Detailed Analysis of the Influencing Parameters on the Self-Healing Behavior of Dynamic Urea-Crosslinked Poly(methacrylate)s
Abstract
:1. Introduction
2. Results and Discussion
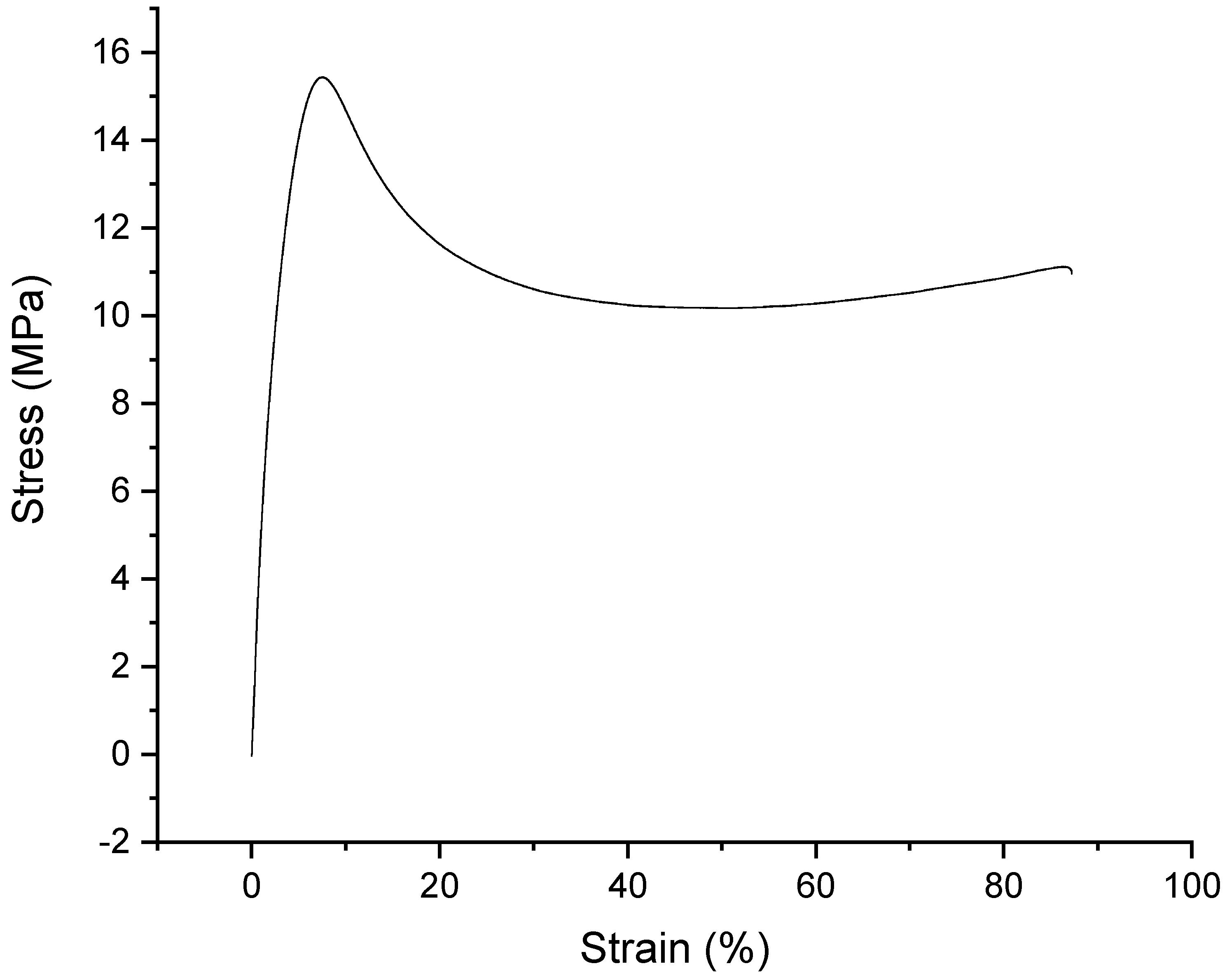
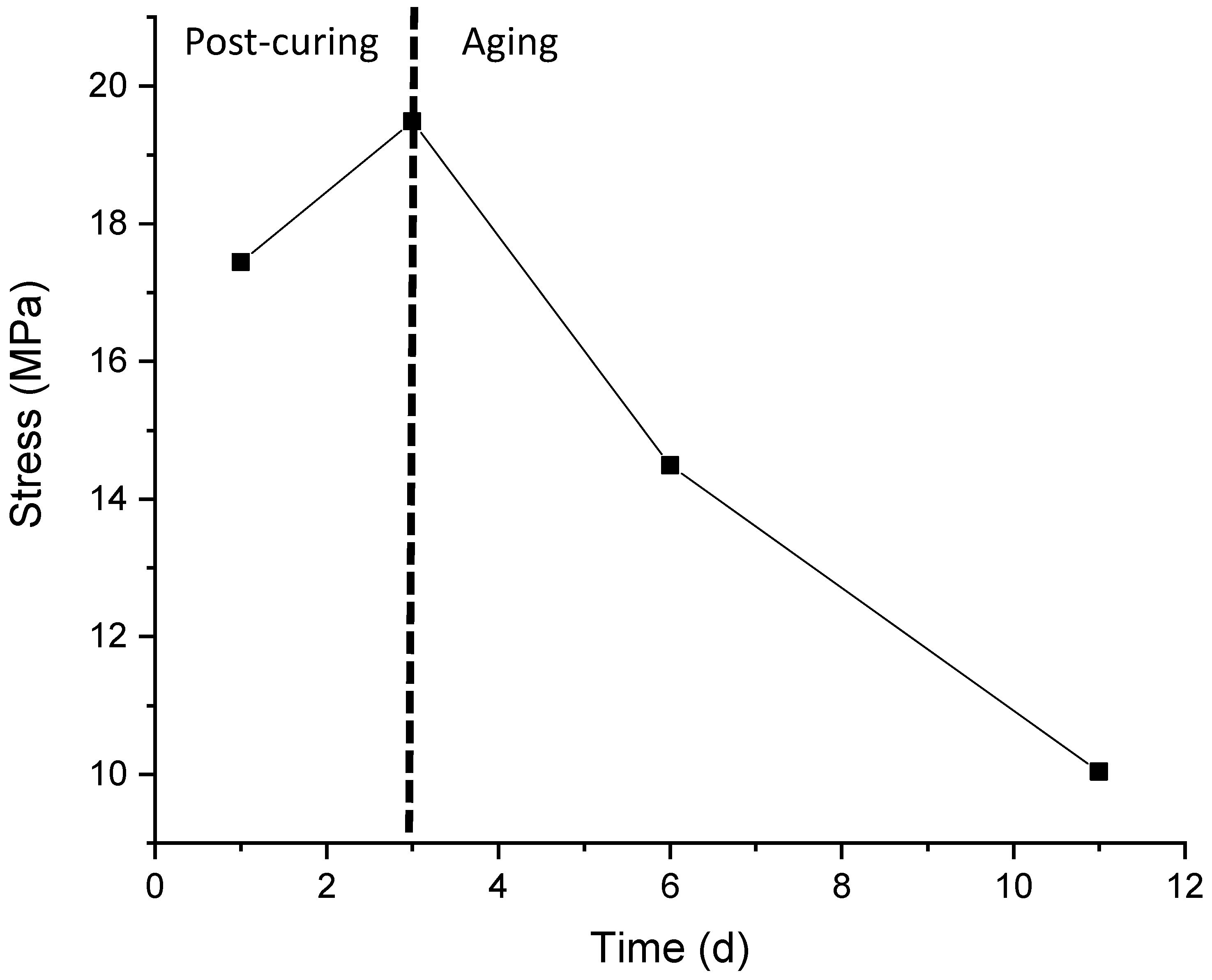
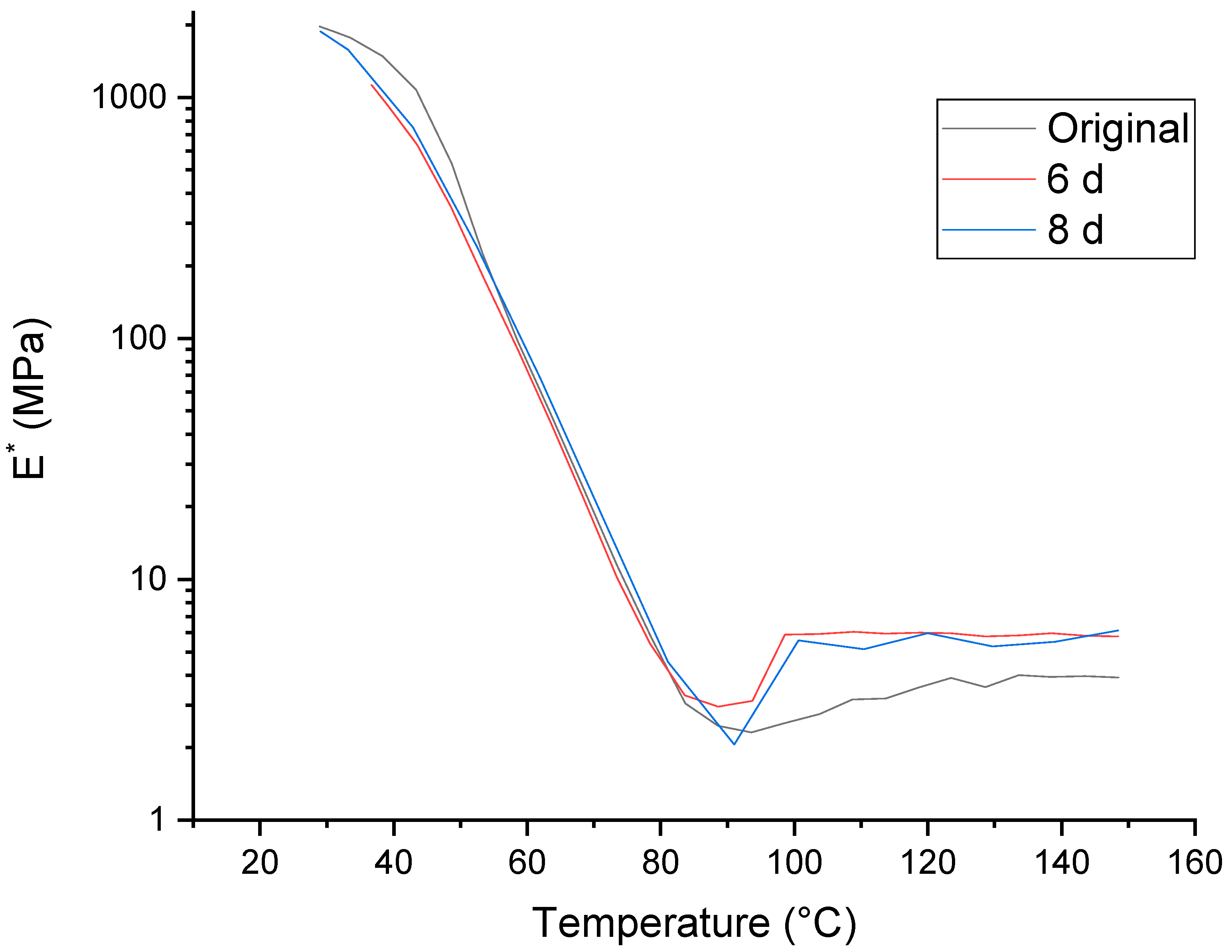
2.1. Time-Dependency of the Original Samples
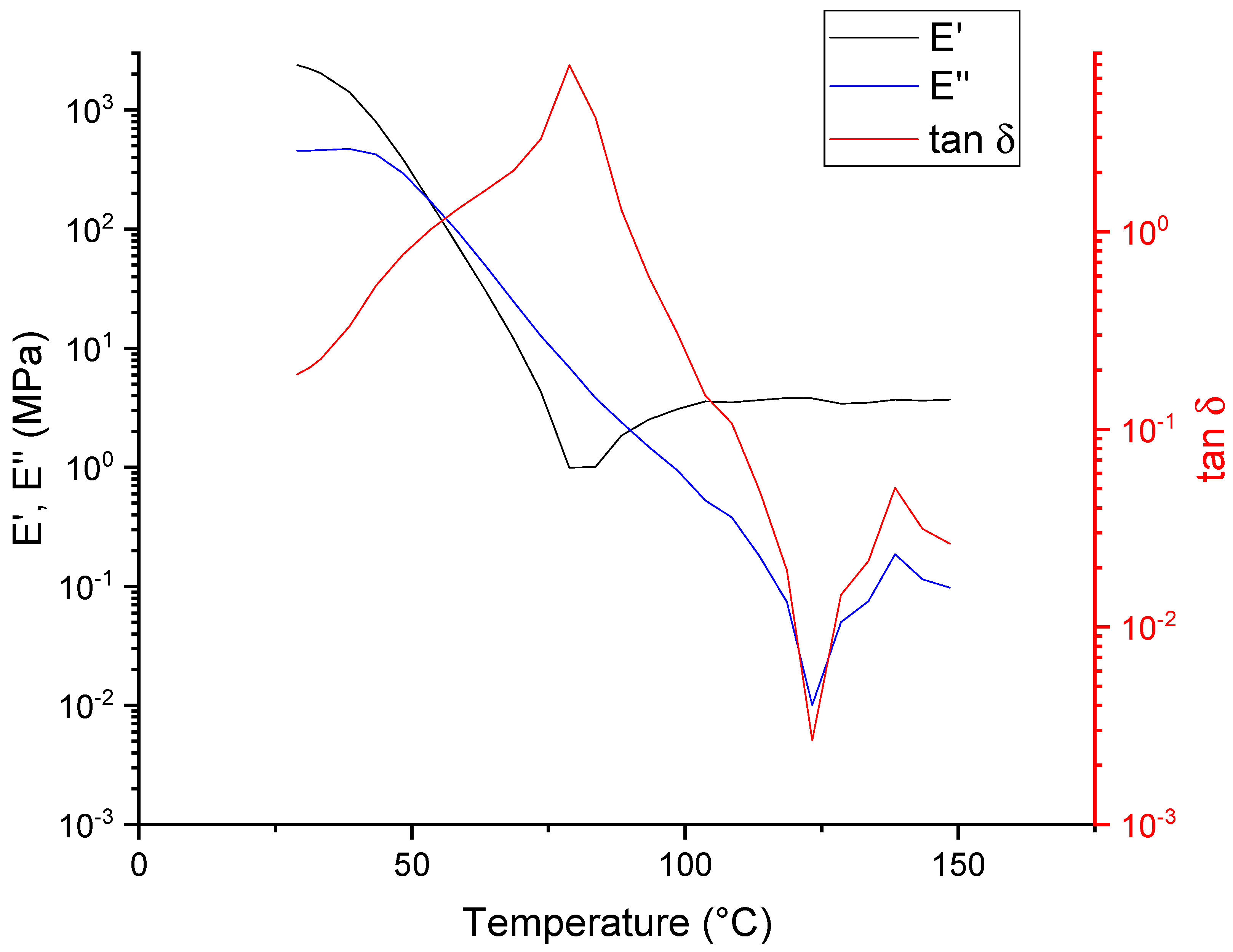
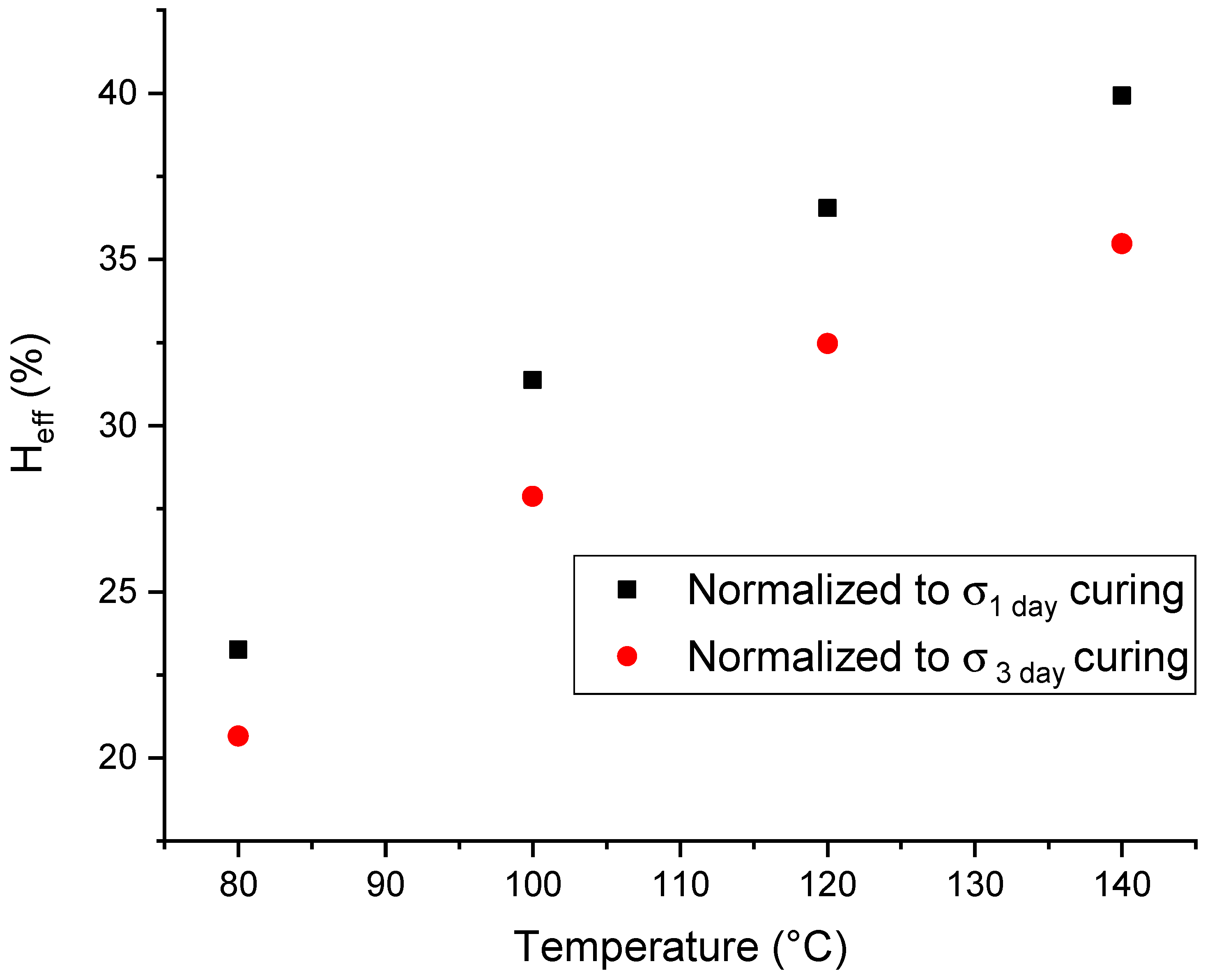
2.2. Temperature-Dependency of the Healing Efficiency
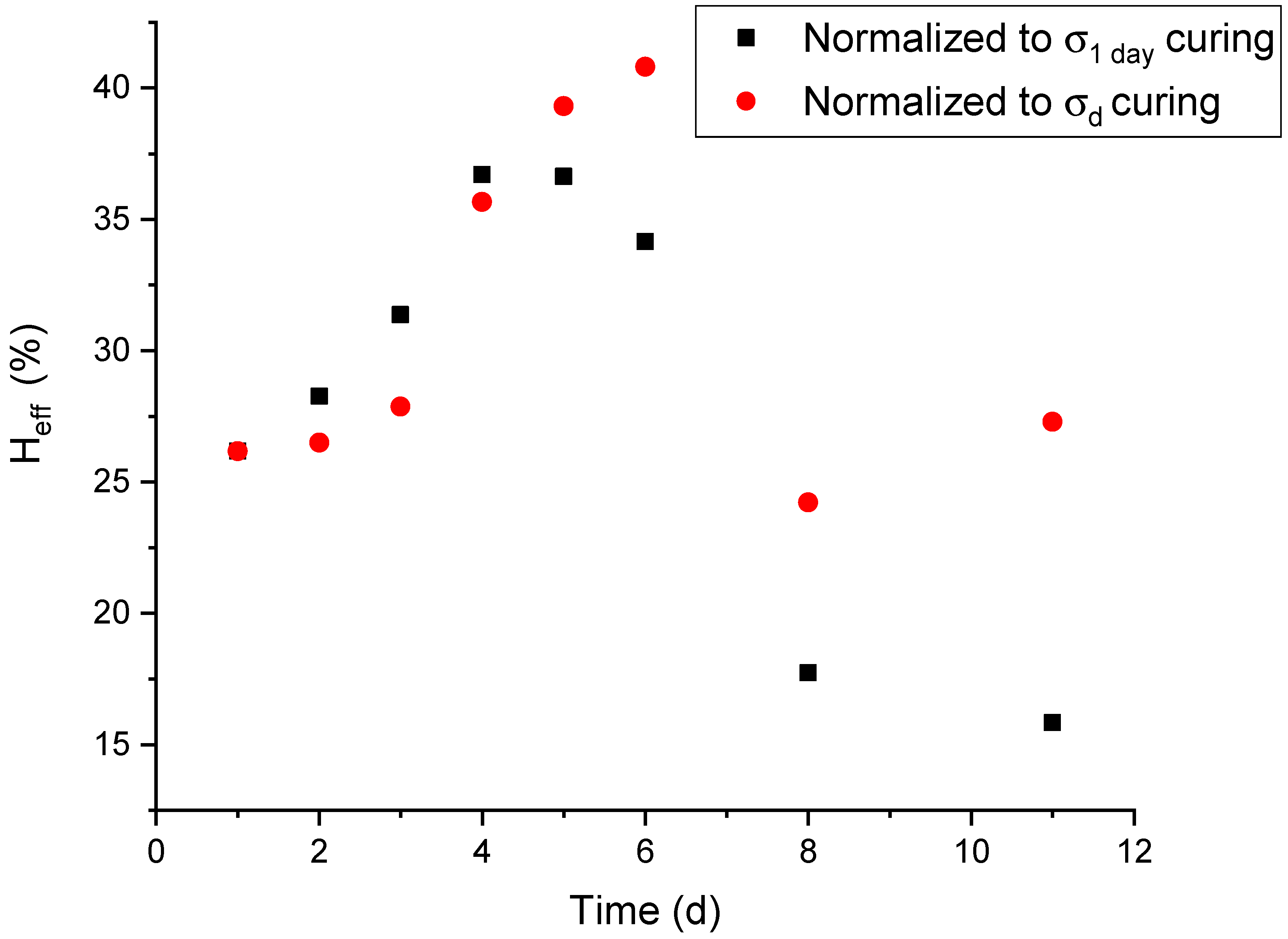
2.3. Time-Dependency of the Healing Efficiency
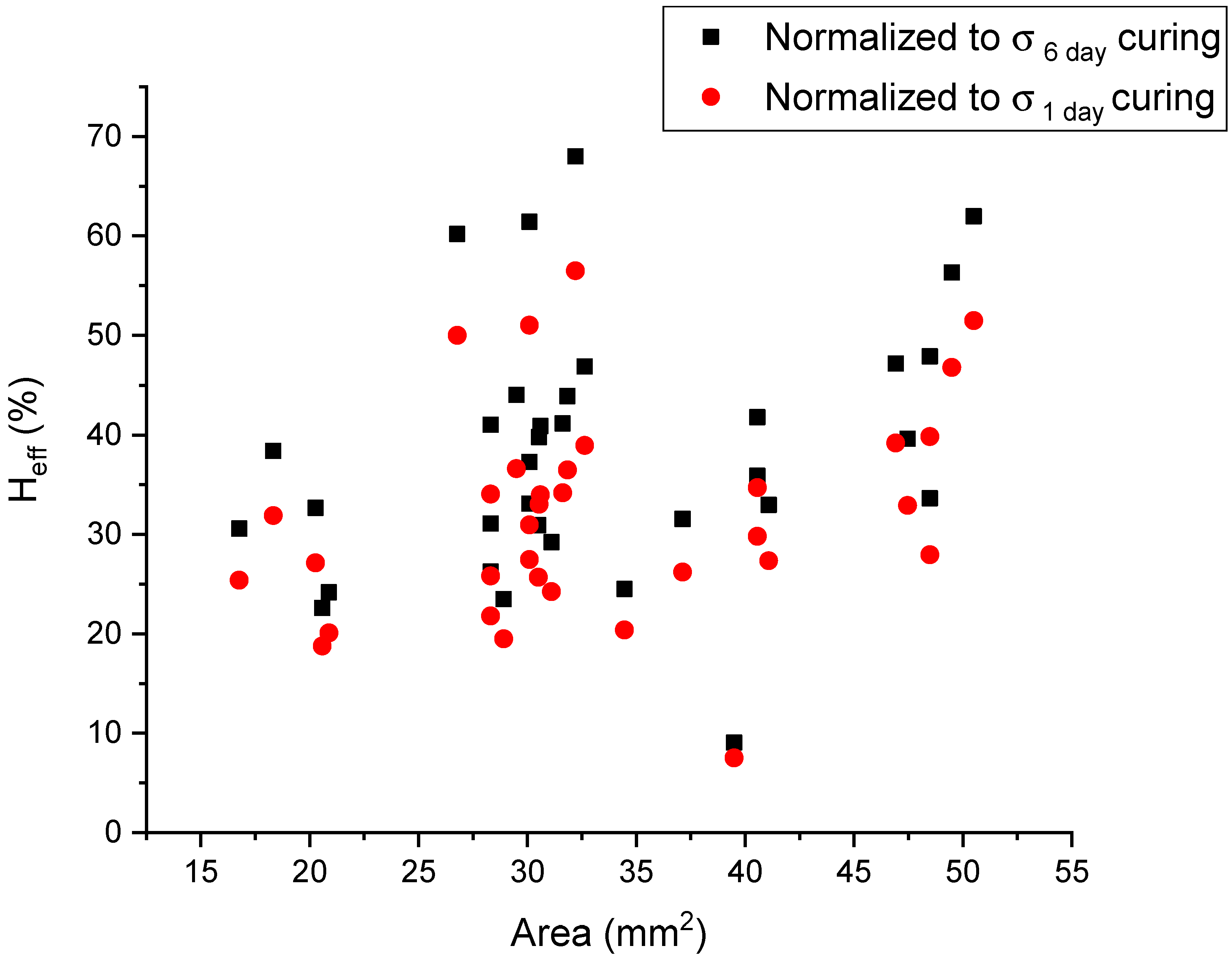
2.4. Area-Dependency of the Healing Efficiency
3. Experimental Section
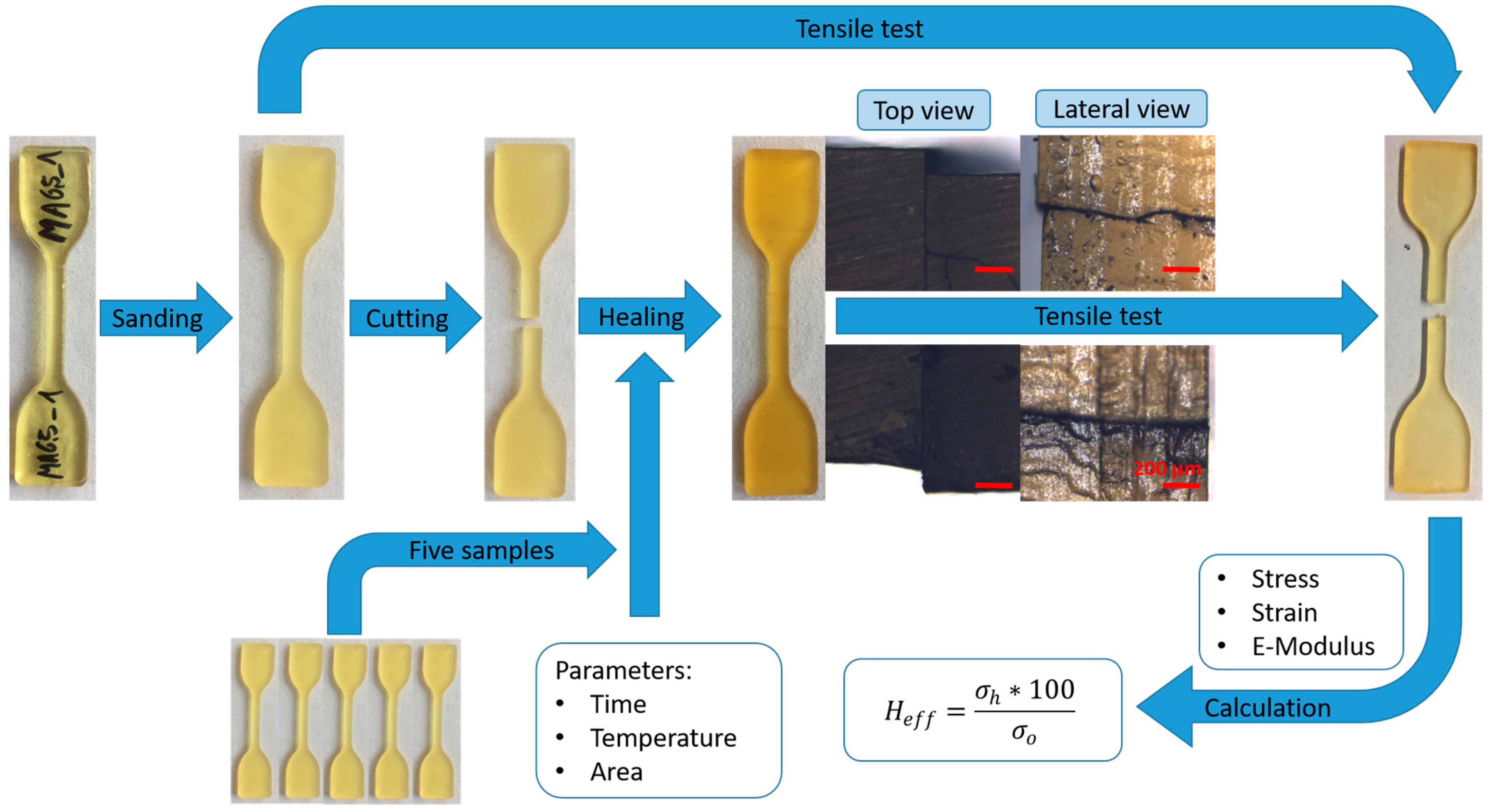
3.1. Materials and Methods
3.2. Polymer Synthesis and Sample Preparation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Overshootday. Available online: www.overshootday.org (accessed on 3 April 2019).
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Guimard, N.K.; Oehlenschlaeger, K.K.; Zhou, J.; Hilf, S.; Schmidt, F.G.; Barner-Kowollik, C. Current trends in the field of self-healing materials. Macromol. Chem. Phys. 2012, 213, 131–143. [Google Scholar] [CrossRef]
- Zwaag, S. Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science; Springer: Dordrecht, The Netherlands, 2007; Volume 1. [Google Scholar]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Matter. Rev. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.; Geubelle, P.; Moore, J.; Kessler, M.R.; Sriram, S.; Brown, E.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, J.; Gu, J.; Qiao, L.; Zhang, B.; Zhang, Q. Rapid and efficient synthesis of isocyanate microcapsules via thiol-ene photopolymerization in Pickering emulsion and its application in self-healing coating. Compos. Sci. Technol. 2016, 123, 250–258. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Li, C.; Kou, K. Synthesis of cyanate ester microcapsules via solvent evaporation technique and its application in epoxy resins as a healing agent. Ind. Eng. Chem. Res. 2016, 55, 10941–10946. [Google Scholar] [CrossRef]
- Dahlke, J.; Zechel, S.; Hager, M.D.; Schubert, U.S. How to design a self-healing polymer: General concepts of dynamic covalent bonds and their application for intrinsic healable materials. Adv. Mater. Interfaces 2018, 5, 1800051. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Polym. Eng. Sci. 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Geitner, R.; Kötteritzsch, J.; Siegmann, M.; Bocklitz, T.; Hager, M.; Schubert, U.; Gräfe, S.; Dietzek, B.; Schmitt, M.; Popp, J. Two-dimensional Raman correlation spectroscopy reveals molecular structural changes during temperature-induced self-healing in polymers based on the Diels–Alder reaction. Phys.Chem.Chem. Phys. 2015, 17, 22587–22595. [Google Scholar] [CrossRef]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Imato, K.; Takahara, A.; Otsuka, H. Self-healing of a cross-linked polymer with dynamic covalent linkages at mild temperature and evaluation at macroscopic and molecular levels. Macromolecules 2015, 48, 5632–5639. [Google Scholar] [CrossRef]
- Herbst, F.; Döhler, D.; Michael, P.; Binder, W.H. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.K.; Hohlbein, N.; Garcia, S.J.; Schmidt, A.M.; van der Zwaag, S. Connecting supramolecular bond lifetime and network mobility for scratch healing in poly (butyl acrylate) ionomers containing sodium, zinc and cobalt. Phys. Chem. Chem. Phys. 2015, 17, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.R.; Hunter, J.H.; Nguyen, N.A.; Harries, J.L.; Greenland, B.W.; Mackay, M.E.; Colquhoun, H.M.; Hayes, W. Multivalency in healable supramolecular polymers: The effect of supramolecular cross-link density on the mechanical properties and healing of non-covalent polymer networks. Polym. Chem. 2014, 5, 3680–3688. [Google Scholar] [CrossRef]
- Bode, S.; Enke, M.; Bose, R.; Schacher, F.; Garcia, S.; van der Zwaag, S.; Hager, M.; Schubert, U. Correlation between scratch healing and rheological behavior for terpyridine complex based metallopolymers. J. Mater. Chem. A 2015, 3, 22145–22153. [Google Scholar] [CrossRef]
- Kupfer, S.; Zedler, L.; Guthmuller, J.; Bode, S.; Hager, M.D.; Schubert, U.S.; Popp, J.; Gräfe, S.; Dietzek, B. Self-healing mechanism of metallopolymers investigated by QM/MM simulations and Raman spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12422–12432. [Google Scholar] [CrossRef]
- Bode, S.; Zedler, L.; Schacher, F.H.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Self-healing polymer coatings based on crosslinked metallosupramolecular copolymers. Adv. Mater. 2013, 25, 1634–1638. [Google Scholar] [CrossRef]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [Green Version]
- Abend, M.; Kunz, C.; Stumpf, S.; Gräf, S.; Zechel, S.; Müller, F.A.; Hager, M.D.; Schubert, U.S. Femtosecond laser-induced scratch ablation as an efficient new method to evaluate the self-healing behavior of supramolecular polymers. J. Mater.Chem. A 2019, 7, 2148–2155. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, Z. Multivalent hydrogen bonding block copolymers self-assemble into strong and tough self-healing materials. Chem. Commun. 2014, 50, 10868–10870. [Google Scholar] [CrossRef] [PubMed]
- Tepper, R.; Bode, S.; Geitner, R.; Jäger, M.; Görls, H.; Vitz, J.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D. Polymeric halogen-bond-based donor systems showing self-healing behavior in thin films. Angew. Chem. Int. Ed. 2017, 56, 4047–4051. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mahmood, N.; Beiner, M.; Binder, W.H. Self-healing materials from V-and H-shaped supramolecular architectures. Angew. Chem. Int. Ed. 2015, 54, 10188–10192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Abend, M.; Geitner, R.; Vitz, J.; Zechel, S.; Schmitt, M.; Popp, J.; Schubert, U.S.; Hager, M.D. Urethanes as reversible covalent moieties in self-healing polymers. Eur. Polym. J. 2018, 104, 45–50. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nature Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Zechel, S.; Geitner, R.; Abend, M.; Siegmann, M.; Enke, M.; Kuhl, N.; Klein, M.; Vitz, J.; Gräfe, S.; Dietzek, B. Intrinsic self-healing polymers with a high E-modulus based on dynamic reversible urea bonds. NPG Asia Mater. 2017, 9, e420. [Google Scholar] [CrossRef]
- Boutin, M.; Lesage, J.; Ostiguy, C.; Pauluhn, J.; Bertrand, M. Identification of the isocyanates generated during the thermal degradation of a polyurethane car paint. J. Anal. Appl. Pyrol. 2004, 71, 791–802. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2012, 113, 80–118. [Google Scholar] [CrossRef]
- Kim, Y.H.; Wool, R.P. A theory of healing at a polymer-polymer interface. Macromolecules 1983, 16, 1115–1120. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Monomer | Tensile Test (mg) |
|---|---|
| Benzoin methyl ether | 100 |
| Butylmethacrylate | 10,000 |
| N,N´-di-tert-butylethylenediamine | 302 |
| 2-isocyanate ethyl methacrylate | 546 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abend, M.; Zechel, S.; Schubert, U.S.; Hager, M.D. Detailed Analysis of the Influencing Parameters on the Self-Healing Behavior of Dynamic Urea-Crosslinked Poly(methacrylate)s. Molecules 2019, 24, 3597. https://doi.org/10.3390/molecules24193597
Abend M, Zechel S, Schubert US, Hager MD. Detailed Analysis of the Influencing Parameters on the Self-Healing Behavior of Dynamic Urea-Crosslinked Poly(methacrylate)s. Molecules. 2019; 24(19):3597. https://doi.org/10.3390/molecules24193597
Chicago/Turabian StyleAbend, Marcus, Stefan Zechel, Ulrich S. Schubert, and Martin D. Hager. 2019. "Detailed Analysis of the Influencing Parameters on the Self-Healing Behavior of Dynamic Urea-Crosslinked Poly(methacrylate)s" Molecules 24, no. 19: 3597. https://doi.org/10.3390/molecules24193597
APA StyleAbend, M., Zechel, S., Schubert, U. S., & Hager, M. D. (2019). Detailed Analysis of the Influencing Parameters on the Self-Healing Behavior of Dynamic Urea-Crosslinked Poly(methacrylate)s. Molecules, 24(19), 3597. https://doi.org/10.3390/molecules24193597








