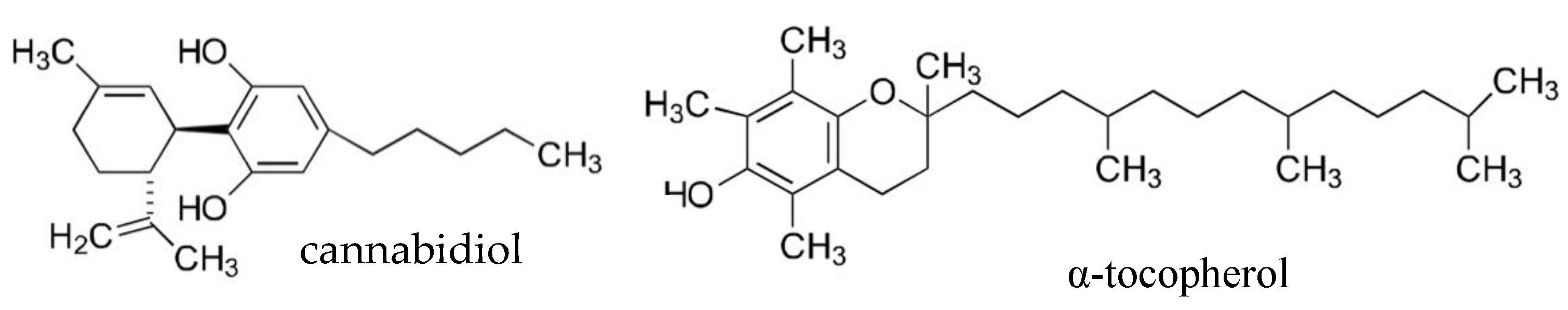
Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils
Abstract
:1. Introduction
2. Results
2.1. Determination of CBD Content
2.2. Determination of α-Tocopherol Content
2.3. Peroxide Value
2.4. Free Acidity
2.5. Oxidative Stability Index (OSI)
2.6. Electron Spin Resonance
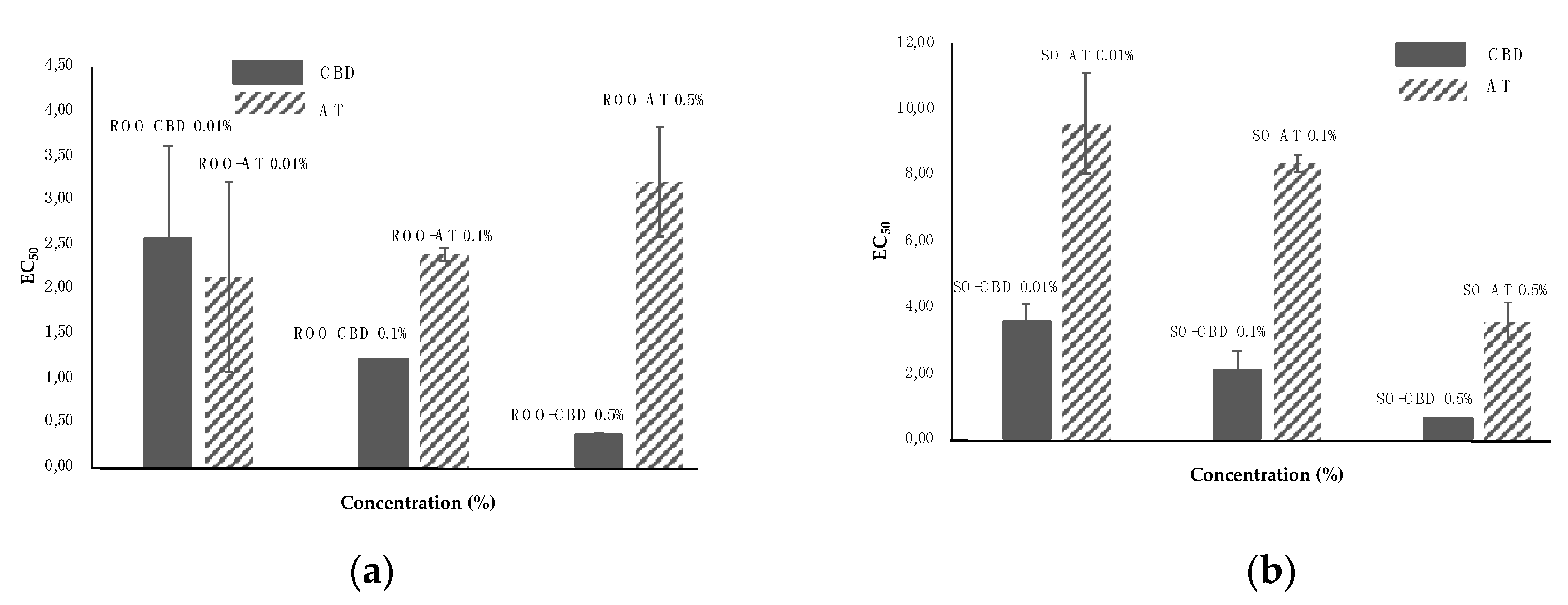
2.7. DPPH• Radical Scavenging Activity Assay
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Determination of CBD Content
4.3. Determination of α-Tocopherol Content
4.4. Peroxide Value
4.5. Free Acidity
4.6. Oxidative Stability Index
4.7. Micro-ESR
4.8. DPPH• Radical Scavenging Activity Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; Esposito, G.; De Filippis, D.; Scuderi, C.; Steardo, L. Cannabidiol: A promising drug for neurodegenerative disorders? CNS Neurosci. Ther. 2009, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules 2019, 24, 1459. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Batista, J.; Viana, R.; Baetas, A.; Orestes, E.; Andrade, M.; da Silva, A. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef]
- Beddows, G.; Jagait, C.; Kelly, M.J. Preservation of alpha-tocopherol in sunflower oil by herbs and spices. Int. J. Food Sci. Nutr. 2000, 51, 327–339. [Google Scholar] [PubMed]
- Muhammad Anjum, F.; Nadeem, M.; Issa Khan, M.; Hussain, S. Nutritional and therapeutic potential of sunflower seeds: A review. Br. Food J. 2012, 114, 544–552. [Google Scholar] [CrossRef]
- Zaunschirm, M.; Pignitter, M.; Kienesberger, J.; Hernler, N.; Riegger, C.; Eggersdorfer, M.; Somoza, V. Contribution of the ratio of tocopherol homologs to the oxidative stability of commercial vegetable oils. Molecules 2018, 23, 206. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Elmore, S. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Gülcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Lercker, G.; Rodriguez-Estrada, M.T.; Bonoli, M. Analysis of the oxidation products of cis-and trans-octadecenoate methyl esters by capillary gas chromatography–ion-trap mass spectrometry: I. Epoxide and dimeric compounds. J. Chromatogr. A 2003, 985, 333–342. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. CRFSFS 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Simó-Alfonso, E.F.; Bendini, A.; Cerretani, L. Rapid evaluation of oxidised fatty acid concentration in virgin olive oil using Fourier-transform infrared spectroscopy and multiple linear regression. Food Chem. 2011, 124, 679–684. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Di Virgilio, F.; Belloni, P.; Lercker, G.; Gallina Toschi, T. In-process monitoring in industrial olive mill by means of FT-NIR. Eur. J. Lipid Sci. Technol. 2007, 109, 498–504. [Google Scholar] [CrossRef]
- Coppin, E.A.; Pike, O.A. Oil stability index correlated with sensory determination of oxidative stability in light-exposed soybean oil. J. Am. Oil Chem. Soc. 2001, 78, 13–18. [Google Scholar] [CrossRef]
- Chen, H.; Cao, P.; Li, B.; Sun, D.; Li, J.; Liu, Y. High sensitive and efficient detection of edible oils adulterated with used frying oil by electron spin resonance. Food Control 2017, 73, 540–545. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Jezierski, A. Pro-and antioxidative effect of α-tocopherol on edible oils, triglycerides and fatty acids. J. Am. Oil Chem. Soc. 2013, 90, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.F.; Spallaci, M.; Cangiotti, M.; Bacchiocca, M.; Ninfali, P. Electron paramagnetic resonance investigations of free radicals in extra virgin olive oils. J. Agric. Food Chem. 2001, 49, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 702/2007 of 21 June 2007 Amending Commission Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Residual Oil and on the Relevant Methods of Analysis. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007R0702&from=IT (accessed on 1 August 2019).
- European Pharmacopeia. Monograph for Heparin Sodium Heparinum Natricum, 9th ed.; 01/2017:0333; Council of Europe: Strasbourg, France, 2017. [Google Scholar]
- FAO-WHO, CODEX ALIMENTARIUS. Codex Standard for Edible Fats and Oils not Covered by Individual Standards, CODEX STAN 19; Codex Alimentarius Commission; Codex Alimentarius; Fats, Oils and Related Products; Food and Agriculture Organization: Rome, Italy, 1981; revisão 1999. [Google Scholar]
- Wagner, K.H.; Elmadfa, I. Effects of tocopherols and their mixtures on the oxidative stability of olive oil and linseed oil under heating. Eur. J. Lipid Sci. Technol. 2000, 102, 624–629. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Ogata, A.; Hazekawa, M.; Liu, A.X.; Fujioka, M.; Abe, K.; Hasebe, N.; Egashira, N.; et al. Repeated treatment with cannabidiol but not Δ9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology 2007, 52, 1079–1087. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Sielicka, M.; Małecka, M.; Purłan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Kemerli-Kalbaran, T.; Ozdemir, M. Multi-response optimization of oil extraction from pine nut (Pinus pinea L.) by response surface methodology: Extraction efficiency, physicochemical properties and antioxidant activity. LWT 2019, 103, 34–43. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Sabolová, M.; Johanidesová, A.; Hasalíková, E.; Fišnar, J.; Doležal, M.; Réblová, Z. Relationship between the composition of fats and oils and their oxidative stability at different temperatures, determined using the Oxipres apparatus. Eur. J. Lipid Sci. Technol. 2017, 119, 1600454. [Google Scholar] [CrossRef]
- Mandrioli, M.; Tura, M.; Scotti, S.; Gallina Toschi, T. Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L. Molecules 2019, 24, 2113. [Google Scholar] [CrossRef] [PubMed]
- Oils and Fats Experimental Station. NGD Fats and Derivatives Regulations (Norme Grassi e Derivati-NGD), Rules Published in the Oils and Fats Area of the Experimental Stations for Industry SRL; Oils and Fats Experimental Station: Milan, Italy, 1976; NGD Method C35. [Google Scholar]
- Jebe, T.A.; Matlock, M.G.; Sleeter, R.T. Collaborative study of the oil stability index analysis. J. Am. Oil Chem. Soc. 1993, 70, 1055–1061. [Google Scholar] [CrossRef]
- Ninfali, P.; Aluigi, G.; Bacchiocca, M.; Magnani, M. Antioxidant capacity of extra-virgin olive oils. J. Am. Oil Chem. Soc. 2001, 78, 243–247. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT-Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Chang, M.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Effects of interaction between α-tocopherol, oryzanol, and phytosterol on the antiradical activity against DPPH radical. LWT 2019, 112, 108206. [Google Scholar] [CrossRef]
- Szabo, M.R.; Idiţoiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Alma, M.H.; Mavi, A.; Yildirim, A.; Digrak, M.; Hirata, T. Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol. Pharm. Bull. 2003, 26, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Sample | Peroxide Value (meq O2/Kg of Oil) | RSD % | Free Acidity (mg KOH/g of Oil) | RSD% | OSI-Time (Hours) | RSD% |
|---|---|---|---|---|---|---|
| ROO | 1.93 ± 0.09 c | 4.66 | 0.15 ± 0.02 b | 12.86 | 23.58 ± 0.32 a | 1.35 |
| ROO-CBD 0.01% | 2.47 ± 0.04 b | 1.59 | 0.18±0.02 b | 11.70 | 23.33 ± 0.18 a | 0.76 |
| ROO-CBD 0.1% | 2.98 ± 0.14 a | 4.66 | 0.18±0.02 b | 10.88 | 21.90 ± 0.07 b | 0.32 |
| ROO-CBD 0.5% | 2.99 ± 0.14 a | 4.68 | 0.27±0.02 a | 7.30 | 17.28 ± 0.18 c | 1.02 |
| SO | 5.00 ± 0.02 X | 3.00 | 0.11±0.00 X | 0.00 | 4.93 ± 0.04 X | 0.72 |
| SO-CBD 0.01% | 6.30 ± 0.01 Y | 0.13 | 0.08±0.00 Y | 0.00 | 4.80 ± 0.21 Y | 4.42 |
| SO-CBD 0.1% | 6.04 ± 0.29 Y | 4.76 | 0.06±0.00 Z | 0.07 | 4.90 ± 0.00 Y | 0.00 |
| SO-CBD 0.5% | 14.73 ± 0.24 Z | 1.60 | 0.06±0.00 Z | 0.07 | 3.98 ± 0.04 Z | 0.89 |
| Sample | Peroxide Value (meqO2/Kg of Oil) | RSD % | Free Acidity (mg KOH/g of Oil) | RSD % | OSI-Time (Hours) | RSD % |
|---|---|---|---|---|---|---|
| ROO | 0.15 ± 0.00 c | 0.08 | 0.17 ± 0.00 b | 0.07 | 28.35 ± 0.28 a | 1.00 |
| ROO-AT 0.01% | 0.52 ± 0.03 b | 6.65 | 0.17 ± 0.00 b | 0.00 | 25.30 ± 0.42 b | 1.68 |
| ROO-AT 0.1% | 0.52 ± 0.04 b | 6.78 | 0.21 ± 0.02 a | 9.43 | 24.88 ± 0.18 b | 0.71 |
| ROO-AT 0.5% | 0.62 ± 0.04 a | 5.67 | 0.21 ± 0.02 a | 9.43 | 28.23 ± 0.81 a | 2.88 |
| SO | 12.31 ± 0.50 X | 4.03 | 0.20 ± 0.00 X | 0.07 | 4.15 ± 0.07 x | 1.70 |
| SO-AT 0.01% | 13.50 ± 0.09 X,Y | 0.64 | 0.21 ± 0.02 X | 9.15 | 4.20 ± 0.07 x | 1.68 |
| SO-AT 0.1% | 13.43 ± 0.70 X,Y | 5.18 | 0.17 ± 0.00 Y | 0.14 | 5.28 ± 0.04 y | 0.67 |
| SO-AT 0.5% | 15.08 ± 1.16 Y | 7.68 | 0.17 ± 0.00 Y | 0.00 | 6.28 ± 0.11 z | 1.69 |
| Sample | Concentration of Free Radicals after 20 min (µM) | RSD % | Concentration of Free Radicals after 240 min (µM) | RSD % |
|---|---|---|---|---|
| ROO | 5.70 ± 0.25 b | 4.47 | 76.94 ± 9.04 a | 11.26 |
| ROO-CBD 0.01% | 10.39 ± 0.57 a | 5.44 | 83.85 ± 5.45 a | 6.50 |
| ROO-CBD 0.1% | 9.27 ± 0.31 a | 3.36 | 70.98 ± 5.62 a | 7.92 |
| ROO-CBD 0.5% | 6.50 ± 0.25 b | 3.81 | 72.25 ± 4.13 a | 5.72 |
| SO | 8.35 ± 0.22 X | 2.66 | 17.91 ± 0.95 X | 5.29 |
| SO-CBD 0.01% | 9.87 ± 0.00 Y | 0.00 | 20.82 ± 0.53 X | 2.57 |
| SO-CBD 0.1% | 9.00 ± 0.53 X,Y | 5.86 | 25.37 ± 2.27 Y | 8.96 |
| SO-CBD 0.5% | 5.41 ± 0.24 Z | 4.51 | 16.84 ± 0.25 Z | 1.47 |
| Sample | Concentration of Free Radicals after 20 min (µM) | RSD % | Concentration of Free Rad icals after 240 min (µM) | RSD % |
|---|---|---|---|---|
| ROO | 3.10 ± 0.15 a | 4.72 | 83.33 ± 4.56 a | 5.48 |
| ROO-AT 0.01% | 3.19 ± 0.03 a | 0.89 | 81.22 ± 6.04 a | 7.43 |
| ROO-AT 0.1% | 1.27 ± 0.14 a | 11.14 | 61.41 ± 5.82 b | 9.48 |
| ROO-AT 0.5% | 0.55 ± 0.03 b | 5.81 | 11.23 ± 0.28c | 2.52 |
| SO | 5.90 ± 0.27 X | 4.55 | 19.21 ± 1.39 X | 7.22 |
| SO-AT 0.01% | 6.66 ± 0.23 Y | 3.43 | 22.61 ± 0.23 Y | 1.00 |
| SO-AT 0.1% | 3.35 ± 0.28 Z | 8.24 | 13.25 ± 0.74 Z | 5.59 |
| SO-AT 0.5% | 2.08 ± 0.20 W | 9.47 | 6.90 ± 0.53 W | 7.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tura, M.; Mandrioli, M.; Gallina Toschi, T. Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils. Molecules 2019, 24, 3485. https://doi.org/10.3390/molecules24193485
Tura M, Mandrioli M, Gallina Toschi T. Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils. Molecules. 2019; 24(19):3485. https://doi.org/10.3390/molecules24193485
Chicago/Turabian StyleTura, Matilde, Mara Mandrioli, and Tullia Gallina Toschi. 2019. "Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils" Molecules 24, no. 19: 3485. https://doi.org/10.3390/molecules24193485
APA StyleTura, M., Mandrioli, M., & Gallina Toschi, T. (2019). Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils. Molecules, 24(19), 3485. https://doi.org/10.3390/molecules24193485