Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview
Abstract
1. Introduction
- Increasing the efficiency of drug delivery and reducing side effects, therefore toxicity;
- Specific targeting of the active components in cell/tissues;
- Improving the properties of pharmacologically active drugs such as stability, solubility, half-life, and tumor aggregation;
- Generating stimuli-responsive drug release;
- Expanding the area of drugs encapsulated/attached to biomacromolecules such as proteins, mRNA;
- Improvement of therapeutic efficiency by delivering multiple active agents to a specific targeted site in order to overcome limitations such as drug resistance;
- Overcoming biological barriers;
- Improving the sensitivity of diagnosis and imaging of tumorous sites;
- Linking anti-cancer active components with imaging molecules in order to attain a real-time assessment of the in vivo efficiency of the drugs;
- Developing new paths for the manufacture of synthetic vaccines; and
- Improving cancer diagnosis and imaging with scaled-down medical devices.
2. Nanomaterials for Drug Delivery
2.1. Properties of Nanoparticles
2.2. Cancer Theranostics
3. Biological Barriers that Influence Drug Delivery
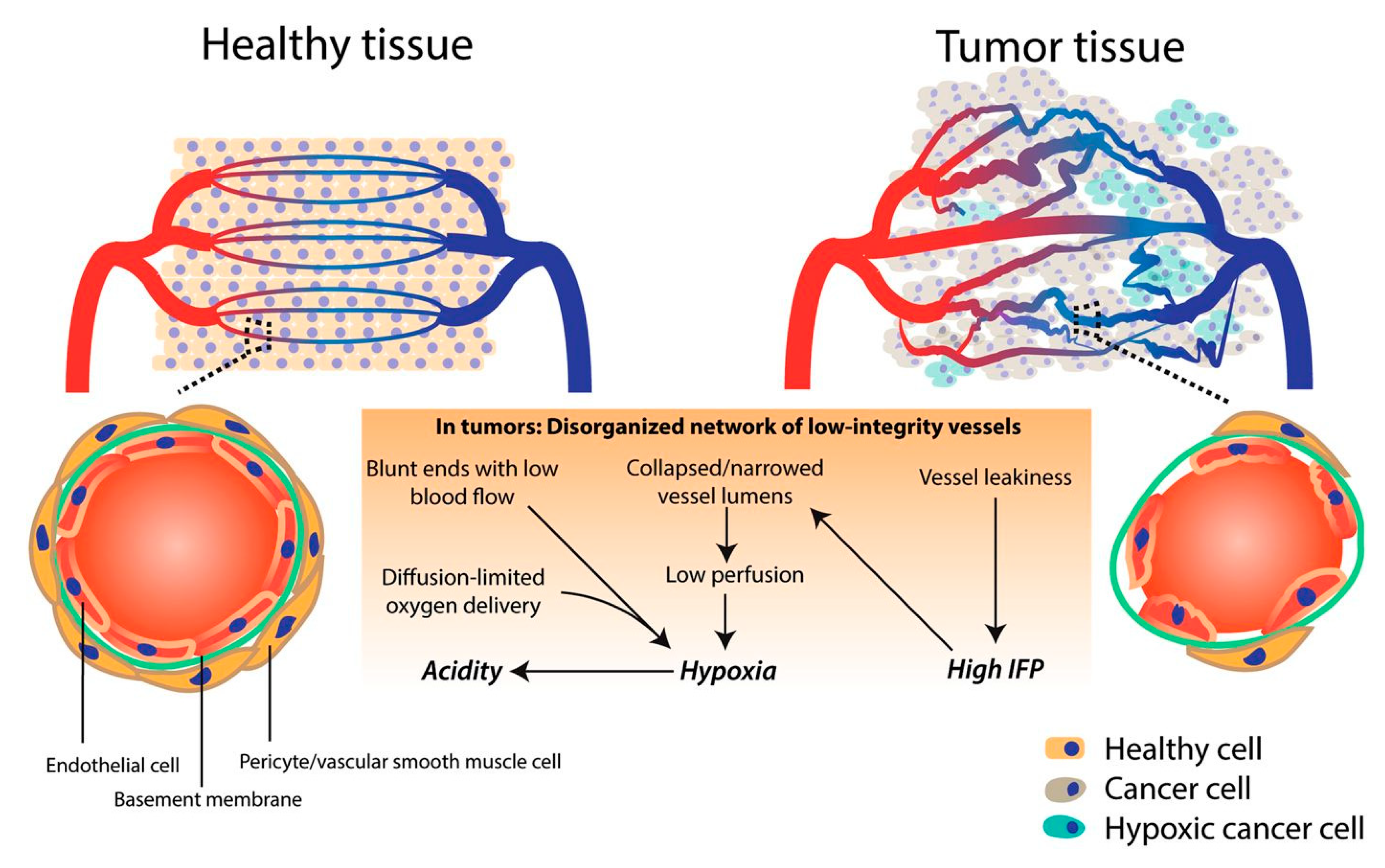
3.1. Tumor Microenvironment and Vasculature
3.2. Reticuloendothelial System (RES)
3.3. Blood–Brain Barrier (BBB)
3.4. Kidney Filtration
4. Types of Nanoparticles
4.1. Liposomes
4.2. Polymeric Nanoparticles
4.3. Gold Nanoparticles
4.4. Magnetic Nanoparticles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, C.Y.; Cheng, R.; Yang, Z.; Tian, Z.M. Nanotechnology for cancer therapy based on chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for cancer therapy and imaging. Mol. Cells 2011, 31, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yasri, S.; Wiwanitkit, V. Applied biointerface technology for medical diagnosis: A summary. Biointerface Res. Appl. Chem. 2018, 8, 3490–3492. [Google Scholar]
- Narayana, A. Applications of nanotechnology in cancer: A literature review of imaging and treatment. J. Nucl. Med. Radiat. Ther. 2014, 5. [Google Scholar] [CrossRef]
- Sabry, N.M.; Tolba, S.; Abdel-Gawad, F.K.; Bassem, S.M.; Nassar, H.F.; El-Taweel, G.E.; Okasha, A.; Ibrahim, M. Interaction between nano silver and bacteria: Modeling approach. Biointerface Res. Appl. Chem. 2018, 8, 3570–3574. [Google Scholar]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Contrast agents delivery: An up-to-date review of nanodiagnostics in neuroimaging. Nanomaterials 2019, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Faisal, N.; Kumar, K. Polymer and metal nanocomposites in biomedical applications. Biointerface Res. Appl. Chem. 2017, 7, 2286–2294. [Google Scholar]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An up-to-date overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Seeta Rama Raju, G.; Benton, L.; Pavitra, E.; Yu, J.S. Multifunctional nanoparticles: Recent progress in cancer therapeutics. Chem. Commun. 2015, 51, 13248–13259. [Google Scholar] [CrossRef]
- Tang, L.; Gabrielson, N.P.; Uckun, F.M.; Fan, T.M.; Cheng, J. Size-dependent tumor penetration and in vivo efficacy of monodisperse drug–silica nanoconjugates. Mol. Pharm. 2013, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Dhakshinamurthy, G.S.; Misra, S.K. Tailoring of physicochemical properties of nanocarriers for effective anti-cancer applications. J. Biomed. Mater. Res. Part A 2017, 105, 2906–2928. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.; Ghaghada, K.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar]
- Srivastava, I.; Misra, S.K.; Ostadhossein, F.; Daza, E.; Singh, J.; Pan, D. Surface chemistry of carbon nanoparticles functionally select their uptake in various stages of cancer cells. Nano Res. 2017, 10, 3269–3284. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wong, S.T.C. Chapter 1—Cancer theranostics: An introduction. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Oxford, MS, USA, 2014; pp. 3–8. [Google Scholar]
- Ayodele, A.T.; Valizadeh, A.; Adabi, M.; Esnaashari, S.S.; Madani, F.; Khosravani, M.; Adabi, M. Ultrasound nanobubbles and their applications as theranostic agents in cancer therapy: A review. Biointerface Res. Appl. Chem. 2017, 7, 2253–2262. [Google Scholar]
- Zhu, L.; Yang, L.; Zhou, Z. Nanomaterials in cancer theranostics. In Bioactivity of Engineered Nanoparticles; Yan, B., Zhou, H., Gardea-Torresdey, J.L., Eds.; Springer: Singapore, 2017; pp. 173–206. [Google Scholar]
- Figueiredo, P.; Bauleth-Ramos, T.; Hirvonen, J.; Sarmento, B.; Santos, H.A. Chapter 1—The emerging role of multifunctional theranostic materials in cancer nanomedicine. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–31. [Google Scholar]
- Gao, H. Shaping tumor microenvironment for improving nanoparticle delivery. Curr. Drug Metab. 2016, 17, 731–736. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomed. (Lond. Engl.) 2010, 5, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Et Biophys. Acta 2009, 1788, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-F. The blood-brain barrier: Brain cancer therapy hits a wall. Oncol. Times 2018, 40, 1–6. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for drug delivery to the central nervous system. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Sharon, A.; Baranes, K.; Motiei, M.; Lellouche, J.P.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: An in-vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomed. (Lond. Engl.) 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C.W. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl. Acad. Sci. USA 2016, 113, E1142–E1151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Du, J.-Z.; Du, X.-J.; Xu, C.-F.; Sun, C.-Y.; Wang, H.-X.; Cao, Z.-T.; Yang, X.-Z.; Zhu, Y.-H.; Nie, S.; et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. USA 2016, 113, 4164–4169. [Google Scholar] [CrossRef]
- Qie, Y.; Yuan, H.; von Roemeling, C.A.; Chen, Y.; Liu, X.; Shih, K.D.; Knight, J.A.; Tun, H.W.; Wharen, R.E.; Jiang, W.; et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci. Rep. 2016, 6, 26269. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Hayden, E.; Camann, A.; Berman, Z.; Vicente, P.; Tran, E.; Meyers, J.; Pansky, J.; Peiris, P.M.; Wu, H.; et al. Multimodal in vivo imaging exposes the voyage of nanoparticles in tumor microcirculation. Acs Nano 2013, 7, 3118–3129. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with t(1)-t(2) switchable magnetic resonance imaging contrast. Acs Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef]
- Heist, R.S.; Duda, D.G.; Sahani, D.V.; Ancukiewicz, M.; Fidias, P.; Sequist, L.V.; Temel, J.S.; Shaw, A.T.; Pennell, N.A.; Neal, J.W.; et al. Improved tumor vascularization after anti-vegf therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Miyamoto, Y.; Kojima, Y.; Maeda, H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br. J. Cancer 1993, 67, 975–980. [Google Scholar] [CrossRef]
- Park, E.-J.; Zhang, Y.-Z.; Vykhodtseva, N.; McDannold, N. Enhanced permeability of tumor blood vessels in brain using focused ultrasound with microbubbles. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; IEEE: New York, NY, USA, 2010. [Google Scholar]
- Snyder, J.W.; Greco, W.R.; Bellnier, D.A.; Vaughan, L.; Henderson, B.W. Photodynamic therapy: A means to enhanced drug delivery to tumors. Cancer Res. 2003, 63, 8126–8131. [Google Scholar]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. Acs Nano 2013, 7, 717–724. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for cancer therapies. Nanotechnol. Rev. 2017, 6, 473–476. [Google Scholar] [CrossRef]
- Iurie, R. Cell drug delivery of fluorescein loaded apob100 functionalized liposomes. Biointerface Res. Appl. Chem. 2015, 5, 1007–1010. [Google Scholar]
- Bahramian, G.; Golestan, L.; Khosravi-Darani, K. Antimicrobial and antioxidant effect of nanoliposomes containing zataria multiflora boiss essential oil on the rainbow trout fillets during refrigeration. Biointerface Res. Appl. Chem. 2018, 8, 3505–3513. [Google Scholar]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Niemann, B.; Massing, U.; Drevs, J.; Unger, C.; Bhamra, R.; Swenson, C.E. Pharmacokinetics of liposomal doxorubicin (tlc-d99; myocet) in patients with solid tumors: An open-label, single-dose study. Cancer Chemother. Pharmacol. 2004, 54, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(r)—The first fda-approved nano-drug: Lessons learned. J. Control. Release. 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Zhang, J.A.; Anyarambhatla, G.; Ma, L.; Ugwu, S.; Xuan, T.; Sardone, T.; Ahmad, I. Development and characterization of a novel cremophor el free liposome-based paclitaxel (lep-etu) formulation. Eur. J. Pharm. Biopharm. 2005, 59, 177–187. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific ph-responsive peptide-modified ph-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control Release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, S.; Li, H.; Zhang, Z.; Zhong, J.; Liu, M.; Zhou, X. Mri-guided liposomes for targeted tandem chemotherapy and therapeutic response prediction. Acta Biomater. 2016, 35, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. Handb. Exp. Pharmacol. 2010, 55–86. [Google Scholar] [CrossRef]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Vilos, C.; Morales, F.A.; Solar, P.A.; Herrera, N.S.; Gonzalez-Nilo, F.D.; Aguayo, D.A.; Mendoza, H.L.; Comer, J.; Bravo, M.L.; Gonzalez, P.A.; et al. Paclitaxel-PHBV nanoparticles and their toxicity to endometrial and primary ovarian cancer cells. Biomaterials 2013, 34, 4098–4108. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, Y.; Ling, Y.; Huang, Y.; Li, T.; Li, X. Improved therapeutic effect of folate-decorated plga–peg nanoparticles for endometrial carcinoma. Bioorganic Med. Chem. 2011, 19, 4057–4066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Xie, S.-X.; Baoum, A.; Yakovleva, T.; Siahaan, T.J.; Berkland, C.J. Icam-1 targeting of doxorubicin-loaded plga nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci. 2009, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Ullah, N.; Choi, M.H.; Kim, M.O.; Yoon, S.C. Amorphous amphiphilic p(3hv-co-4hb)-b-mpeg block copolymer synthesized from bacterial copolyester via melt transesterification: Nanoparticle preparation, cisplatin-loading for cancer therapy and in vitro evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized pt(iv) prodrug-plga-peg nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Dhas, N.; Ige, P.; Kudarha, R. Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized plga nanoparticle for delivery of bicalutamide in prostate cancer. Power Technol. 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Garg, N.K.; Tyagi, R.K.; Singh, B.; Katare, O.P.; Webster, T.J.; Soni, V. Surface engineered polymeric nanocarriers mediate the delivery of transferrin–methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater. 2015, 24, 140–151. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, W.; Bao, Y.; Xu, H.; Qin, S.; Tu, Y. Bsa capped au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. Rsc Adv. 2015, 5, 40514–40520. [Google Scholar] [CrossRef]
- Kong, T.; Zeng, J.; Wang, X.; Yang, X.; Yang, J.; McQuarrie, S.; McEwan, A.; Roa, W.; Chen, J.; Xing, J.Z. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small 2008, 4, 1537–1543. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of iron oxide nanoparticles in cancer therapy: Amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image-guided approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef]
- Frounchi, M.; Shamshiri, S. Magnetic nanoparticles-loaded pla/peg microspheres as drug carriers. J. Biomed. Mater. Res. Part A 2015, 103, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Behera, B.; Sahu, S.K.; Ananthakrishnan, R.; Maiti, T.K.; Pramanik, P. Design of dual stimuli responsive polymer modified magnetic nanoparticles for targeted anti-cancer drug delivery and enhanced mr imaging. New J. Chem. 2016, 40, 545–557. [Google Scholar] [CrossRef]
- Parsian, M.; Unsoy, G.; Mutlu, P.; Yalcin, S.; Tezcaner, A.; Gunduz, U. Loading of gemcitabine on chitosan magnetic nanoparticles increases the anti-cancer efficacy of the drug. Eur. J. Pharmacol. 2016, 784, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase i study of mrx34, a liposomal mir-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Dey, G.; Banerjee, I.; Dey, K.K.; Parida, S.; Kumar, B.N.P.; Das, C.K.; Pal, I.; Mukherjee, M.; Misra, M.; et al. Somatostatin receptor targeted liposomes with diacerein inhibit il-6 for breast cancer therapy. Cancer Lett. 2017, 388, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-j.; Wang, H.-y.; Peng, H.-g.; Chen, B.-f.; Zhang, W.-y.; Wu, A.-h.; Xu, Q.; Huang, Y.-z. Codelivery of dihydroartemisinin and doxorubicin in mannosylated liposomes for drug-resistant colon cancer therapy. Acta Pharmacol. Sin. 2017, 38, 885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liang, C.; Yi, X.; Song, G.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.; Liu, Z. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Xing, S.; He, Y.; Cao, W.; Zhu, R.; Gao, D. Gold nanoshell coated thermo-ph dual responsive liposomes for resveratrol delivery and chemo-photothermal synergistic cancer therapy. J. Mater. Chem. B 2017, 5, 2161–2171. [Google Scholar] [CrossRef]
- Chen, D.; Wu, I.C.; Liu, Z.; Tang, Y.; Chen, H.; Yu, J.; Wu, C.; Chiu, D.T. Semiconducting polymer dots with bright narrow-band emission at 800 nm for biological applications. Chem. Sci. 2017, 8, 3390–3398. [Google Scholar] [CrossRef]
- Cui, D.; Xie, C.; Lyu, Y.; Zhen, X.; Pu, K. Near-infrared absorbing amphiphilic semiconducting polymers for photoacoustic imaging. J. Mater. Chem. B 2017, 5, 4406–4409. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Zhang, R.; Chen, R.; Zhang, Z.; Zhang, W.; Peng, S.-H.; Chen, X.; Liu, G.; Hsu, C.-S.; et al. Biocompatible D–A Semiconducting Polymer Nanoparticle with Light-Harvesting Unit for Highly Effective Photoacoustic Imaging Guided Photothermal Therapy. Adv. Funct. Mater. 2017, 27, 1605094. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. Ph-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Liu, Y.; Ayyash, D.; Rasmussen, J.; Huo, M.; et al. ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy. Adv. Mater. 2017, 29, 1700141. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 2017, 29, 1604894. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.K.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Pathak, S.; Oh, K.T.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Pegylated thermosensitive lipid-coated hollow gold nanoshells for effective combinational chemo-photothermal therapy of pancreatic cancer. Colloids Surf. B Biointerfaces 2017, 160, 73–83. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Zhang, M.; Ma, Y.; Liu, Y.; Gao, W.; Zhang, J.; Gu, Y. Laser-triggered small interfering rna releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv. Sci. 2017, 4, 1600327. [Google Scholar] [CrossRef]
- Yin, D.; Li, X.; Ma, Y.; Liu, Z. Targeted cancer imaging and photothermal therapy via monosaccharide-imprinted gold nanorods. Chem. Commun. 2017, 53, 6716–6719. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, H.S.; Jin, T.; Woo, J.; Piao, Y.J.; Moon, W.K. Near-infrared photothermal therapy using anti-egfr-gold nanorod conjugates for triple negative breast cancer. Oncotarget 2017, 8, 86566–86575. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.T.; Yang, G.; Mo, S.J.; Wang, Z.Y.; Li, B.J.; Ma, W.; Guo, Y.X.; Chen, X.; Zhao, X.; Liu, J.Q.; et al. Atomically precise gold-levonorgestrel nanocluster as a radiosensitizer for enhanced cancer therapy. ACS Nano 2019, 13, 8320–8328. [Google Scholar] [CrossRef]
- Bera, K.; Maiti, S.; Maity, M.; Mandal, C.; Maiti, N.C. Porphyrin-gold nanomaterial for efficient drug delivery to cancerous cells. Acs Omega 2018, 3, 4602–4619. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.C.; Michiels, C.; Lucas, S. Thioredoxin reductase activity predicts gold nanoparticle radiosensitization effect. Nanomaterials 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.M.; Mehdizadeh, A.; Koosha, F.; Eslahi, N.; Mahabadi, V.P.; Ghaznavi, H.; Shakeri-Zadeh, A. Investigating the photo-thermo-radiosensitization effects of folate-conjugated gold nanorods on kb nasopharyngeal carcinoma cells. Photodiagnosis Photodyn. Ther. 2018, 24, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of gold nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi Malekzadeh, A.; Ramazani, A.; Tabatabaei Rezaei, S.J.; Niknejad, H. Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and mri in cancer theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-M.; Fu, C.-P.; Fang, J.-Z.; Xu, X.-D.; Wei, X.-H.; Tang, W.-J.; Jiang, X.-Q.; Zhang, L.-M. Hyaluronan-modified superparamagnetic iron oxide nanoparticles for bimodal breast cancer imaging and photothermal therapy. Int. J. Nanomed. 2016, 12, 197–206. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Davaran, S.; Danafar, H.; Manjili, H.K. Methotrexate-conjugated l-lysine coated iron oxide magnetic nanoparticles for inhibition of mcf-7 breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Ghaznavi, H.; Hosseini-Nami, S.; Kamrava, S.K.; Irajirad, R.; Maleki, S.; Shakeri-Zadeh, A.; Montazerabadi, A. Folic acid conjugated peg coated gold–iron oxide core–shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1594–1604. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine serum albumin (bsa) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorganic Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; de Silva, N.; Bhandari, S.; Yap, Y.K.; Costha, N.P. Ph responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Trabulo, S.; Aires, A.; Aicher, A.; Heeschen, C.; Cortajarena, A.L. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite coated iron oxide nanoparticles: A promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef] [PubMed]

| Reference | Year | Type of Nanoparticles (NP) | Use | Results |
|---|---|---|---|---|
| Lyposomes | ||||
| Muhammad et al. [78] | 2017 | MRX34 – a liposoman formulation that mimics the tumor suppressor microRNA-34a | Phase I study for advanced solid tumors | Anti-tumoral activity |
| Bharti et al. [79] | 2017 | Liposome encapsulating diacerein (therapeutic molecule) and decorated with synthetic somatostatin analogue (receptor overexpressed in breast cancer cells) | Breast cancer therapy | Enhanced circulation time and apoptosis in breast cells, tumor growth inhibition |
| Kang et al. [80] | 2017 | Liposomal system loaded with dihydroartemisinin and doxorubicin | Co-delivery system for drug-resistant colon cancer therapy | High cytotoxicity to cancer cells and tumor inhibition rate above 80% compared to free drugs |
| Zhang et al. [81] | 2017 | Cisplatin-prodrug-constructed liposomes loaded with an antioxidant enzyme (catalase) | Enhanced chemoradiotherapy | Tumor hypoxia relief and DNA damage in cancer cells |
| Wang et al. [82] | 2017 | Chitosan-modified liposomes loaded with resveratrol and coated with gold nanshells | Chemophotothermal cancer therapy | High stability and photothermal conversion capacity, higher therapeutic effect on cancer cells compared to single therapies |
| Polymeric Nanoparticles | ||||
| Chen et al. [83] | 2017 | NIR800 polymer | In vivo imaging | High-contrast imaging of lymph nodes and tumors |
| Cui et al. [84] | 2017 | Polyethylene glycol (PEG)-grafted poly(cyclopentadithiophene-alt-diketopyrrolopyrrole) semiconducting polymeric NPs | Photoacoustic imaging agents | Imaging of tumor in living mice with a high ratio of tumor signal to background |
| Zhang et al. [85] | 2017 | Biocompatible electron donor–acceptor conjugated semiconducting polymer nanoparticles (PPor-PEG) with light-harvesting unit | Photoacoustic imaging-guided photothermal therapy | Complete tumor regression in tumor-bearing mice |
| Cheng et al. [86] | 2017 | Polydopamine-modified mesoporous silica NPs coated with poly(ethylene glycol)-folic acid, loaded with doxorubicin | Drug delivery system for cervical cancer therapy | High targeting efficiency and anti-tumor efficacy in vivo |
| Xu et al. [87] | 2017 | NP platform containing mitoxantrone core, poly(ethylene glycol) shell, and arginylglycylaspartic acid (RGD) peptide | Reactive oxygen species (ROS)-responsive polydrug NPs | Responsive to intracellular ROS and significant inhibitory activity on tumor cell growth |
| Gold Nanoparticles | ||||
| Cheng et al. [88] | 2017 | Diazirine-decorated gold nanoparticles | Photothermal therapy and photoacousting imaging of tumors | Negligible cytotoxicity, impressive photothermal ablation effect |
| Poudel et al. [89] | 2017 | PEGylated thermosensitive lipid-coated plasmonic hollow gold nanoshells, loaded with gemcitabine and bortezomib | Chemotherapy combined with photothermal therapy of pancreatic cancer | Efficient cellular uptake and apoptosis of cancer cells, specific drug delivery, exhibited photothermal effect |
| Wang et al. [90] | 2017 | Hollow gold nanoshell functionalized with small interfering RNAs against heat shock protein 70 (Hsp70) | Photothermal platform for induced hyperthermia therapy | Enhanced cellular uptake, efficient siRNA delivery and Hsp70 silencing |
| Yin et al. [91] | 2017 | Sialic acid-imprinted gold nanorods | Targeted near-infrared (NIR) cancer photothermal therapy | Biocompatibility, selectivity of targeted cancer cells and high photothermal effect |
| Zhang et al. [92] | 2017 | Anti-epidermal growth factor receptor-conjugated gold nanorods | Epidermal growth factor receptor therapy for triple-negative breast cancer using photoacoustic imaging-guided NIR photothermal therapy | Strong anti-proliferation, apoptotic activity of cancer cells, and tumor regression |
| Jia et al. [93] | 2019 | Gold-levonorgestrel nanoclusters | Radiosensitizer for enhanced cancer therapy | ROS production that leads to cell death significantly inhibited tumorigenicity after one treatment |
| Bera et al. [94] | 2018 | Porphyrin-coated gold NPs loaded with Doxorucibin (DOX) | Nanochemotherapeutic system | High encapsulation efficicency, selective internalization inside cancerous cells with increased retention time, targeted delivery, |
| Penninckx et al. [95] | 2019 | Amino-PEG functionalized gold nanoparticles | Radiosensitizer and effect on residual thioredoxin reductase | Rediosensitization effect dependent on cell type, thioredoxin reductase activity inhibition |
| Movahedi et al. [96] | 2018 | Folic acid-conjugated gold nanorods | Multimodal cancer therapy | Improved photosensitivity and radiosensitivity of cancerous cells, induced cell death in nasopharyngeal carcinoma cells (KB) |
| Mendes et al. [97] | 2017 | 14-nm gold NPs loaded with DOX | Photothermal agents | Induced cell death in breast cancer cells |
| Magnetic Nanoparticles | ||||
| Rao et al. [98] | 2017 | Red blood cell membrane-derived vesicles-coated Fe3O4 NPs | Magnetic resonance imaging (MRI) and photothermal therapy | Enhanced stability and performance in in vivo MRI and photothermal therapy |
| Malekzadeh et al. [99] | 2017 | Fe3O4 NPs functionalized with poly citric acid, PEG, and folic acid | MRI for cancer therapy | Selective cellular uptake, increased NPs cytotoxicity on HeLa cells, enhanced magnetic resonance signal |
| Huang et al. [100] | 2017 | Superparamagnetic iron oxide NPs (SPIONs) coated with PEG, polyethyleimine (PEI) and folic acid and loaded with DOX | Drug delivery platforms for cancer theranostics | Low toxicity, specific targeting of cancer cell, and inhibition of tumor growth |
| Yang et al. [101] | 2017 | Hyaluronan-modified SPIONs | Breast cancer imaging and photothermal therapy | Specific cellular uptake and accumulation, meaningful contrast enhancement in MRI, concentration-dependent photothermal effect |
| Nosrati et al. [102] | 2018 | Fe3O4 conjugated with l-lysine and loaded with methotrexate | Drug delivery vehicle for breast cancer | Targeted delivery and internalization of the NPs, cytotoxic effect on human breast cancer cells, possible real-time montorization of drug delivery |
| Ghaznavi et al. [103] | 2017 | Au@Fe3O4 coated with PEG and folic acid | Photothermal therapeutic agent | Induced apopotosis in cancer cells |
| Nosrati et al. [104] | 2018 | Bovine serum albumin-coated Fe3O4 loaded with curcumin | Drug delivery carriers | Sustained release at body temperature, semnificative toxicity effect against breast cancer cells |
| Manatunga et al. [105] | 2017 | Fe3O4 coated with a bi-layer of sodium alginate and hydroxyapatite and loaded with curcumin and 6-gingerol | Delivery of hydrophobic drugs | High loading efficiency, sustained and controlled release at low pH |
| Trabulo et al. [106] | 2017 | Iron oxide MNPs conjugated with anti-CD47 antibody (CD47 – primary receptor expressed in pancreatic ductal adenocarcinoma) loaded with gemcitabine | Targeted delivery agent for pancreatic cancer cells | Selective and targeted delivery, induced apoptosis in pancreatic cancer cells |
| Mondal et al. [107] | 2017 | Fe3O4 coated hydroxyapatite NPs | Magnetic hyperthermia | No cytotoxicity without magnetic field, hyperthermia-mediated cell death on cancer cells |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules 2019, 24, 3547. https://doi.org/10.3390/molecules24193547
Lungu II, Grumezescu AM, Volceanov A, Andronescu E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules. 2019; 24(19):3547. https://doi.org/10.3390/molecules24193547
Chicago/Turabian StyleLungu, Iulia Ioana, Alexandru Mihai Grumezescu, Adrian Volceanov, and Ecaterina Andronescu. 2019. "Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview" Molecules 24, no. 19: 3547. https://doi.org/10.3390/molecules24193547
APA StyleLungu, I. I., Grumezescu, A. M., Volceanov, A., & Andronescu, E. (2019). Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules, 24(19), 3547. https://doi.org/10.3390/molecules24193547







