Cannabinoid Receptor Interacting Protein 1a (CRIP1a): Function and Structure
Abstract
1. Introduction
2. Functional Modulation of CB1 Cellular Signaling by CRIP1a
2.1. G Protein Selectivity and Effect on Signal Transduction Via cAMP Inhibition and ERK1/2 Phosphorylation
2.2. β-Arrestins and Internalization of the CB1 Receptor
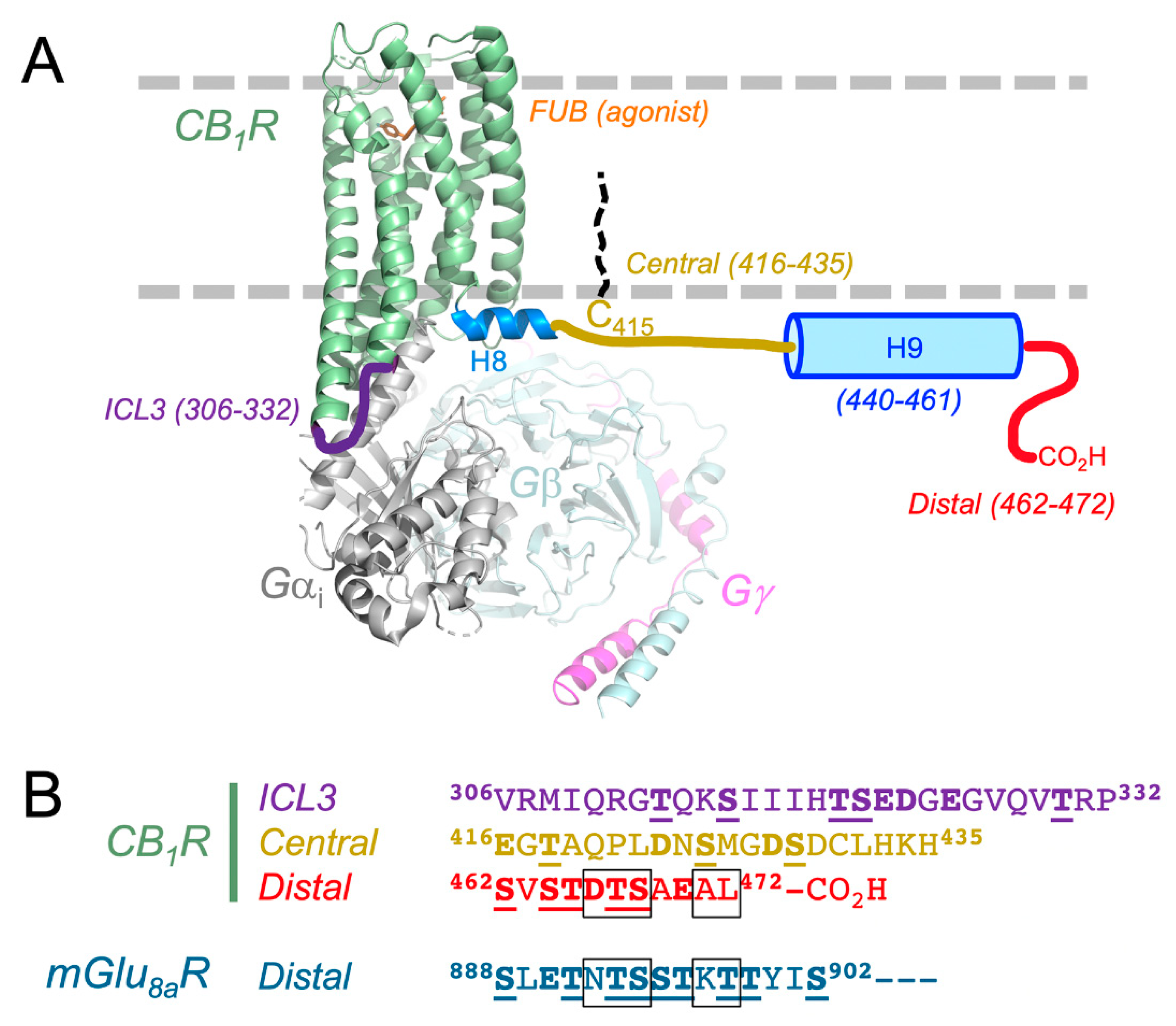
3. Structure of CB1 Receptor and CRIP1a Interactions
3.1. CB1 Receptor Structure: What is Known About How CRIP1a Interacts?
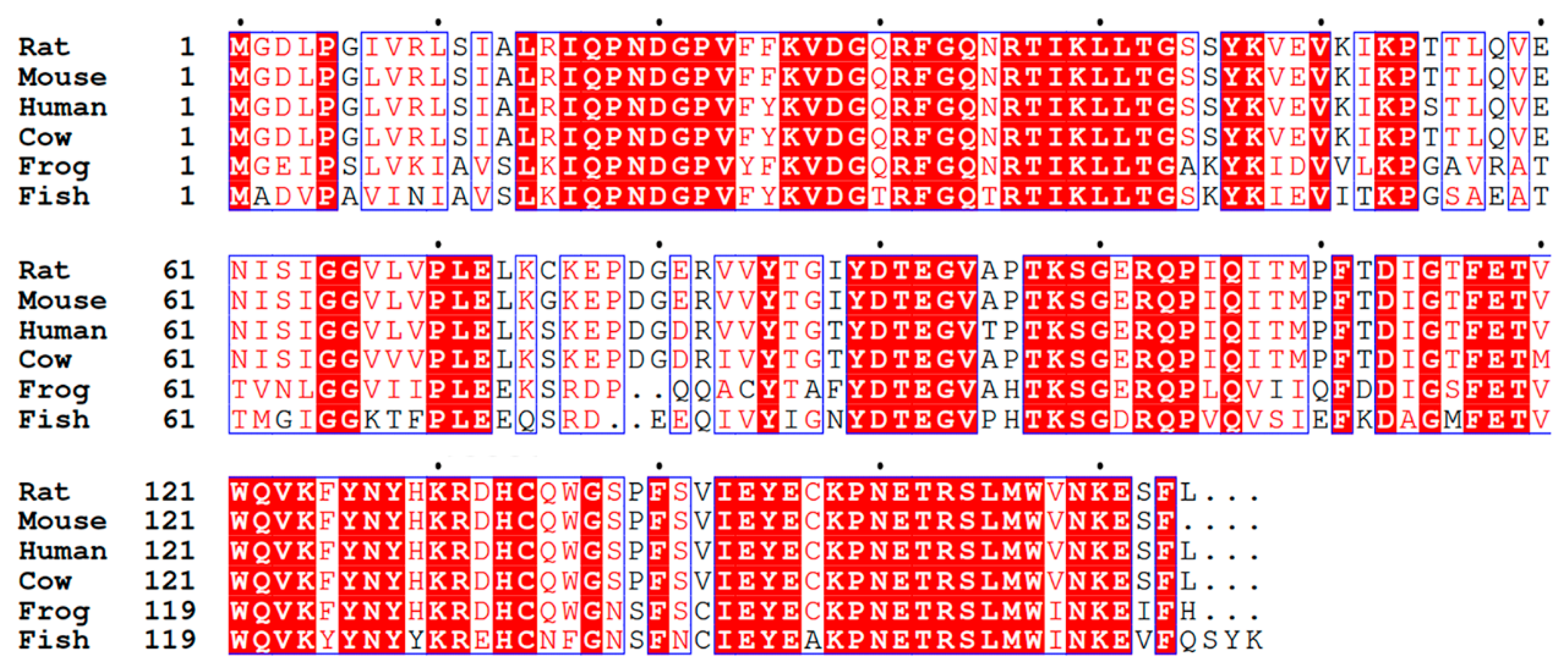
3.2. Predicted Structure of CRIP1a
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ligresti, A.; De, P.L.; Di Marzo, V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharm. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; Starowicz, K.; Maione, S.; Di Marzo, V. Allodynia lowering induced by cannabinoids and endocannabinoids (ALICE). Pharm. Res. 2017, 119, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Bradshaw, H.B. Endocannabinoid analytical methodologies: Techniques that drive discoveries that drive techniques. Adv. Pharm. 2017, 80, 1–30. [Google Scholar]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 receptor pharmacology. Adv. Pharm. 2017, 80, 169–206. [Google Scholar]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB1 cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef]
- Smith, T.H.; Sim-Selley, L.J.; Selley, D.E. Cannabinoid CB1 receptor-interacting proteins: Novel targets for central nervous system drug discovery? Br. J. Pharm. 2010, 160, 454–466. [Google Scholar] [CrossRef]
- Nie, J.; Lewis, D.L. The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience 2001, 107, 161–167. [Google Scholar] [CrossRef]
- Nie, J.; Lewis, D.L. Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J. Neurosci. 2001, 21, 8758–8764. [Google Scholar] [CrossRef]
- Niehaus, J.L.; Liu, Y.; Wallis, K.T.; Egertova, M.; Bhartur, S.G.; Mukhopadhyay, S.; Shi, S.; He, H.; Selley, D.E.; Howlett, A.C.; et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol. Pharm. 2007, 72, 1557–1566. [Google Scholar] [CrossRef]
- Ludanyi, A.; Eross, L.; Czirjak, S.; Vajda, J.; Halasz, P.; Watanabe, M.; Palkovits, M.; Magloczky, Z.; Freund, T.F.; Katona, I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008, 28, 2976–2990. [Google Scholar] [CrossRef] [PubMed]
- Monory, K.; Massa, F.; Egertova, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Bojnik, E.; Turunc, E.; Armagan, G.; Kanit, L.; Benyhe, S.; Yalcin, A.; Borsodi, A. Changes in the cannabinoid (CB1) receptor expression level and G-protein activation in kainic acid induced seizures. Epilepsy Res. 2012, 99, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Guggenhuber, S.; Alpar, A.; Chen, R.; Schmitz, N.; Wickert, M.; Mattheus, T.; Harasta, A.E.; Purrio, M.; Kaiser, N.; Elphick, M.R.; et al. Cannabinoid receptor-interacting protein Crip1a modulates CB1 receptor signaling in mouse hippocampus. Brain Struct. Funct. 2016, 221, 2061–2074. [Google Scholar] [CrossRef]
- Perez, S.M.; Donegan, J.J.; Boley, A.M.; Aguilar, D.D.; Giuffrida, A.; Lodge, D.J. Ventral hippocampal overexpression of cannabinoid receptor interacting protein 1 (CNRIP1) produces a schizophrenia-like phenotype in the rat. Schizophr. Res. 2019, 206, 263–270. [Google Scholar] [CrossRef]
- Xie, J.; Gizatullin, R.; Vukojevic, V.; Leopardi, R. The CCDC55 couples cannabinoid receptor CNR1 to a putative DISC1 schizophrenia pathway. Neuroscience 2015, 310, 723–730. [Google Scholar] [CrossRef]
- Blume, L.C.; Bass, C.E.; Childers, S.R.; Dalton, G.D.; Roberts, D.C.; Richardson, J.M.; Xiao, R.; Selley, D.E.; Howlett, A.C. Striatal CB1 and D2 receptors regulate expression of each other, CRIP1A and delta opioid systems. J. Neurochem. 2013, 124, 808–820. [Google Scholar] [CrossRef]
- Blume, L.C.; Eldeeb, K.; Bass, C.E.; Selley, D.E.; Howlett, A.C. Cannabinoid receptor interacting protein (CRIP1a) attenuates CB1R signaling in neuronal cells. Cell Signal. 2015, 27, 716–726. [Google Scholar] [CrossRef]
- Blume, L.C.; Leone-Kabler, S.; Luessen, D.J.; Marrs, G.S.; Lyons, E.; Bass, C.E.; Chen, R.; Selley, D.E.; Howlett, A.C. Cannabinoid receptor interacting protein suppresses agonist-driven CB1 receptor internalization and regulates receptor replenishment in an agonist-biased manner. J. Neurochem. 2016, 139, 396–407. [Google Scholar] [CrossRef]
- Blume, L.C.; Patten, T.; Eldeeb, K.; Leone-Kabler, S.; Ilyasov, A.A.; Keegan, B.M.; O’Neal, J.E.; Bass, C.E.; Hantgan, R.R.; Lowther, W.T.; et al. Cannabinoid receptor interacting protein 1a competition with beta-arrestin for CB1 receptor binding sites. Mol. Pharm. 2017, 91, 75–86. [Google Scholar] [CrossRef]
- Smith, T.H.; Blume, L.C.; Straiker, A.; Cox, J.O.; David, B.G.; McVoy, J.R.; Sayers, K.W.; Poklis, J.L.; Abdullah, R.A.; Egertova, M.; et al. Cannabinoid receptor-interacting protein 1a modulates CB1 receptor signaling and regulation. Mol. Pharm. 2015, 87, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.D.; Howlett, A.C. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br. J. Pharm. 2012, 165, 2497–2511. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.B.; Howlett, A.C. Solubilization of the cannabinoid receptor from rat brain and its functional interaction with guanine nucleotide-binding proteins. Mol. Pharm. 1993, 43, 17–22. [Google Scholar]
- Houston, D.B.; Howlett, A.C. Differential receptor-G-protein coupling evoked by dissimilar cannabinoid receptor agonists. Cell Signal. 1998, 10, 667–674. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; McIntosh, H.H.; Houston, D.B.; Howlett, A.C. The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol. Pharm. 2000, 57, 162–170. [Google Scholar]
- Mukhopadhyay, S.; Howlett, A.C. CB1 receptor-G protein association. Subtype selectivity is determined by distinct intracellular domains. Eur. J. Biochem. 2001, 268, 499–505. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Cowsik, S.M.; Lynn, A.M.; Welsh, W.J.; Howlett, A.C. Regulation of Gi by the CB1 cannabinoid receptor C-terminal juxtamembrane region: Structural requirements determined by peptide analysis. Biochemistry 1999, 38, 3447–3455. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Shim, J.Y.; Assi, A.A.; Norford, D.; Howlett, A.C. CB(1) cannabinoid receptor-G protein association: A possible mechanism for differential signaling. Chem. Phys. Lipids 2002, 121, 91–109. [Google Scholar] [CrossRef]
- Eldeeb, K.; Leone-Kabler, S.; Blume, L.C.; Howlett, A.C. CB1 Receptor intracellular loop4 mutation modulates G protein activation and cAMP production in human neuroblastoma cells. Int. Cannabinoid Res. Soc. Symp. Cannabinoids 2014, 24, 35. [Google Scholar]
- Mukhopadhyay, S.; Howlett, A.C. Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol. Pharm. 2005, 67, 2016–2024. [Google Scholar] [CrossRef]
- Eldeeb, K.; Leone-Kabler, S.; Howlett, A.C. CB1 cannabinoid receptor-mediated increases in cyclic AMP accumulation are correlated with reduced Gi/o function. J. Basic Clin. Physiol. Pharm. 2016, 27, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Latorraca, N.R.; Kato, H.E.; Hilger, D.; Maeda, S.; et al. Structure of a signaling cannabinoid receptor 1-G protein complex. Cell 2019, 176, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Pellegrini, M.; Tsomaia, N.; Yatawara, A.K.; Kendall, D.A.; Mierke, D.F. Structural analysis of the human cannabinoid receptor one carboxyl-terminus identifies two amphipathic helices. Biopolymers 2009, 91, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Dainese, E.; Sandiford, S.; Fezza, F.; Lanuti, M.; Chiurchiu, V.; Totaro, A.; Catanzaro, G.; Barcaroli, D.; De, L.V.; et al. Effects of palmitoylation of Cys(415) in helix 8 of the CB(1) cannabinoid receptor on membrane localization and signalling. Br. J. Pharm. 2012, 165, 2635–2651. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Totaro, A.; Scipioni, L.; Dufrusine, B.; Stepniewski, T.M.; Selent, J.; Maccarrone, M.; Dainese, E. Role of palmitoylation of cysteine 415 in functional coupling CB1 receptor to Galphai2 protein. Biotechnol. Appl. Biochem. 2018, 65, 16–20. [Google Scholar] [CrossRef]
- Al-Zoubi, R.; Morales, P.; Reggio, P.H. Structural insights into CB1 receptor biased signaling. Int. J. Mol. Sci. 2019, 20, 1837. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal structure of the human cannabinoid receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef]
- Nobles, K.N.; Xiao, K.; Ahn, S.; Shukla, A.K.; Lam, C.M.; Rajagopal, S.; Strachan, R.T.; Huang, T.Y.; Bressler, E.A.; Hara, M.R.; et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci. Signal. 2011, 4, ra51. [Google Scholar] [CrossRef]
- Gyombolai, P.; Boros, E.; Hunyady, L.; Turu, G. Differential beta-arrestin2 requirements for constitutive and agonist-induced internalization of the CB1 cannabinoid receptor. Mol. Cell Endocrinol. 2013, 372, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Schmid, C.L.; Raehal, K.M.; Selley, D.E.; Bohn, L.M.; Sim-Selley, L.J. beta-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol. Psychiatry 2012, 71, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Vigano, D.; Premoli, F.; Castiglioni, C.; Bianchessi, S.; Zippel, R.; Parolaro, D. Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: A role for RAS-ERK cascade. Mol. Neurobiol. 2006, 33, 199–213. [Google Scholar] [CrossRef]
- Breivogel, C.S.; Lambert, J.M.; Gerfin, S.; Huffman, J.W.; Razdan, R.K. Sensitivity to delta9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2 -/- mice. Behav. Pharm. 2008, 19, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Davis, B.J.; Kearn, C.S.; Marcus, D.; Cook, A.J.; Wager-Miller, J.; Straiker, A.; Myoga, M.H.; Karduck, J.; Leishman, E.; et al. Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J. Neurosci. 2014, 34, 5152–5163. [Google Scholar] [CrossRef]
- Jin, W.; Brown, S.; Roche, J.P.; Hsieh, C.; Celver, J.P.; Kovoor, A.; Chavkin, C.; Mackie, K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999, 19, 3773–3780. [Google Scholar] [CrossRef]
- Hsieh, C.; Brown, S.; Derleth, C.; Mackie, K. Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem. 1999, 73, 493–501. [Google Scholar] [CrossRef]
- Daigle, T.L.; Kwok, M.L.; Mackie, K. Regulation of CB1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J. Neurochem. 2008, 106, 70–82. [Google Scholar] [CrossRef]
- Daigle, T.L.; Kearn, C.S.; Mackie, K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 2008, 54, 36–44. [Google Scholar] [CrossRef]
- Bakshi, K.; Mercier, R.W.; Pavlopoulos, S. Interaction of a fragment of the cannabinoid CB1 receptor C-terminus with arrestin-2. Febs Lett. 2007, 581, 5009–5016. [Google Scholar] [CrossRef]
- Singh, S.N.; Bakshi, K.; Mercier, R.W.; Makriyannis, A.; Pavlopoulos, S. Binding between a distal C-terminus fragment of cannabinoid receptor 1 and arrestin-2. Biochemistry 2011, 50, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, M.; Kelley, B.; Shen, M.; Thayer, S.A. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Mol. Pharm. 2002, 61, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Mascia, F.; Klotz, L.; Lerch, J.; Ahmed, M.H.; Zhang, Y.; Enz, R. CRIP1a inhibits endocytosis of G-protein coupled receptors activated by endocannabinoids and glutamate by a common molecular mechanism. J. Neurochem. 2017, 141, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Kellogg, G.E.; Selley, D.E.; Safo, M.K.; Zhang, Y. Predicting the molecular interactions of CRIP1a-cannabinoid 1 receptor with integrated molecular modeling approaches. Bioorg. Med. Chem. Lett. 2014, 24, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ganjiwale, A.; Howlett, A.C.; Cowsik, S.M. In silico interaction analysis of cannabinoid receptor interacting protein 1b (CRIP1b) - CB1 cannabinoid receptor. J. Mol. Graph Model 2017, 77, 311–321. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Booth, W.T.; Walker, N.B.; Lowther, W.T.; Howlett, A.C. Cannabinoid Receptor Interacting Protein 1a (CRIP1a): Function and Structure. Molecules 2019, 24, 3672. https://doi.org/10.3390/molecules24203672
Booth WT, Walker NB, Lowther WT, Howlett AC. Cannabinoid Receptor Interacting Protein 1a (CRIP1a): Function and Structure. Molecules. 2019; 24(20):3672. https://doi.org/10.3390/molecules24203672
Chicago/Turabian StyleBooth, William T., Noah B. Walker, W. Todd Lowther, and Allyn C. Howlett. 2019. "Cannabinoid Receptor Interacting Protein 1a (CRIP1a): Function and Structure" Molecules 24, no. 20: 3672. https://doi.org/10.3390/molecules24203672
APA StyleBooth, W. T., Walker, N. B., Lowther, W. T., & Howlett, A. C. (2019). Cannabinoid Receptor Interacting Protein 1a (CRIP1a): Function and Structure. Molecules, 24(20), 3672. https://doi.org/10.3390/molecules24203672





