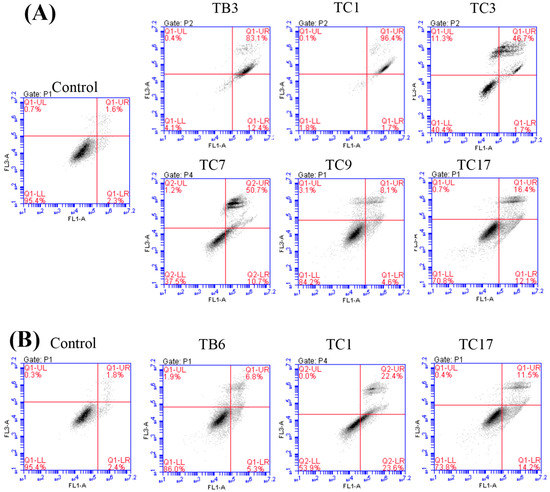
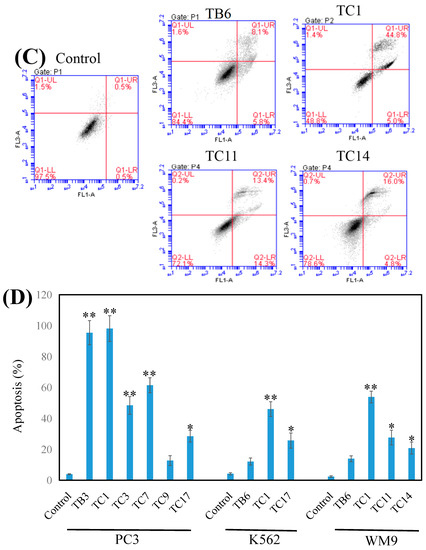
Figure 4.
Effects of the active compounds on PC3 (A), K562 (B), and WM9 (C) cell growth and apoptosis. Cell apoptosis induced by the compounds and tested by flow cytometry and the date was analyzed with Origin Pro 9.0 (D) and presented as means ± SEM from at least three independent experiments. * p < 0.05, ** p < 0.01 (n = 3) as compared with the control.
The K562 cells treated with TC1 were obviously dead and dispersed, with the appearance of apoptotic bodies as comparing with the control group (Figure 3B). The cells treated with TB6 had a significantly reduced number of cells and most cells clumped growth similar to the control cells. For the cells treated with TC17 we observed both dead cells and fewer clumps of cells. Furthermore, for the WM9 cell lines treated with TB6, TC1, TC11, and TC14 (Figure 3C), we observed that the cells treated with TC1 and TC11 were obviously dead with a large number of apoptotic bodies and dispersed cells; the cells treated with TB6 showed a significant decrease in the number of cells and fewer dead cells. Observation of the cells treated with TC14 showed that the number of cells was significantly reduced, while some cells were obviously dead with apoptotic bodies appearing, and the morphology of some cells also became an irregular shape of spindle length. The above results indicated that TC1 can induce apoptosis for K562 and WM9 cells to inhibit the growth; TB6, TC11, TC14, and TC17 can jointly inhibit the proliferation of cell through a variety of mechanisms.
Flow cytometry analyzed results (Figure 4) confirmed that TB3 (p < 0.01), TC1 (p < 0.01), TC3 (p < 0.01), TC7 (p < 0.01), and TC17 (p < 0.05) could significantly induce the apoptosis of PC3 cells (Figure 4A), while TC9 did not. TC1 (p < 0.01) and TC17 (p < 0.05) significantly induced apoptosis of K562 cells (Figure 4B), while TB6 had no significant effect on apoptosis of K562 and WM9 cells. TC1 (p < 0.01), TC11 (p < 0.05), and TC14 (p < 0.01) could potently induce apoptosis for WM9 cells (Figure 4C).
3. Materials and Methods
3.1. Instruments and Materials
High-resolution mass spectra (HRMS) were obtained on an electrospray ionization (ESI) mode on a Bruker ESI-QTOF mass spectrometry. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance NEO (1H NMR, 600 MHz; 13C NMR, 150 MHz, Bruker, Switzerland) with TMS as an internal standard. The IR spectra were recorded by using a FTIR Spectrometer (IR 200 Fourier Energy Spectrum Technology Co., Ltd., TianJin, China) and the KBr disk method was adopted. The melting points (mp) were determined on an WRX-4 microscope melting point apparatus. The column chromatography was performed on silica gel (Qingdao, 200–300 mesh) and the thin-layer (0.25 mm) chromatography (TLC) analysis was carried out on silica gel plates (Qingdao, China). Other reagents were analytical grade or guaranteed reagent commercial product and used without further purification, unless otherwise noted.
3.2. Methods of Synthesis
3.2.1. Synthesis of 2-Allyl-3-hydroxy-1,4-naphthoquinone (7)
A mixture of lawsone 6 (10.0 g, 57.42 mmol) and anhydrous K2CO3 (7.94 g, 57.42 mmol) in anhydrous DMF (100 mL) were stirred for 15 min at room temperature. Allyl bromide (17.37 g, 143.55 mmol) in DMF (5 mL) was added dropwise and stirred for 15 min at 0 °C. The mixture was refluxed at 120 °C for 3 h and then cooled to room temperature before it was poured into water and extracted with EA. The organic phase was washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (eluent: petroleum ether/EtOAc 15:1) to afford 7 (7.8 g, 63% yield) as a light yellow solid. Other data was found in reference [26].
3.2.2. Synthesis of 2-Methyl-2,3-dihydrolnaphthol[1,2-b]furan-4,5-dione (8)
NbCl5 (18.92 g, 70.02 mmol) was added into 7 (3.0 g, 14.00 mmol) in anhydrous DCM (50 mL) at 0 °C. After stirring for 45 min at 30 °C, the mixture was poured into ice water and extracted with DCM. The organic phase was washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (eluent: petroleum ether/EtOAc 4:1) to afford 8 (2.16 g, 72% yield) as red solid. Other data was found in reference [26].
3.2.3. Synthesis of 2-Bromomethyl-naphtho[1-b]furan-4,5-dione (9)
A mixture of 8 (1.5 g, 7.00 mmol), anhydrous N-bromosuccinimide (2.49 g, 1.40mmol), and 2,2′-azobis(2-methylpropionitrile) (114.98 mg, 1.40 mmol) in anhydrous CCl4 (50 mL) was stirred under argon at 70 °C, until 8 were disappeared. Then, the mixture was cooled to room temperature, and anhydrous N-bromosuccinimide (2.49 g, 1.40 mmol) and 2,2′-azobis(2-methylpropionitrile) (114.98 mg, 1.40 mmol) were added and stirred at 70 °C for 2 h. The mixture was cooled to room temperature, and poured into water, extracted with EA, washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (eluent: petroleum ether/EtOAc 12:1) to afford 9 (1.0 g, 67% yield) as red solid. Mp: 169–170 °C. 1H NMR (600 MHz, CDCl3) δ 8.09 (d, J = 7.8 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), and 6.83 (s, 1H), 4.55 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 180.06, 174.13, 160.94, 153.46, 135.56, 130.74, 130.69, 129.03, 127.98, 122.69, 122.34, 108.04, and 21.87. IR (ν, cm−1): 3118.33, 2920.28, 2339.32, 1670.84, 1551.16, and 691.42. HRMS (ESI) calcd. for [M + Na]+ C13H7O3BrNa+: 312.9471, found 312.9460.
3.2.4. Synthesis of TB1–9 and TC1–18
A mixture of the corresponding amines or alcohols (0.52 mmol), K2CO3 (142 mg, 1.03 mmol), and 9 (100 mg, 0.34 mmol) in THF (5 mL) was stirred at 30 °C to 50 °C for 4 h. After cooling, the mixture was poured into water and extracted with EA. The combined organic layer was washed with brine and dried over anhydrous Na2SO4, filtered, and concentrated to afford a crude product which was purified through column chromatography on silica gel.
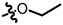
TB1: 2-(((4-methoxyphenyl)oxy)methyl)naphtho[1-b]furan-4,5-dione. Red solid, yield: 34%. Mp: 112–113 °C. 1H NMR (600 MHz, CDCl3) δ 8.09 (d, J = 7.6 Hz, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 6.94–6.92 (m, 2H), 6.87–6.85 (m, 2H), 6.83 (s, 1H), 5.04 (s, 2H), and 3.78 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 180.34, 174.38, 160.88, 154.72, 153.82, 151.95, 135.47, 130.66, 130.50, 128.98, 128.28, 122.59, 122.12, 116.34, 114.81, 108.37, 63.03, and 55.73. IR (ν, cm−1): 3445.32, 2358.40, 2339.32, 1671.47, 1598.48, 1253.20, 1219.81, 1161.58, and 1057.34. HRMS (ESI) calcd. for [M + Na]+ C20H14O5Na+: 357.0733, found 357.0723.
TB2: 2-(((4-acetylphenyl)oxy)methyl)naphtho[1,2-b]furan-4,5-dione. Light orange solid, yield: 40%. Mp: 190–191 °C. 1H NMR (600 MHz, CDCl3) δ 8.13 (d, J = 6.4 Hz, 1H), 8.00 (d, J = 8.9 Hz, 2H), 7.78 (d, J = 9.1 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 8.9 Hz, 2H), 6.94 (s, 1H), 5.19 (s, 2H), and 2.60 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 196.71, 180.20, 174.31, 161.58, 161.10, 152.60, 135.53, 131.28, 130.74, 130.70, 129.02, 128.11, 122.64, 122.08, 114.48, 108.98, 61.92, and 26.43. IR (ν, cm−1): 3445.93, 2358.51, 2341.16, 1671.86, 1599.84, 1249.41, 1217.01, 1178.57, and 1008.47. HRMS (ESI) calcd. for [M + Na]+ C21H14O5Na+: 369.0733, found 369.0721.
TB3: 2-(((4-propiophenyl)oxy)methyl)naphtho[1,2-b]furan-4,5-dione. Orange solid, yield: 37%. Mp: 179–180 °C. 1H NMR (600 MHz, CDCl3) δ 8.11 (d, J = 7.6 Hz, 1H), 8.00 (d, J = 8.9 Hz, 2H), 7.77 (d, J = 6.4 Hz, 1H), 7.71–7.68 (m, 1H), 7.53–7.50 (m, 1H), 7.06 (d, J = 8.9 Hz, 2H), 6.93 (s, 1H), 5.18 (s, 2H), 2.99 (q, J = 7.2 Hz, 2H), and 1.24 (t, J = 7.3 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 199.42, 180.20, 174.30, 161.40, 161.06, 152.68, 135.53, 130.98, 130.72, 130.68, 130.36, 129.01, 128.11, 122.63, 122.08, 114.47, 108.92, 61.90, 31.53, and 8.38. IR (ν, cm−1): 3447.03, 2358.62, 2341.16, 1669.65, 1601.07, 1222.31, 1180.35, and 1002.86. HRMS (ESI) calcd. for [M + Na]+ C22H16O5Na+: 383.0890, found 383.0876.
TB4: 2-(((4-nitrophenyl)oxy)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 48%. Mp: 205–206 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.25 (d, J = 9.3 Hz, 2H), 7.96 (d, J = 7.6 Hz, 1H), 7.77–7.74 (m, 2H), 7.59–7.55 (m, 1H), 7.34 (d, J = 9.3 Hz, 2H), 7.18 (s, 1H), and 5.42 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 179.58, 174.56, 163.29, 160.07, 152.42, 141.83, 135.43, 130.84, 130.17, 129.83, 127.89, 126.41, 122.50, 122.36, 115.94, 110.32, and 62.45. IR (ν, cm−1): 3445.75, 2358.70, 2341.16, 1681.37,1592.17, 1507.95, 1384.15, 1340.19, 1277.67, and 1110.13. HRMS (ESI) calcd. for [M + Na]+ C19H11O6NNa+: 372.0479, found 372.0465.
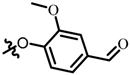
TB5: 2-(((2-methoxyl-4-formyl)phenyl)oxy)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 34%. Mp: 203–204 °C. 1H NMR (600 MHz, CDCl3) δ 9.91 (s, 1H), 8.13 (d, J = 6.4 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.72–7.68 (m, 1H), 7.54–7.48 (m, 3H), 7.15 (d, J = 8.0 Hz, 1H), 6.96 (s, 1H), 5.28 (s, 2H), and 3.97 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 190.88, 180.19, 174.31, 161.15, 152.50, 152.42, 150.30, 135.52, 131.28, 130.75, 129.67, 129.03, 128.10, 122.68, 122.09, 115.30, 112.93, 109.87, 109.40, 62.85, and 56.09. IR (ν, cm−1): 3446.32, 2358.18, 2341.16, 1702.84, 1676.79, 1586.53, 1508.23, 1267.05, 1236.25, 1137.10, and 999.76. HRMS (ESI) calcd. for [M + Na]+ C21H14O6Na+: 385.0683, found 385.0668.
TB6: 2-(((4-formylphenyl)oxy)methyl)naphtho[1,2-b]furan-4,5-dione. Orange solid, yield: 30%. Mp: 212–213 °C. 1H NMR (600 MHz, CDCl3) δ 9.95 (s, 1H), 8.13 (d, J = 7.8 Hz, 1H), 7.92 (d, J = 8.9 Hz, 2H), 7.78 (d, J = 7.8 Hz, 1H), 7.73–7.69 (m, 1H), 7.56–7.51 (m, 1H), 7.14 (d, J = 8.7 Hz, 2H), 6.96 (s, 1H), and 5.21 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 190.71, 180.17, 174.30, 162.67, 161.14, 152.36, 135.53, 132.10, 130.82, 130.77, 130.74, 129.05, 128.09, 122.64, 122.08, 115.06, 109.11, and 61.99. IR (ν, cm−1): 3446.21, 2358.24, 2337.30, 1687.41, 1671.45, 1598.48, 1253.19, 1219.95, 1161.49, and 1057.40. HRMS (ESI) calcd. for [M + Na]+ C20H12O5Na+: 355.0577, found 355.0565.
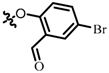
TB7: 2-(((4-bromo-2-formyl)phenyl)oxy)methyl)naphtho[1,2-b]furan-4,5-dione. Light orange solid, yield: 33%. Mp: 201–202 °C. 1H NMR (600 MHz, CDCl3) δ 10.43 (s, 1H), 8.14 (d, J = 7.6 Hz, 1H), 7.99 (d, J = 2.7 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.73–7.69 (m, 2H), 7.57–7.51 (m, 1H), 7.06 (d, J = 8.7 Hz, 1H), 6.96 (s, 1H), and 5.24 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 187.86, 180.06, 174.25, 161.30, 158.94, 151.80, 138.27, 135.59, 131.52, 130.87, 130.82, 129.07, 127.95, 126.84, 122.64, 121.99, 115.01, 114.95, 109.43, and 62.80. IR (ν, cm−1): 3445.63, 2358.34, 2337.30, 1671.90, 1599.56, 1556.29, 1249.42, 1217.50, 1178.60, and 1008.51. HRMS (ESI) calcd. for [M + Na]+ C20H11O5BrNa+: 432.9682, found 432.9673.
TB8: 2-(methoxymethyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 12%. Mp: 53–54 °C. 1H NMR (600 MHz, CDCl3) δ 8.11 (dd, J = 7.8, 1.5 Hz, 1H), 7.78 (dd, J = 7.7, 1.4 Hz, 1H), 7.70–7.66 (m, 1H), 7.52–7.48 (m, 1H), 6.80 (s, 1H), 4.51 (s, 2H), and 3.46 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 180.43, 174.46, 160.91, 154.77, 135.45, 130.64, 130.44, 128.96, 128.34, 122.59, 122.05, 107.97, 66.02, and 58.32. IR (ν, cm−1): 3446.10, 2358.55, 2337.96, 1677.60, 1276.65, 1216.21, 1153.22, and 1082.89. HRMS (ESI) calcd. for [M + Na]+ C14H10O4Na+: 265.0471, found 265.0463.
TB9: 2-(ethoxymethyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 11%. Mp: 60–61 °C. 1H NMR (600 MHz, CDCl3) δ 8.10 (dd, J = 8.2, 1.3 Hz, 1H), 7.77 (dd, J = 7.6, 1.2 Hz, 1H), 7.71–7.65 (m, 1H), 7.52–7.46 (m, 1H), 6.78 (s, 1H), 4.55 (s, 2H), 3.63 (q, J = 7.0 Hz, 2H), and 1.29 (t, J = 7.0 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 180.47, 174.45, 160.81, 155.23, 135.45, 130.61, 130.37, 128.90, 128.39, 122.59, 122.08, 107.66, 66.26, 64.25, and 15.10. IR (ν, cm−1): 3446.08, 2358.74, 2342.78, 1681.54, 1275.43, 1215.49, 1159.01, and 1083.47. HRMS (ESI) calcd. for [M + Na]+ C15H12O4Na+: 279.0628, found 279.0621.
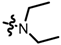
TC1: 2-(diethylaminomethyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 51%. Mp: 78–79 °C. 1H NMR (600 MHz, CDCl3) δ 8.07 (d, J = 7.6 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.65 (t, J = 7.0 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 6.66 (s, 1H), 3.77 (s, 2H), 2.62 (q, J = 7.2 Hz, 4H), and 1.14 (t, J = 7.2 Hz, 6H). 13C NMR (150 MHz, CDCl3) δ 180.65, 174.55, 160.34, 156.44, 135.41, 130.55, 130.12, 128.74, 128.58, 122.45, 122.21, 107.11, 48.69, 47.10, and 12.01. IR (ν, cm−1): 3445.24, 2953.88, 2358.48, 2339.23, 1700.91, 1676.52, 1216.10, and 1111.39. HRMS (ESI) calcd. for [M + Na]+ C17H18O3Na+: 284.1281, found 284.1271.
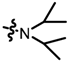
TC2: 2-(diisopropylaminomethyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 18%. Mp: 69–70 °C. 1H NMR (600 MHz, CDCl3) δ 8.06 (d, J = 7.8 Hz, 1H), 7.68 (d, J = 6.2 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.44 (t, J = 6.7 Hz, 1H), 6.66 (s, 1H), 3.72 (s, 2H), 3.13 (p, J = 6.5 Hz, 2H), and 1.08 (d, J = 6.7 Hz, 12H). 13C NMR (150 MHz, CDCl3) δ 180.88, 174.66, 161.17, 159.76, 135.37, 130.50, 129.81, 128.91, 128.65, 122.50, 122.09, 105.12, 49.12, 42.36, and 20.81. IR (ν, cm−1): 3445.80, 2966.49, 2358.61, 2337.30, 1676.07, 1558.32, 1215.85, and 1149.51. HRMS (ESI) calcd. for [M + Na]+ C19H22O3Na+: 312.1594, found 312.1583.
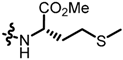
TC3: 2-((l-methionine methyl ester-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 12%. Mp: 52–53 °C. 1H NMR (600 MHz, CDCl3) δ 8.08 (d, J = 7.8 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.66 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 1H), 6.66 (s, 1H), 3.95 (d, J = 15.1 Hz, 1H), 3.79 (d, J = 15.1 Hz, 1H), 3.74 (s, 3H), 3.52 (dd, J = 8.4, 5.0 Hz, 1H), 2.64 (t, J = 7.2 Hz, 2H), 2.10 (s, 3H), 2.02–1.97 (m, 1H) and 1.89–1.82 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 180.55, 175.18, 174.44, 160.36, 157.00, 135.44, 130.58, 130.19, 128.80, 128.47, 122.35, 122.17, 106.00, 59.14, 52.14, 44.65, 32.68, 30.48, and 15.38. IR (ν, cm−1): 3446.14, 2923.56, 2358.62, 2335.37, 1698.61, 1670.40, 1215.45, and 1147.65. HRMS (ESI) calcd. for [M + Na]+ C19H19O5NSNa+: 396.0876, found 396.0865.
TC4: 2-((l-alanine methyl ester-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 27%. Mp: 83–84 °C. 1H NMR (600 MHz, CDCl3) δ 8.07 (d, J = 6.9 Hz, 1H), 7.73 (d, J = 4.9 Hz, 1H), 7.65 (d, J = 8.6 Hz, 1H), 7.46 (t, J = 6.6 Hz, 1H), 6.66 (s, 1H), 3.94 (d, J = 14.1 Hz, 1H), 3.81 (d, J = 14.9 Hz, 1H), 3.74 (s, 3H), 3.46 (q, J = 6.9 Hz, 1H), and 1.37 (d, J = 6.6 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 180.52, 175.63, 174.42, 160.36, 156.93, 135.40, 130.56, 130.18, 128.80, 128.46, 122.39, 122.18, 105.99, 55.69, 52.05, 44.26, and 19.13. IR (ν, cm−1): 3328.35, 2958.60, 2358.44, 2337.30, 1735.12, 1676.39, 1216.30, and 1139.72. HRMS (ESI) calcd. for [M + Na]+ C17H15O5NNa+: 336.0842, found 336.0831.
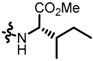
TC5: 2-((l-isoleucinate methyl ester-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 39%. Mp: 112–113 °C. 1H NMR (600 MHz, CDCl3) δ 8.07 (d, J = 7.7 Hz, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.67–7.62 (m, 1H), 7.48–7.43 (m, 1H), 6.65 (s, 1H), 3.92 (d, J = 15.1 Hz, 1H), 3.72 (d, J = 16.4 Hz, 4H), 3.17 (d, J = 5.8 Hz, 1H), 1.57–1.50 (m, 1H), 1.26–1.16 (m, 2H), and 0.93–0.88 (m, 6H). 13C NMR (150 MHz, CDCl3) δ 180.60, 175.16, 174.46, 160.27, 157.37, 135.45, 130.55, 130.15, 128.78, 128.52, 122.32, 122.19, 105.82, 65.31, 51.64, 45.08, 38.47, 25.39, 15.66, and 11.49. IR (ν, cm−1): 3445.72, 2924.70, 2358.43, 2341.16,1732.42, 1682.86, 1209.15, and 1150.10. HRMS (ESI) calcd. for [M + Na]+ C20H21O5NNa+: 378.1312, found 378.1299.
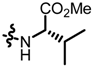
TC6: 2-((l-valine methyl ester-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 41%. Mp: 54–55 °C. 1H NMR (600 MHz, CDCl3) δ 8.09 (d, J = 6.2 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.70–7.64 (m, 1H), 7.50–7.45 (m, 1H), 6.67 (s, 1H), 3.95 (d, J = 15.3 Hz, 1H), 3.77–3.72 (m, 4H), 3.11 (d, J = 5.8 Hz, 1H), 2.02–1.95 (m, 1H), and 0.98 (t, J = 6.3 Hz, 7H). 13C NMR (150 MHz, CDCl3) δ 180.62, 175.22, 174.49, 160.28, 157.39, 135.43, 130.59, 130.14, 128.82, 128.56, 122.32, 122.22, 105.83, 66.36, 51.70, 45.16, 31.75, 19.28, and 18.35. IR (ν, cm−1): 3425.42, 2923,62, 2358.56, 2337.30, 1698.57, 1670.37, 1187.10, and 1118.00. HRMS (ESI) calcd. for [M + Na]+ C19H19O5NNa+: 364.1155, found 364.1141.
TC7: 2-((l-glycine methyl ester-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 17%. Mp: 55–56 °C. 1H NMR (600 MHz, CDCl3) δ 8.06 (d, J = 7.6 Hz, 1H), 7.71 (d, J = 6.4 Hz, 1H), 7.68–7.62 (m, 1H), 7.49–7.43 (m, 1H), 6.67 (s, 1H), 3.93 (s, 2H), 3.75 (s, 3H), and 3.50 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 180.49, 174.42, 172.45, 160.49, 156.62, 135.44, 130.57, 130.25, 128.79, 128.39, 122.41, 122.14, 106.25, 52.03, 49.50, and 45.38. IR (ν, cm−1): 3328.36, 2958.26, 2358.57, 2337.30, 1735.10, 1676.24, 1216.29, and 1180.25. HRMS (ESI) calcd. for [M + Na]+ C16H13O5NNa+: 322.0686, found 322.0680.
TC8: 2-((l-leucinate methyl ester-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 19%. Mp: 54–55 °C. 1H NMR (600 MHz, CDCl3) δ 8.09 (d, J = 7.6 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 1H), 6.66 (s, 1H), 3.94 (d, J = 15.1 Hz, 1H), 3.77 (d, J = 15.1 Hz, 1H), 3.73 (s, 3H), 3.38 (t, J = 7.2 Hz, 1H), 1.82–1.77 (m, 1H), 1.52 (t, J = 7.4 Hz, 2H), 0.95 (d, J = 6.7 Hz, 3H), and 0.90 (d, J = 6.7 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 180.59, 176.01, 174.47, 160.32, 157.18, 135.42, 130.59, 130.17, 128.84, 128.51, 122.32, 122.20, 105.94, 59.08, 51.88, 44.63, 42.75, 24.89, 22.78, and 22.09. IR (ν, cm−1): 3434.68, 2958.49, 2358.68, 2339.23, 1670.06, 1518.72, 1211.01, and 1107.62. HRMS (ESI) calcd. for [M + Na]+ C20H21O5NNa+: 378.1312, found 378.1302.
TC9: 2-((4-boc-piperazin-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 26%. Mp: 56–57 °C. 1H NMR (600 MHz, CDCl3) δ 8.10 (d, J = 7.4 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 6.70 (s, 1H), 3.68 (s, 2H), 3.49 (s, 4H), 2.52 (s, 4H), and 1.47 (s, 9H). 13C NMR (150 MHz, CDCl3) δ 180.53, 174.48, 160.57, 155.01, 154.68, 135.44, 130.64, 130.29, 128.81, 128.46, 122.53, 122.17, 107.66, 79.88, 54.60, 52.60, and 28.42. IR (ν, cm−1): 3434.73, 2358.58, 2339.23, 1669.96, 1518.77, 1211.18, and 1107.93. HRMS (ESI) calcd. for [M + H]+ C22H25O5N2+: 397.1758, found 397.1751.
TC10: 2-((pyrrolidin-1-yl)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 24%. Mp: 62–63 °C. 1H NMR (600 MHz, CDCl3) δ 8.08 (d, J = 7.8 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.65 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 6.68 (s, 1H), 3.76 (s, 2H), 2.69–2.63 (m, 4H), and 1.89–1.83 (m, 4H). 13C NMR (150 MHz, CDCl3) δ 180.64, 174.54, 160.39, 156.44, 135.40, 130.56, 130.14, 128.80, 128.56, 122.55, 122.24, 106.71, 54.01, 51.84, and 23.53. IR (ν, cm−1): 3388.30, 3110.62, 2358.68, 2337.30, 1660.65, 1510.49, 1222.52, and 1091.51. HRMS (ESI) calcd. for [M + H]+ C17H16O3N+: 282.1125, found 282.1115.
TC11: 2-(morpholinomethyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 38%. Mp: 98–99 °C. 1H NMR (600 MHz, CDCl3) δ 8.09 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.69–7.64 (m, 1H), 7.51–7.45 (m, 1H), 6.70 (s, 1H), 3.78–3.74 (m, 4H), 3.66 (s, 2H), and 2.61–2.55 (m, 4H). 13C NMR (150 MHz, CDCl3) δ 180.50, 174.46, 160.50, 155.01, 135.36, 130.59, 130.23, 128.86, 128.47, 122.46, 122.21, 107.64, 66.79, 54.93, and 53.28. IR (ν, cm−1): 3438.84, 2807.27, 2358.54, 2342.76, 1676.47, 1557.77, 1215.99, 1111.32, and 1006.63. HRMS (ESI) calcd. for [M + Na]+ C17H15O4NNa+: 320.0893, found 320.0886.
TC12: 2-(((4-fluorophenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Dark red solid, yield: 51%. Mp: 174–175 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.92 (d, J = 7.3 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.65 (d, J = 7.6 Hz, 1H), 7.52 (t, J = 7.5 Hz, 1H), 6.94 (t, J = 8.9 Hz, 2H), 6.74 (s, 1H), 6.73–6.68 (m, 2H), 6.20 (t, J = 6.4 Hz, 1H), and 4.37 (d, J = 6.0 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 179.86, 174.62, 159.03, 157.32, 155.16 (d, J = 231.0 Hz), 145.06, 135.47, 130.37, 129.85, 129.69, 128.27, 122.42, 122.06, 115.77 (d, J = 21.0 Hz), 113.88 (d, J = 6.0 Hz), and 105.93. IR (ν, cm−1): 3378.05, 2923.56, 2358.52, 2335.37, 1662.70, 1514.18, 1215.45, and 1161.45. HRMS (ESI) calcd. for [M + Na]+ C19H12O3NFNa+: 344.0693, found 344.0681.
TC13: 2-(((3-fluorophenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Dark red solid, yield: 45%. Mp: 169–170 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.92 (d, J = 7.6 Hz, 1H), 7.74 (t, J = 7.5 Hz, 1H), 7.65 (d, J = 7.6 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.10 (q, J = 8.0 Hz, 1H), 6.78 (s, 1H), 6.61 (t, J = 6.3 Hz, 1H), 6.54 (d, J = 8.2 Hz, 1H), 6.50 (d, J = 12.2 Hz, 1H), 6.35 (t, J = 8.4 Hz, 1H), and 4.41 (d, J = 6.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 179.83, 174.63, 163.90 (d, J = 238.5 Hz), 159.09, 156.86, 150.51 (d, J = 10.5 Hz), 135.46, 130.79 (d, J = 9.0 Hz), 130.40, 129.85, 129.73, 128.25, 122.42, 122.04, 109.34, 106.10, 102.99 (d, J = 21.0 Hz), and 99.24 (d, J = 27.0 Hz). IR (ν, cm−1): 3390.68, 3105.83, 2360.44, 2337.30, 1665.28, 1618.38, 1220.09, and 1152.73. HRMS (ESI) calcd. for [M + Na]+ C19H12O3NFNa+: 344.0693, found 344.0688.
TC14: 2-(((2-fluorophenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Dark red solid, yield: 46%. Mp: 170–171 °C. 1H NMR (600 MHz, CDCl3) δ 8.08 (d, J = 7.8 Hz, 1H), 7.69 (d, J = 7.4 Hz, 1H), 7.66 (t, J = 7.4 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.05–7.00 (m, 2H), 6.79 (t, J = 8.5 Hz, 1H), 6.75–6.72 (m, 1H), 6.71 (s, 1H), and 4.51 (d, J = 6.4 Hz, 2H). 13C NMR (150 MHz, CDCl3) δ 180.39, 174.38, 160.36, 155.98, 151.74 (d, J = 237.0 Hz), 135.45, 135.22 (d, J = 12.0 Hz), 130.63, 130.29, 128.79, 128.36, 124.67 (d, J = 4.5 Hz), 122.26 (d, J = 15.0 Hz), 118.16 (d, J = 6.0 Hz), 114 (d, J = 19.5 Hz), 112.52, 112.50, 106.03, and 40.74. IR (ν, cm−1): 3425.04, 2923.94, 2358.97, 2854,13, 1670.34, 1513.50, 1186.96, and 1117.57. HRMS (ESI) calcd. for [M + Na]+ C19H12O3NFNa+: 344.0693, found 344.0684.
TC15: 2-(((2,4-difluorophenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 33%. Mp: 154–155 °C. 1H NMR (600 MHz, CDCl3) δ 8.10 (d, J = 7.6 Hz, 1H), 7.71 (d, J = 7.6 Hz, 1H), 7.67 (t, J = 7.4 Hz, 1H), 7.49 (t, J = 7.4 Hz, 1H), 7.14 (t, J = 8.1 Hz, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.71 (s, 2H), 6.59 (d, J = 8.2 Hz, 1H), and 4.46 (d, J = 6.4 Hz, 2H). 13C NMR (150 MHz, CDCl3) δ 180.36, 174.38, 160.42, 155.83 (d, J = 9.0 Hz), 154.24 (d, J = 10.5 Hz), 151.95 (d, J = 12.0 Hz), 150.34 (d, J = 12.0 Hz), 135.49, 131.73 (d, J = 12.0 Hz), 130.52 (d, J = 46.5 Hz), 129.67, 128.55 (d, J = 73.5 Hz), 122.24 (d, J = 24 Hz), 115.33, 112.73 (dd, J = 12.0 Hz), 110.86 (dd, J = 25.5 Hz), 106.09, 103.90 (dd, J = 49.5 Hz), and 41.22. IR (ν, cm−1): 3434.72, 2358.46, 2337.30, 1689.86, 1518.53, 1210.93, 1107.68, and 957.02. HRMS (ESI) calcd. for [M + Na]+ C19H11O3NF2Na+: 362.0599, found 362.0589.
TC16: 2-(((2,4,6-trimethylphenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Red solid, yield: 33%. Mp: 173–174 °C. 1H NMR (600 MHz, CDCl3) δ 8.10 (d, J = 7.6 Hz, 1H), 7.67 (d, J = 3.8 Hz, 2H), 7.50–7.46 (m, 1H), 6.85 (s, 2H), 6.60 (s, 1H), 4.21 (s, 2H), 2.29 (s, 6H), and 2.25 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 180.53, 174.47, 160.18, 157.50, 141.61, 135.47, 132.42, 130.65, 130.23, 130.13, 129.64, 128.84, 128.47, 122.23, 122.18, 105.72, 44.94, 20.62, and 18.23. IR (ν, cm−1): 3445.76, 2357.38, 2327.66, 1670.28, 1557.44, 1215.32, 1147.47, and 1025.94. HRMS (ESI) calcd. for [M + Na]+ C22H19O3NNa+: 368.1257, found 368.1250.
TC17: 2-(((4-chlorophenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Dark red solid, yield: 28%. Mp: 205–206 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.92 (d, J = 6.8 Hz, 1H), 7.74 (t, J = 7.5 Hz, 1H), 7.65 (d, J = 7.6 Hz, 1H), 7.52 (t, J = 7.1 Hz, 1H), 7.12 (d, J = 8.9 Hz, 2H), 6.75 (s, 1H), 6.72 (d, J = 8.9 Hz, 2H), 6.49 (t, J = 6.3 Hz, 1H), and 4.40 (d, J = 6.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ: 179.82, 174.61, 159.06, 156.95, 147.33, 135.46, 130.39, 129.84, 129.72, 129.09, 128.24, 122.41, 122.05, 120.26, 114.42, and 106.08. IR (ν, cm−1): 3388.22, 3110.62, 2358.84, 2342.68, 1660.69, 1510.19, 1222.68 and 1118.65. HRMS (ESI) calcd. for [M + Na]+ C19H12O3NClNa+, 360.0398, found 360.0384.
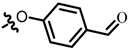
TC18: 2-(((4-methoxyphenyl)amino)methyl)naphtho[1,2-b]furan-4,5-dione. Dark solid, yield: 64%. Mp: 112–113 °C. 1H NMR (600 MHz, CDCl3) δ 8.08 (d, J = 6.4 Hz, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.69–7.63 (m, 1H), 7.50–7.44 (m, 1H), 6.82 (d, J = 8.9 Hz, 2H), 6.70–6.67 (m, 3H), 4.42 (s, 2H), and 3.77 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 180.49, 174.43, 160.22, 156.83, 152.98, 140.82, 135.43, 130.62, 130.21, 128.77, 128.46, 122.27, 115.00, 114.74, 105.84, 55.77, and 42.13. IR (ν, cm−1): 3370.45, 3105.23, 2358.89, 2337.30, 1660.16, 1514.41, 1234.80, and 1040.25. HRMS (ESI) calcd. for [M + Na]+ C20H15O4NNa+: 356.0893, found 356.0883.
3.3. In Vitro Cytotoxicity Assay
The human cancer cell lines, including prostate cancer cells PC3, leukemia cells K562, and melanoma cells WM9, were stored in the biology laboratory of the Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences (Guiyang, China). All cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (Sijiqing, Hangzhou, China) and incubated at 37 °C under 5% CO2, 95% air, and 95% humidity. Cytotoxicity was evaluated by performing the MTT assay [24]. Briefly, the cells were seeded in 96-well microculture plates at a density from 4 × 103 to 8 × 103 cells/well. Cells were then exposed to different concentrations of the assayed compounds for 48 h. Then, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated at 37 °C for an additional 4 h. The medium was then removed and 200 µL Tris-DMSO solution was added. Plates were lightly shaking up to dissolve the dark blue formazan crystals and the absorbance was measured in an ELISA plate reader at 570 nm.
3.4. Flow Cytometry Assay
Cell apoptosis was determined by an inverted fluorescence microscope observation and flow cytometry as describe in our previous study [28]. Briefly, the cancer cells treated with compounds were harvested for centrifugation at 1000 rpm for 5 min at room temperature, washed twice with PBS and resuspended with binding buffer, and then PI (Sigma, St. Louis, MO, USA) was added to a final concentration of 20 mg/mL. The cell lines were analyzed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
3.5. Statistical Analysis
The IC50 values were calculated from the semilogarithmic dose-response curves. The data were analyzed using SPSS 18.0 and reported as mean ± SD of the number of experiments indicated. For all measurements, one-way ANOVA followed by Student’s t-test was used to assess the statistical significance of the difference between each group. The LSD method was used to assess the statistical significance of the difference between the two groups. A statistically significant difference was considered at the level of P < 0.05. The data are presented as the mean ± SEM of three assays.
4. Conclusions
In this study, 27 novel L-shaped ortho-quinone analogs were synthesized and evaluated for their anti-cancer activities. Compounds TB1, TB3, TB4, TB6, TC1, TC3, TC5, TC9, TC11, TC12, TC14, TC15, TC16, and TC17 possessed broad-spectrum potent cytotoxicity against PC3, K562, and WM9 cells. With more than a 70% inhibitory rate, TB7 showed better inhibitory activity of K562 cells as compared with other cells. Moreover, we observed that TC7 inhibited the growth of PC3 cells more efficiently than other cells. Some of the active compounds such as TB3, TC1, TC3, and TC7 inhibited cell proliferation mainly through inducing apoptosis. The structure-activity relationships evaluation showed that removing methyl at C-3 of the furan ring and introducing diverse side chains at C-2 of the furan ring is an effective strategy for improving the anticancer activity of L-shaped ortho-quinone analogs.
Author Contributions
W.-D.P. and H.L. conceived and designed the experiments; S.-Y.L. and X.-Y.Z. performed part of chemical experiment; Z.-K.S., Y.Z., M.-L.W. performed the biology experiment; S.-C.H., J.L., J.-R.S. and C.C. contributed reagents and materials and revised the paper; S.-Y.L. and H.L. wrote the paper.
Funding
This work was supported by the Science and Technology Department of Guizhou Province (no. QKHJC (2017)1412, QKHRC (2016)4037, QKHZC (2019)2757, QKHJC (2016)1099, and QKHPTRC (2017) 5737), the National Science Foundation of China (NSFC no. 81660580 and 81702914), and Financial support from Guizhou Provincial Engineering Research Center for Natural Drugs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.M.; Zong, Y.N.; Cao, S.M.; Xu, R.H. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Weerink, L.B.; Gant, C.M.; Leeuwen, B.L.V.; De Bock, G.H.; Kouwenhoven, E.A.; Faneyte, I.F. Long-term survival in octogenarians after surgical treatment for colorectal cancer: Prevention of postoperative complications is key. Ann. Surg. Oncol. 2018, 25, 3874–3882. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the Last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Tan, Y.H.; Xiao, X.; Yao, J.N.; Han, F.; Lou, H.Y.; Luo, H.; Liang, G.Y.; Ben-David, Y.; Pan, W.D. Syntheses and Anti-cancer Activities of Glycosylated Derivatives of Diosgenin. Chem. Res. Chin. Univ. 2017, 33, 80–86. [Google Scholar] [CrossRef]
- Lan, J.J.; Huang, L.; Lou, H.Y.; Chen, C.; Liu, T.J.J.; Hu, S.C.; Yao, Y.; Song, J.R.; Luo, J.; Liu, Y.Z.; et al. Design and synthesis of novel C-14-urea-tetrandrine derivatives with potent anti-cancer activity. Eur. J. Med. Chem. 2018, 143, 1968–1980. [Google Scholar] [CrossRef]
- Lan, J.J.; Wang, N.; Huang, L.; Liu, Y.Z.; Ma, X.P.; Lou, H.Y.; Chen, C.; Feng, Y.P.; Pan, W.D. Design and synthesis of novel tetrandrine derivatives as potential anti-tumor agents against human hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 127, 554–566. [Google Scholar] [CrossRef]
- Song, J.R.; Lan, J.J.; Chen, C.; Hu, S.C.; Song, J.L.; Liu, W.L.; Zeng, X.Y.; Lou, H.Y.; Ben-David, Y.; Pan, W.D. Design, synthesis and bioactivity investigation of tetrandrine derivatives as potential anti-cancer agents. MedChemComm 2018, 9, 1131–1141. [Google Scholar] [CrossRef]
- Liu, F.; Yu, G.; Wang, G.; Liu, H.; Wu, X.; Wang, Q.; Liu, M.; Liao, K.; Wu, M.; Cheng, X.; et al. An NQO1-initiated and p53-independent apoptotic pathway determines the anti-tumor effect of tanshinone IIA against non-small cell lung cancer. PLoS ONE 2012, 7, e42138. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Chen, L.; Xiao, Z.G.; Zhu, Y.H.; Jiang, H.; Jin, Y.; Gu, C.; Wu, Y.L.; Wang, L.; Zhang, W.; et al. Potentiation of the anticancer effect of doxorubicinin drug-resistant gastric cancer cells by tanshinone IIA. Phytomedicine 2018, 51, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, L.; Wang, H.; Yang, L.Q.; Guo, H.J.; Wang, X.J. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling pathways. J. Cell Biochem. 2013, 114, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Huang, C.C.; Huang, W.L.; Lin, T.K.; Liao, P.L.; Wang, P.W.; Liou, C.W.; Chuang, J.H. Tanshinone IIA induces intrinsic apoptosis in osteosarcoma cells both in vivo and in vitro associated with mitochondrial dysfunction. Sci. Rep. 2017, 7, 40382. [Google Scholar] [CrossRef] [PubMed]
- Bentle, M.S.; Reinicke, K.E.; Bey, E.A.; Spitz, D.R.; Boothman, D.A. Calcium-dependent modulation of poly (ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J. Biol. Chem. 2006, 281, 33684–33696. [Google Scholar] [CrossRef]
- Bey, E.A.; Bentle, M.S.; Reinicke, K.E.; Dong, Y.; Yang, C.R.; Girard, L.; Minna, J.D.; Bornmann, W.G.; Gao, J.M.; Boothman, D.A. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by β-lapachone. Proc. Natl. Acad. Sci. USA 2007, 104, 11832–11837. [Google Scholar] [CrossRef]
- Cheng, X.F.; Liu, F.; Yan, T.T.; Zhou, X.Y.; Wu, L.; Liao, K.; Wang, G.J.; Hao, H.P. , Metabolic profile, enzyme kinetics, and reaction phenotyping of β-lapachone metabolism in human liver and intestine in vitro. Mol. Pharm. 2012, 9, 3476–3485. [Google Scholar] [CrossRef]
- Huang, W.G.; Li, J.Y.; Zhang, W.; Zhou, Y.Y.; Xie, C.M.; Luo, Y.; Li, Y.F.; Wang, J.L.; Li, J.; Lu, W. Synthesis of miltirone analogues as inhibitors of Cdc25 phosphatases. Bioorg. Med. Chem. Lett. 2006, 16, 1905–1908. [Google Scholar] [CrossRef]
- Huang, W.G.; Jiang, Y.Y.; Li, Q.; Li, J.; Li, J.Y.; Lu, W.; Cai, J.C. Synthesis and biological evaluation of (±)-cryptotanshinone and its simplified analogues as potent CDC25 inhibitors. Tetrahedron 2005, 61, 1863–1870. [Google Scholar] [CrossRef]
- Bian, J.L.; Deng, B.; Xu, L.L.; Xu, X.L.; Wang, N.; Hu, T.H.; Yao, Z.Y.; Du, J.Y.; Yang, L.; Lei, Y.H.; et al. 2-Substituted 3-methylnaphtho[1,2-b]furan-4,5-diones as novel L-shaped ortho-quinone substrates for NAD(P)H: Quinone oxidoreductase (NQO1). Eur. J. Med. Chem. 2014, 82, 56–67. [Google Scholar] [CrossRef]
- Bian, J.L.; Li, X.; Wang, N.; Wu, X.S.; You, Q.D.; Zhang, X.J. Discovery of quinone-directed antitumor agents selectively bioactivated by NQO1 over CPR with improved safety profile. Eur. J. Med. Chem. 2017, 129, 27–40. [Google Scholar] [CrossRef]
- Deniz, N.G.; Ozyurek, M.; Tufan, A.N.; Apak, R. One-pot synthesis, characterization, and antioxidant capacity of sulfur-and oxygen-substituted 1,4-naphthoquinones and a structural study. Monatshefte für Chemie-Chemical Monthly 2015, 146, 2117–2126. [Google Scholar] [CrossRef]
- Arenas, P.; Peña, A.; Rios, D.; Benites, J.; Muccioli, G.G.; Calderon, P.B.; Valderrama, J.A. Eco-friendly synthesis and antiproliferative evaluation of some oxygen substituted diaryl ketones. Molecules 2013, 18, 9818–9832. [Google Scholar] [CrossRef]
- Li, X.; Bian, J.L.; Wang, N.; Qian, X.; Gu, J.; Mu, T.; Fan, J.; Yang, X.W.; Li, S.Z.; Yang, T.T.; et al. Novel naphtho[2,1-d]oxazole-4,5-diones as NQO1 substrates with improved aqueous solubility: Design, synthesis, and in vivo antitumor evaluation. Bioorg. Med. Chem. 2016, 24, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Hu, K.Z.; Wang, H.; Huang, T.; Tang, G.; Liang, X.; He, S.; Tang, X. Synthesis and biological evaluation of N-(2-[(18)F]Fluoropropionyl)-l-methionine for tumor imaging. Nucl. Med. Biol. 2013, 40, 926–932. [Google Scholar] [CrossRef]
- Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P. Potent antitumor activity of synthetic 1,2-Naphthoquinones and 1,4-Naphthoquinones. Bioorg. Med. Chem. 2003, 11, 3179–3191. [Google Scholar] [CrossRef]
- Weerawarna, S.A.; Guha-Biswas, M.; Nelson, W.L. Improved Syntheses of Bufuralol, 7-Ethyl-2-(2-tertbutylamino-1-hydroxyethyl)benzofuran, and 1 Oxobufuralol, 7-Acetyl-2-(2-tert-butylamino-1-hydroxyethyl)benzofuran. Heterocycl 1991, 28, 1395–1403. [Google Scholar] [CrossRef]
- Wen, Z.H.; Zhang, Y.Q.; Wang, X.H.; Zeng, X.P.; Hu, Z.X.; Liu, Y.; Xie, Y.X.; Liang, G.Y.; Zhu, J.G.; Luo, H.; et al. Novel 3′,5′-diprenylated chalcones inhibited the proliferation of cancer cells in vitro by inducing cell apoptosis and arresting cell cycle phase. Eur. J. Med. Chem. 2017, 133, 227–239. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).