Removal of Ammonia from the Municipal Waste Treatment Effluents using Natural Minerals
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of the Commercial Minerals (Non-Modified)
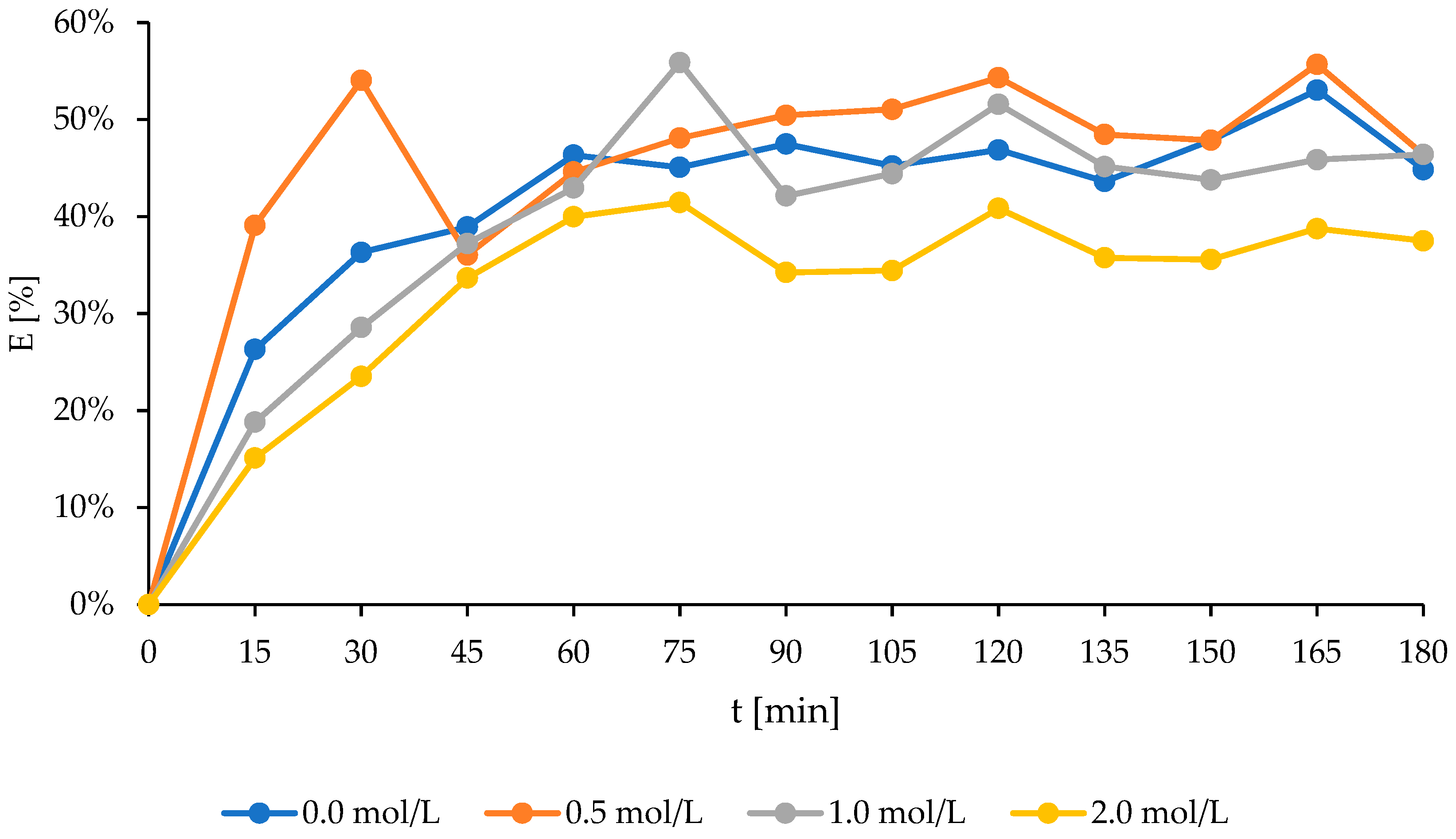
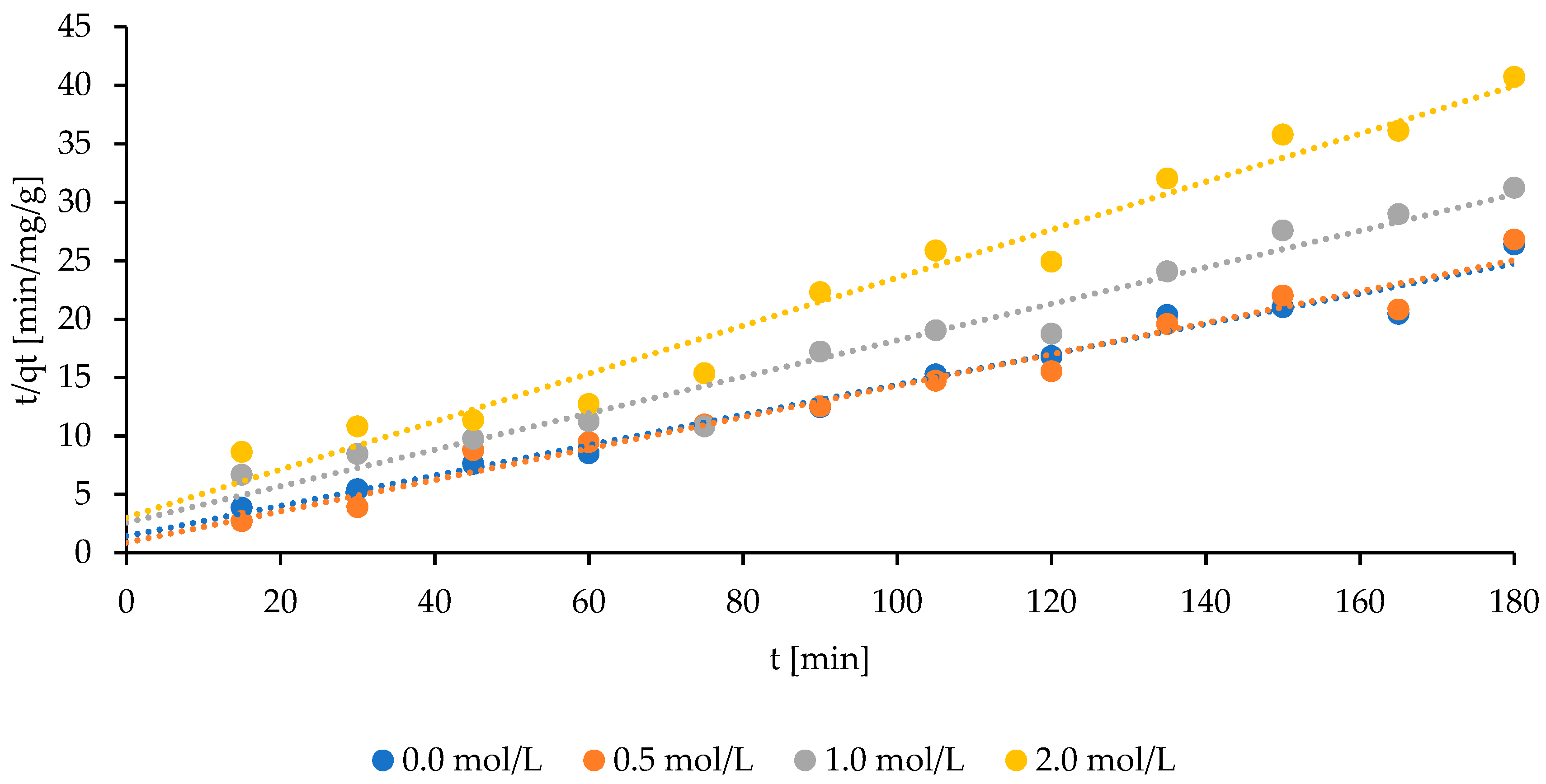
2.2. The Studies on Activated Sorbent
3. Materials and Methods
3.1. The Commercial Minerals
3.2. The Industrial Wastewater
3.3. Ammonium ion Exchange
3.4. The Sorbent Activation
3.5. Analytical Methods
3.6. Ammonium Ion-Exchange Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, Q.; Liu, S.; Cao, Z.; Wang, Y. Ammonium removal from aqueous solution using natural Chinese clinoptilolite. Sep. Purif. Technol. 2005, 44, 223–234. [Google Scholar] [CrossRef]
- Taddeo, R.; Prajapati, S.; Lepisto, R. Optimizing ammonium removal by natural zeolite from wastewater with high loads of ammonium and solids. J. Porous Mater. 2017, 24, 1545–1554. [Google Scholar] [CrossRef]
- Chen, P.; Xie, Q.; Addy, M.; Zhou, W.; Liu, Y.; Wang, Y.; Cheng, Y.; Li, K.; Ruan, R. Utilization of municipal solid and liquid wastes for bioenergy and bioproducts production. Bioresour. Technol. 2016, 215, 163–172. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Alimoradi, M.; Ganjian, E. Old landfill leachate treatment through multistage process: Membrane adsorption bioreactor and nanofitration. Bioprocess Biosyst. Eng. 2016, 39, 1803–1816. [Google Scholar] [CrossRef]
- Omar, H.; Rohani, S. Treatment of landfill waste, leachate and landfill gas: A review. Front. Chem. Sci. Eng. 2015, 9, 15–32. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pang, C.; Wu, S.; Dong, R. Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf. Environ. Prot. 2014, 94, 350–357. [Google Scholar] [CrossRef]
- Jorgensen, T.C.; Weatherley, L.R. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Mahvi, A.H.; Mesdaghinia, A.R.; Nasseri, S. Investigation of ammonia removal from polluted waters by Clinoptilolite zeolite. Int. J. Environ. Sci. Technol. 2004, 1, 125–133. [Google Scholar] [CrossRef]
- Zaghouane-Boudiaf, H.; Boutahala, M. Kinetic analysis of 2,4,5-trichlorophenol adsorption onto acid-activated montmorillonite from aqueous solution. Int. J. Miner. Process. 2011, 100, 72–78. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; An, Y.; Wang, Z.; Yang, S.; Chen, H.; Zhou, Z.; Mai, S. Study on zeolite enhanced contact–adsorption regeneration–stabilization process for nitrogen removal. J. Hazard Mater. 2008, 156, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, D.; Zhang, Q.; Ding, L. Removal of ammonia from landfill leachate by struvite precipitation with the use of low-cost phosphate and magnesium sources. J. Environ. Manag. 2014, 145, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; Ibrahim, A.; Assabri, A.M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism and thermodynamics. Powder Technol. 2014, 258, 20–31. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Xue, Q.; Liu, J.; Hou, L.; Ding, L. Removal of ammonium from swine wastewater by zeolite combined with chlorination for regeneration. J. Environ. Manag. 2015, 160, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianocelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; Yan, C.; Al-Ani, Y.; Salman, A.; Ibrahim, A.; Zhou, C.; Wang, H. An investigation into the adsorption removal of ammonium by salt activated Chinese (Hulaodu) natural zeolite: Kinetics, isotherms and thermodynamics. J. Taiwan Inst. Chem. E 2014, 45, 554–564. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Chawakitchareon, P.; Anuwattana, R.; Buates, J. Production of Slow Release Fertilizer from Waste Materials. In Advanced Materials; Parinov, I.A., Chang, S.H., Topolov, V.Y., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 129–137. [Google Scholar]
- Leggo, P.J. The Efficacy of the Organo-Zeolitic Bio-fertilizer. Agrotechnology 2015, 4, 130. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef]
- Wang, Y.; Kmiya, Y.; Okuhara, T. Removal of low-concentration ammonia in water by ion-exchange using Na-mordenite. Water Res. 2007, 41, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Angaye, S.S.; Wankasi, D.; Dikio, E.D. Synthesis, characterization and application of Mg/Al layered double hydroxide for the degradation of congo red in aqueous solution. Open J. Phys. Chem. 2015, 5, 56–70. [Google Scholar] [CrossRef]
- Ayawei, N.; Ekubo, A.T.; Wankasi, D.; Dikio, E.D. Adsorption of congo red by Ni/Al-CO3: Equilibrium, thermodynamic and kinetic studies. Orient. J. Chem. 2015, 31, 1307–1318. [Google Scholar] [CrossRef]
- Brinques, G.; do Carmo Peralba, M.; Ayub, M. Optimization of probiotic and lactic acid production by Lactobacillus plantarum in submerged bioreactor systems. J. Ind. Microbiol. Biotechnol. 2010, 37, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Seruga, P.; Krzywonos, M. Screening of Medium Components and Process Parameters for Sugar Beet Molasses Vinasse Decolorization By Lactobacillus Plantarum Using Plackett-Burman Experimental Design. Pol. J. Environ. Stud. 2015, 24, 683–688. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, I.; Rodríguez-Iglesias, J.; Marañón, E.; Castrillón, L.; Alvarez, M. Removal of residual phenols from coke wastewater by adsorption. J. Hazard. Mater. 2007, 147, 395–400. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, WA, USA, 2012. [Google Scholar]
- ANON. Handbook of Photometrical Operation Analysis; Dr Lange BDB 079; Lange GmbH: Berlin, Germany, 2000. (In German) [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
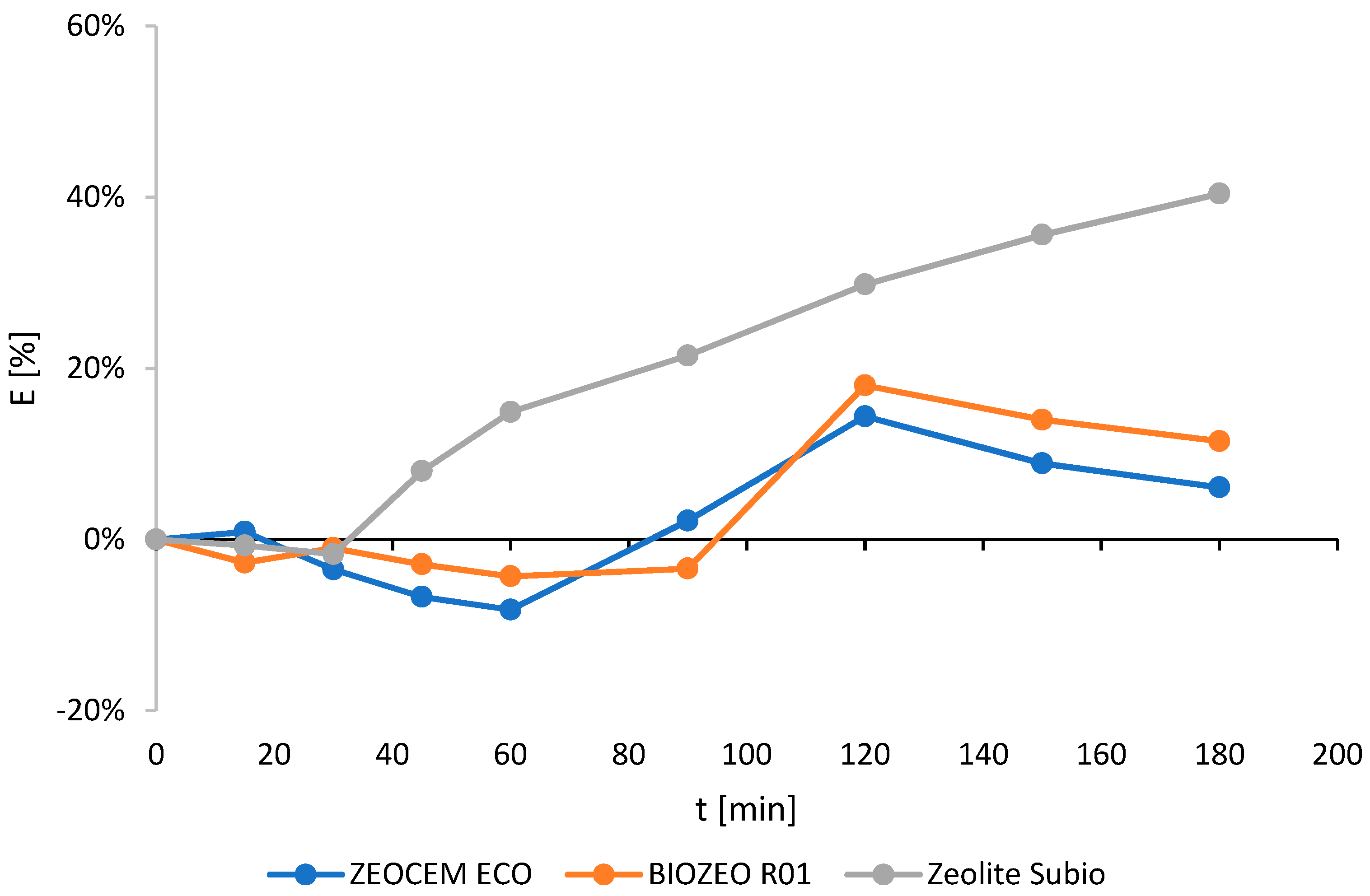
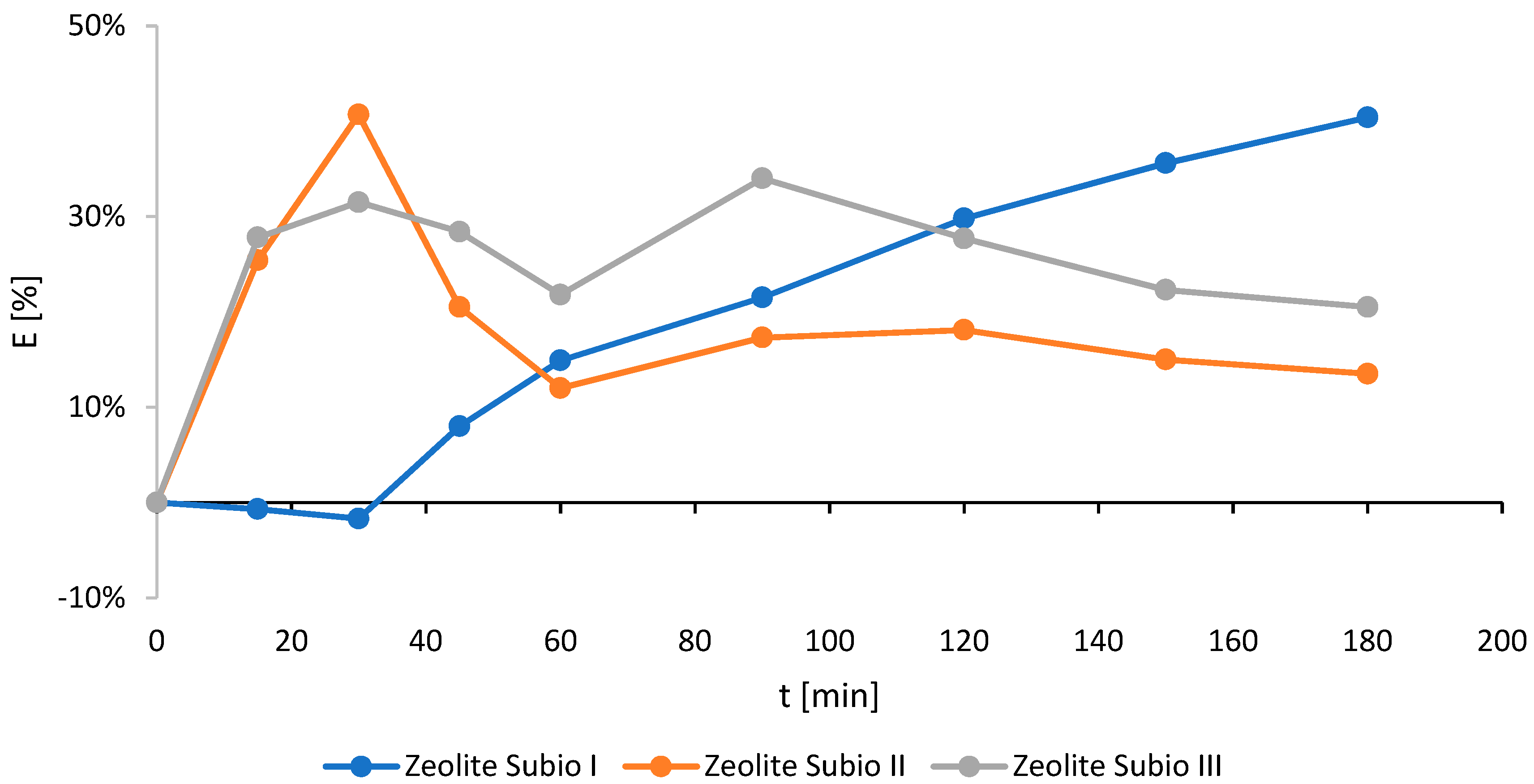
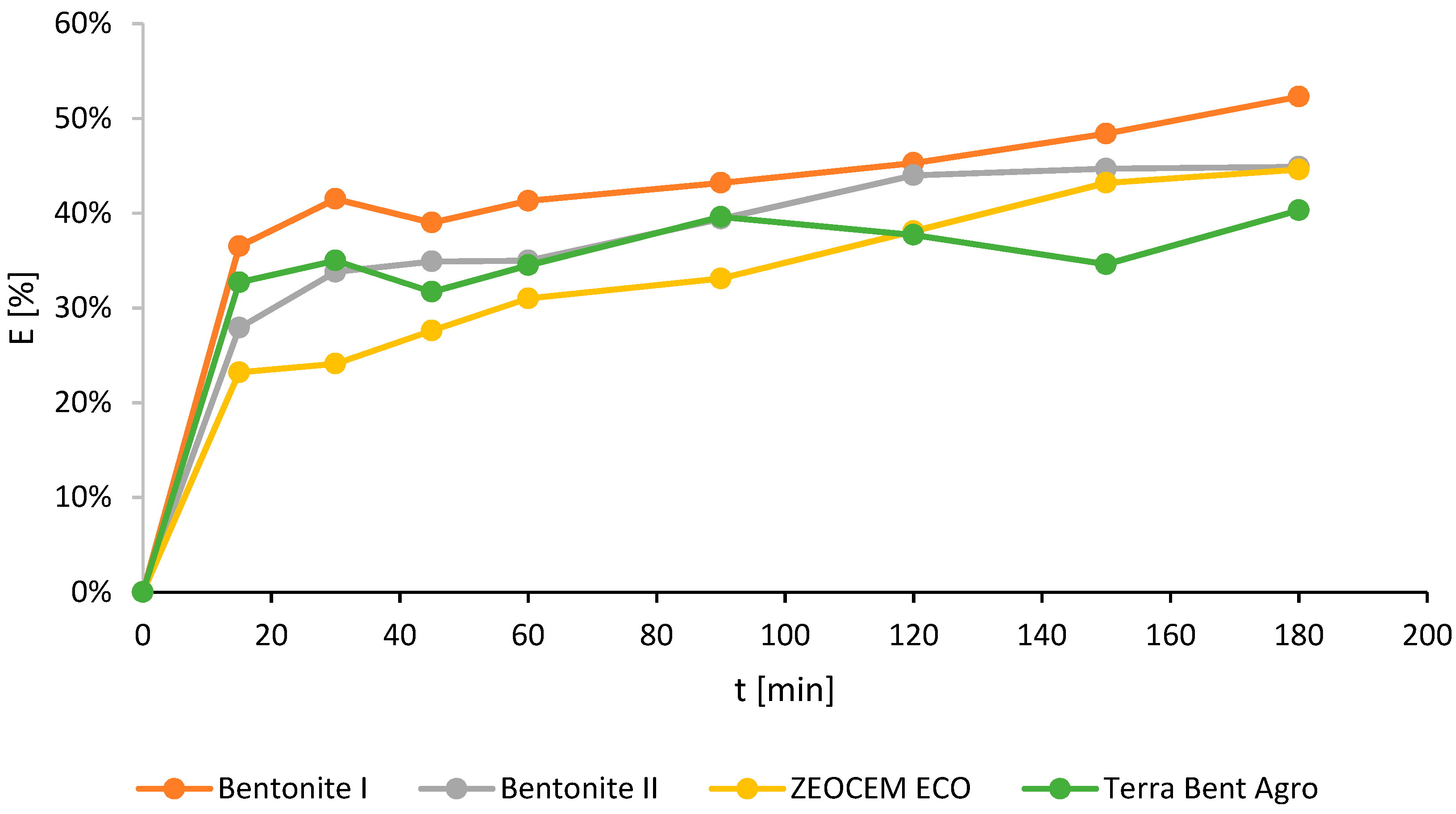

| Mineral Commercial Name | Particular Size [mm] | Adsorption Capacity (q) [mgN/gz] | Maximal Removal Efficiency (E) [%] | Needed Contact Time to Achieve Maximum E [min] |
|---|---|---|---|---|
| Zeocem Eco | 0.5–1 | 0.39 | 14.4 | 120 |
| Biozeo R.01 | 0.5–1 | 0.77 | 18.0 | 120 |
| Zeolite Subio I | 0.5–1 | 3.05 | 40.4 | 180 |
| Zeolite Subio II | 0.2–0.5 | 0.99 | 40.7 | 30 |
| Zeolite Subio III | 0–0.2 | 1.43 | 34.0 | 90 |
| Bentonite I | 0–0.05 | 4.92 | 52.3 | 180 |
| Bentonite II | 0–0.05 | 4.22 | 44.9 | 180 |
| Zeocem Eco | 0–0.05 | 4.2 | 44.6 | 180 |
| Terra Bent Agro | 0–0.05 | 3.79 | 40.3 | 180 |
| Mineral | k1 [min−1] | qe [mgN/gz] | R2 |
|---|---|---|---|
| Natural bentonite I | 0.013 | 7.164 | 0.772 |
| 0.5 M activated bentonite I | 0.030 | 7.285 | 0.840 |
| 1 M activated bentonite I | 0.021 | 5.828 | 0.924 |
| 2 M activated bentonite I | 0.013 | 4.748 | 0.947 |
| Mineral | k2 [g/mg·min] | qe [mgN/gz] | h [mg/g·min] | R2 |
|---|---|---|---|---|
| Natural Bentonite I | 0.013 | 7.164 | 6.657 | 0.979 |
| 0.5 M activated Bentonite I | 0.134 | 7.285 | 7.127 | 0.972 |
| 1 M activated Bentonite I | 0.156 | 5.828 | 5.298 | 0.966 |
| 2 M activated Bentonite I | 0.205 | 4.748 | 4.626 | 0.969 |
| Mineral and Chemical Composition [%] | Zeocem Eco | Zeolite Subio | Bentonite I | Bentonite II | Terra Bent Agro | Biozeo R01 |
|---|---|---|---|---|---|---|
| Clinoptilolite | 84 | 84 | 60 | |||
| Cristobalite | 8 | 8 | ||||
| Clayish mica | 4 | 4 | ||||
| Plagioclase | 3–4 | 3–4 | ||||
| Edisonite | 0.1–0.3 | 0.1–0.3 | ||||
| Montmorillonite | 85 | 65 | 65 | |||
| SiO2 | 65–71.3 | 65–71.3 | 70–80 | 60–65 | 60–80 | 70.6 |
| Al2O3 | 11.5–13.1 | 11.5–13.1 | 13–17 | 15–20 | 11–20 | 12.32 |
| Fe2O3 | 0.7–1.9 | 0.7–1.9 | 1–2 | 5–7 | ND | 1.48 |
| CaO | 2.7–5.2 | 2.7–5.2 | 0.5–1.5 | 2–4 | 1.5–5.2 | 3.42 |
| TiO2 | 0.1–0.3 | 0.1–0.3 | 0.05–0.15 | 0.5–1.0 | ND | 0.71 |
| MgO | 0.6–1.2 | 0.6–1.2 | 0.8–1.8 | 1–2 | ND | 0.96 |
| MnO | ND | ND | 0.05 | <0.05 | ND | 0.02 |
| K2O | 2.2–3.4 | 2.2–3.4 | 0.5–2.0 | 0.5–1.0 | 0.5–3.4 | 2.83 |
| Na2O | 0.2–1.3 | 0.2–1.3 | <0.01 | <0.01 | ND | 0.68 |
| P2O5 | ND | ND | <0,01 | <0,1 | ND | ND |
| ZrO2 | ND | ND | <0.01 | <0.01 | ND | ND |
| Cr2O3 | ND | ND | <0.01 | <0.02 | ND | ND |
| SO3 | ND | ND | <0.01 | <0.01 | ND | ND |
| Si/Al. | 4.8–5.4 | 4.8–5.4 | ND | ND | ND | ND |
| Parameter | Value |
|---|---|
| pH [-] | 7.8 ± 0.3 |
| Chemical Oxygen Demand (COD) [g/L] | 18.7 ± 1.3 |
| Biological Oxygen Demand (BOD) [g/L] | 6.0 ± 0.5 |
| Ammonium nitrogen [g/L] | 0.8 ± 0.2 |
| Total nitrogen [g/L] | 1.1 ± 0.3 |
| Total Organic Carbon (TOC) [g/L] | 3.1 ± 0.2 |
| Total suspended solids (TSS) [g/L] | 0.6 ± 0.2 |
| Cl− [g/L] | 1.3 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seruga, P.; Krzywonos, M.; Pyżanowska, J.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł. Removal of Ammonia from the Municipal Waste Treatment Effluents using Natural Minerals. Molecules 2019, 24, 3633. https://doi.org/10.3390/molecules24203633
Seruga P, Krzywonos M, Pyżanowska J, Urbanowska A, Pawlak-Kruczek H, Niedźwiecki Ł. Removal of Ammonia from the Municipal Waste Treatment Effluents using Natural Minerals. Molecules. 2019; 24(20):3633. https://doi.org/10.3390/molecules24203633
Chicago/Turabian StyleSeruga, Przemysław, Małgorzata Krzywonos, Justyna Pyżanowska, Agnieszka Urbanowska, Halina Pawlak-Kruczek, and Łukasz Niedźwiecki. 2019. "Removal of Ammonia from the Municipal Waste Treatment Effluents using Natural Minerals" Molecules 24, no. 20: 3633. https://doi.org/10.3390/molecules24203633
APA StyleSeruga, P., Krzywonos, M., Pyżanowska, J., Urbanowska, A., Pawlak-Kruczek, H., & Niedźwiecki, Ł. (2019). Removal of Ammonia from the Municipal Waste Treatment Effluents using Natural Minerals. Molecules, 24(20), 3633. https://doi.org/10.3390/molecules24203633










