Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-O Results for Truffles
2.2. Quantitative Analysis and OAV of Volatile Compounds
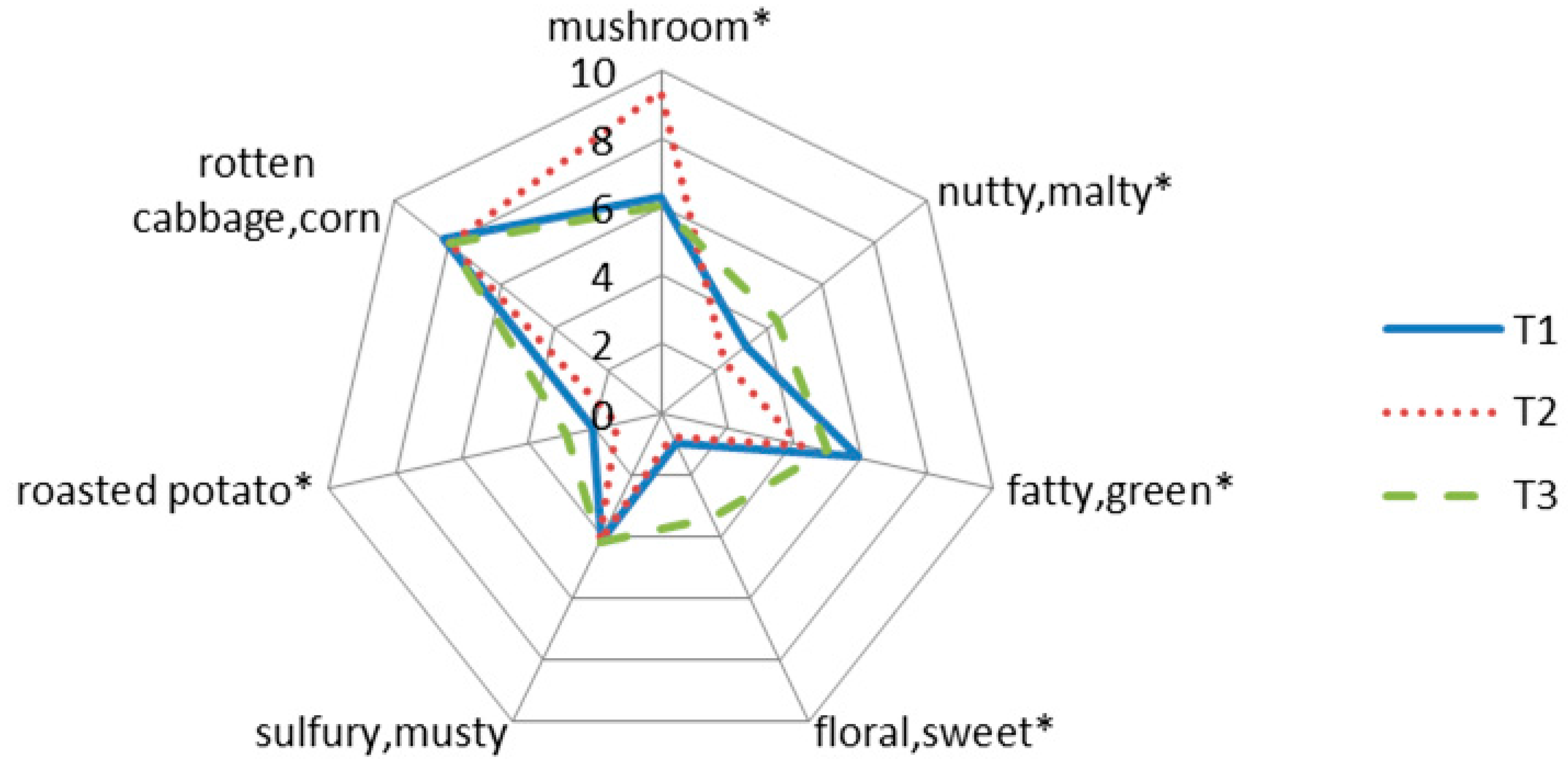
2.3. Sensory Analysis
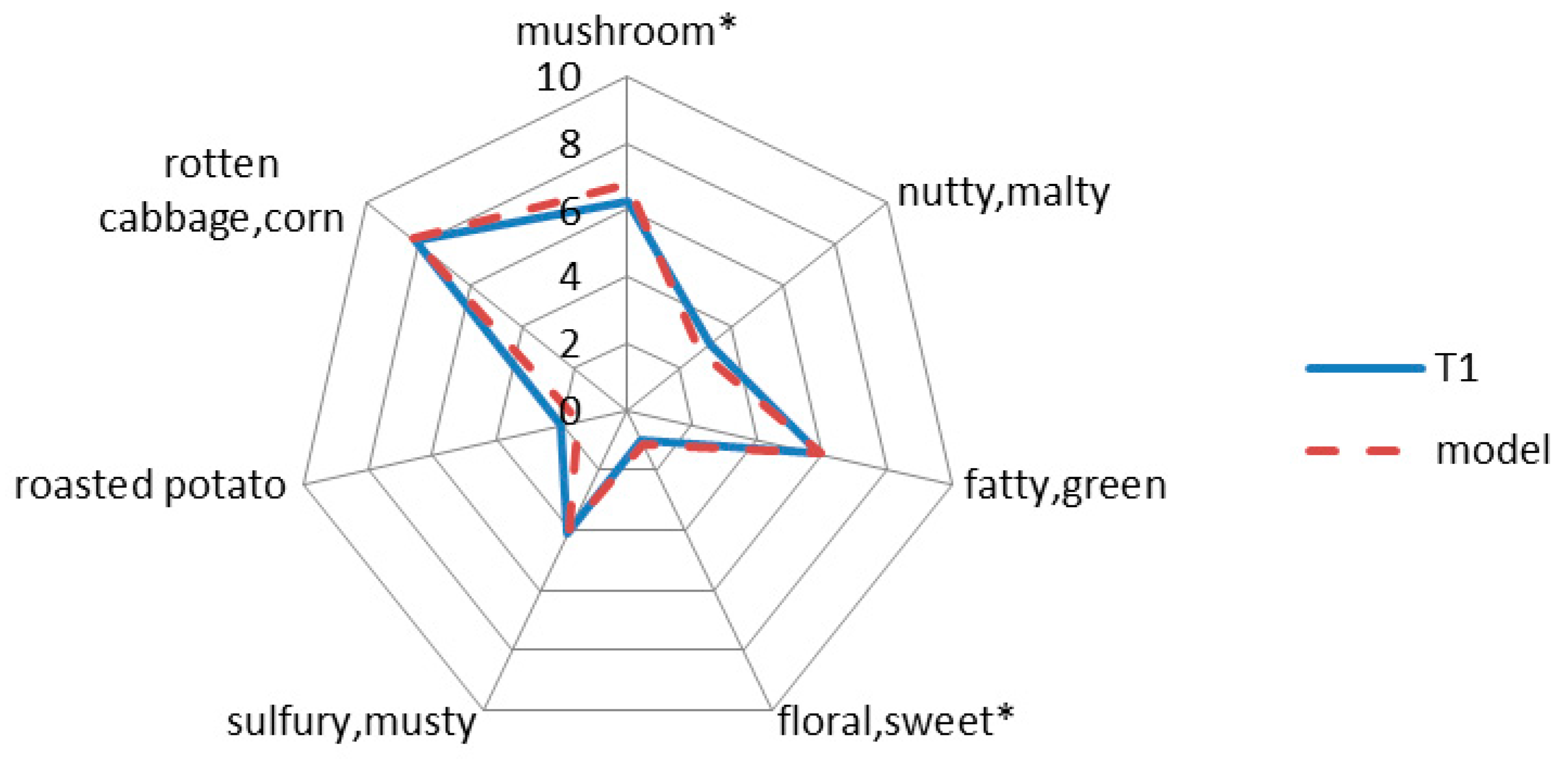
2.4. Aroma Recombination
3. Materials and Methods
3.1. Chemicals
3.2. Materials
3.3. Solid Phase Microextraction (SPME) Absorption of Aroma Compounds
3.4. SPME-GC-FID-O Analysis of Truffle
3.5. Calibration of Standard Curves
3.6. SPME-GC–MS of Volatile Compounds in Truffle
3.7. SPME-GC-FPD Detection of Sulfur Containing Volatile Compounds in Truffle
3.8. Odor Activity Value (OAV)
3.9. Sensory Evaluation
3.10. Aroma Recombination of Truffle
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle volatiles: from chemical ecology to aroma biosynthesis. New Phytol. 2011, 189, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Schmidberger, P.; Schieberle, P. Characterization Of The Key Aroma Compounds in White Alba Truffle (Tuber magnatum pico) and Burgundy Truffle (Tuber uncinatum) By Means Of The Sensomics Approach. J. Agric. Food Chem. 2017, 65, 9287–9296. [Google Scholar] [CrossRef] [PubMed]
- Molinier, V.; Murat, C.; Frochot, H.; Wipf, D.; Splivallo, R. Fine-scale spatial genetic structure analysis of the black truffle, Tuber aestivum, and its link to aroma variability. Environ. Microbiol. 2015, 17, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Al-Rawi, S.S.; Ibrahim, A.H.; Majid, A.S.A.; Majid, A.M.S.A. Antioxidant, anticancer, apoptosis properties and chemical composition of black truffle Terfezia claveryi. Saudi J. Biol. Sci. 2016, 25, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Savini, S.; Loizzo, M.R.; Tundis, R.; Mozzon, M.; Foligni, R.; Longo, E.; Morozova, K.; Scampicchio, M.; Martinvertedor, D.; Boselli, E. Fresh refrigerated Tuber melanosporum truffle: effect of the storage conditions on the antioxidant profile, antioxidant activity and volatile profile. Eur. Food Res. Technol. 2017, 243, 2255–2263. [Google Scholar] [CrossRef]
- Splivallo, R.; Culleré, L. The Smell of Truffles: From Aroma Biosynthesis to Product Quality. In True Truffle (Tuber spp.) in the World; Springer: Cham, Switzerland, 2016; Volume 47, pp. 393–407. [Google Scholar]
- Liu, R.; Jin, G.; Xiao, D.; Li, H.; Bai, F.; Tang, Y. Screening of the key volatile organic compounds of Tuber melanosporum fermentation by aroma sensory evaluation combination with principle component analysis. Sci. Rep. 2015, 5, 17954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, W.; Xu, Y. Comparison on aroma compounds in Chinese soy sauce and strong aroma type liquors by gas chromatography–olfactometry, chemical quantitative and odor activity values analysis. Eur. Food Res. Technol. 2014, 239, 813–825. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Harris, J. Odor intensities of hop oil components. J. Sci. Food Agric. 2010, 17, 142–144. [Google Scholar] [CrossRef]
- Fiecchi, A.; Kienle, M.G.; Scala, A.; Cabella, P. Bis-methylthiomethane, an odorous substance from white truffle, tuber magnatum pico. Tetrahedron Lett. 1967, 8, 1681–1682. [Google Scholar] [CrossRef]
- Talou, T.; Delmas, M.; Gaset, A. Direct Capture of Volatiles Emitted From Entire Black Perigord Truffle. J. Essential Oil Res. 1989, 1, 281–286. [Google Scholar] [CrossRef]
- Liu, T.; Feng, T.; Chen, W. Identification of volatile flavor components of Tuber melanosporum using simultaneous distillation-extraction. Czech J. Food Sci. 2017, 35, 483–487. [Google Scholar]
- Pacioni, G.; Cerretani, L.; Procida, G.; Cichelli, A. Composition of commercial truffle flavored oils with GC–MS analysis and discrimination with an electronic nose. Food Chem. 2014, 146, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Li, D.C.; Li, H.M.; Tang, Y. Evaluation of aroma active compounds in Tuber fruiting bodies by gas chromatography–olfactometry in combination with aroma reconstitution and omission test. Appl. Microbiol. Biot. 2012, 94, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Sun, B.; Mao, X.; Zhang, Y.; Zhou, Y. Comparative Analysis of Volatile Composition in Chinese Truffles via GC Ã-GC/HR-TOF/MS and Electronic Nose. Int. J. Mol. Sci. 2016, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- Martín, D.A.; Osorio, C.; Sinuco, D.C. Flavoromics approach to differentiate three edible Tacsonia (Passifloraceae) fruit species. Eur. Food Res. Tech. 2017, 244, 695–703. [Google Scholar]
- Gracka, A.; Jeleń, H.H.; Majcher, M.; Siger, A.; Kaczmarek, A. Flavoromics approach in monitoring changes in volatile compounds of virgin rapeseed oil caused by seed roasting. J. Chromatogr. A 2016, 1428, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Andujar-Ortiz, I.; Peppard, T.L.; Reineccius, G. Flavoromics for Determining Markers of Cooked and Fermented Flavor in Strawberry Juices. In The Chemical Sensory Informatics of Food: Measurement, Analysis, Integration; American Chemical Society: Washington, DC, USA, 2015; Volume 1191, pp. 293–312. [Google Scholar]
- Ronningen, I.G.; Peterson, D.G. Application of Untargeted LC/MS Techniques (Flavoromics) To Identify Changes Related to Freshness of Food. In The Chemical Sensory Informatics of Food: Measurement, Analysis, Integration; American Chemical Society: Washington, DC, USA, 2015; Volume 1191, pp. 269–277. [Google Scholar]
- NIST Chemistry WebBook Home Page. Available online: https://webbook.nist.gov/chemistry/# (accessed on 4 March 2019).
- Xiao, Z.; Ma, S.; Niu, Y.; Chen, F.; Yu, D. Characterization of odour-active compounds of sweet orange essential oils of different regions by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. Flavour Fragr. J. 2016, 31, 41–50. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the key aroma volatile compounds in cranberry (Vaccinium macrocarpon Ait.) using gas chromatography–olfactometry (GC-O) and odor activity value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, Z. Characterization of the major odor-active compounds in dry jujube cultivars by application of gas chromatography–olfactometry and odor activity value. J. Agric. Food Chem. 2018, 66, 7722–7734. [Google Scholar] [CrossRef] [PubMed]
- Pelusio, F.; Nilsson, T.; Montanarella, L.; Tilio, R.; Larsen, B.; Facchetti, S.; Madsen, J. Headspace solid-phase microextraction analysis of volatile organic sulfur compounds in black and white truffle aroma. J. Agric. Food Chem. 1995, 43, 2138–2143. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; De Grazia, S.; Grasso, E.; Trozzi, A. Headspace-solid-phase microextraction-gas chromatography as analytical methodology for the determination of volatiles in wild mushrooms and evaluation of modifications occurring during storage. J. Anal. Methods Chem. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Pu, B.; Chen, A.; Ao, X.; Xu, D. A Box-Behnken design for characterizing Chinese truffles (Tuber indicum) aroma by HS-SPME-GC-MS. J. Food Res. 2012, 1, 219. [Google Scholar] [CrossRef][Green Version]
- Kamle, M.; Bar, E.; Lewinsohn, D.; Shavit, E.; Roth-Bejerano, N.; Kagan-Zur, V.; Barak, Z.E.; Guy, O.; Zaady, E.; Lewinsohn, E.; et al. Characterization of morphology, volatile profiles, and molecular markers in edible desert truffles from the Negev Desert. J. Agric. Food Chem. 2017, 65, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.I. Compilation of odour threshold values in air and water: Edited by L. J. van Gemert and A. H. Nettenbreijer, National Institute for Water Supply, Voorburg, The Netherlands, 1977. Price: DFl. 22.00. Sci. Total Environ. 1978, 9, 300–301. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredient, 6th ed.; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Paterson, R. Book Review: J.S. Tkacz and L. Lange, eds. Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine, Kluwer Academic/Plenum Publishers, New York, NY, 445 pp, $175. Mycopathologia 2005, 159, 473–474. [Google Scholar] [CrossRef]
- Bellesia, F.; Pinetti, A.; Bianchi, A.; Tirillini, B. Volatile Compounds of the White Truffle (Tuber magnatum Pico) from Middle Italy. Flavour Fragr. J. 1996, 11, 239–243. [Google Scholar] [CrossRef]
- Talou, T.; Gaset, A.; Delmas, M.; Kulifaj, M.; Montant, C. Dimethyl sulphide: the secret for black truffle hunting by animals. Mycol. Res. 1990, 94, 277–278. [Google Scholar] [CrossRef]
- Díaz, P.; Señoráns, F.J.; Reglero, G.; Ibañez, E. Truffle aroma analysis by headspace solid phase microextraction. J. Agric. Food Chem. 2002, 50, 6468–6472. [Google Scholar]
- Talou, T.; Delmas, M.; Gaset, A. Principal constituents of black truffle (Tuber melanosporum) aroma. J. Agric. Food Chem. 1987, 35, 774–777. [Google Scholar] [CrossRef]
- Cullere, L.; Ferreira, V.; Marco, P.; Venturini, M.E.; Blanco, D. Does the host tree exert any influence on the aromatic composition of the black truffle (Tuber melanosporum). Flavour Frag. 2017, 32, 133–140. [Google Scholar] [CrossRef]
- Splivallo, R.; Deveau, A.; Valdez, N.; Kirchhoff, N.; Freyklett, P.; Karlovsky, P. Bacteria associated with truffle-fruiting bodies contribute to truffle aroma. Environ. Microbiol. 2015, 17, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Cullere, L.; Ferreira, V.; Chevret, B.; Venturini, M.E.; Sanchezgimeno, A.C.; Blanco, D. Characterisation of aroma(Tuber melanosporum) active compounds in black truffles and summer truffles (Tuber aestivum) by gas chromatography-olfactometry. Food Chem. 2010, 122, 300–306. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Cristinzio, M. High-Field Nuclear Magnetic Resonance (NMR) Study of Truffles (Tuber aestivum vittadini). J. Agric. Food Chem. 2004, 52, 7988–7996. [Google Scholar]
- Gao, J.-M.; Zhu, W.-M.; Zhang, S.-Q.; Zhang, X.; Zhang, A.-L.; Chen, H.; Sun, Y.-Y.; Tang, M. Sphingolipids from the edible fungus Tuber indicum. J. Lipid Sci. Tech. 2010, 106, 815–821. [Google Scholar]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
Sample Availability: 2-methylbutanal, 3-methylbutanal, pentanal, isopropyl alcohol, 1-propanol, 1-butanol, hexanal, 2-methyl-1-propanol, (E)-2-methyl-2-butenal, limonene, heptenal, 2-butenal, 2-methylbutanol, 3-methylbutanol, 2-pentyl-furan, 3-octanone, 1-pentanol, 2-octanone, 4-isopropyltoluene, octanal, 1-octen-3-one, isobutyl hexanoate, nonanal, heptanoic acid ethyl ester, 1-hexanol, 3-octanol, octanoic acid ethyl ester, (E)-2-octenal, (E)-2-nonenal, P-cresyl methyl ether, 1-heptanol, 1-octen-3-ol, 1-octanol, benzaldehyde, 2-methyl-butanoic acid, 3-methyl-butanoic acid, 2-acetylthiazole, benzeneacetaldehyde, benzyl alcohol, phenylethyl alcohol, γ-nonalactone, Methanethiol, dimethyl sulfide, dimethyl disulfide, bis(methylthio)methane, 3-(methylthio)propanal, dimethyl sulfoxide, dipropyl trisulfide, 3-(methylthio)propanol, dimethyl sulfone, bis(2-methyl-3-furyl)disulfide, these samples are available from the authors. |
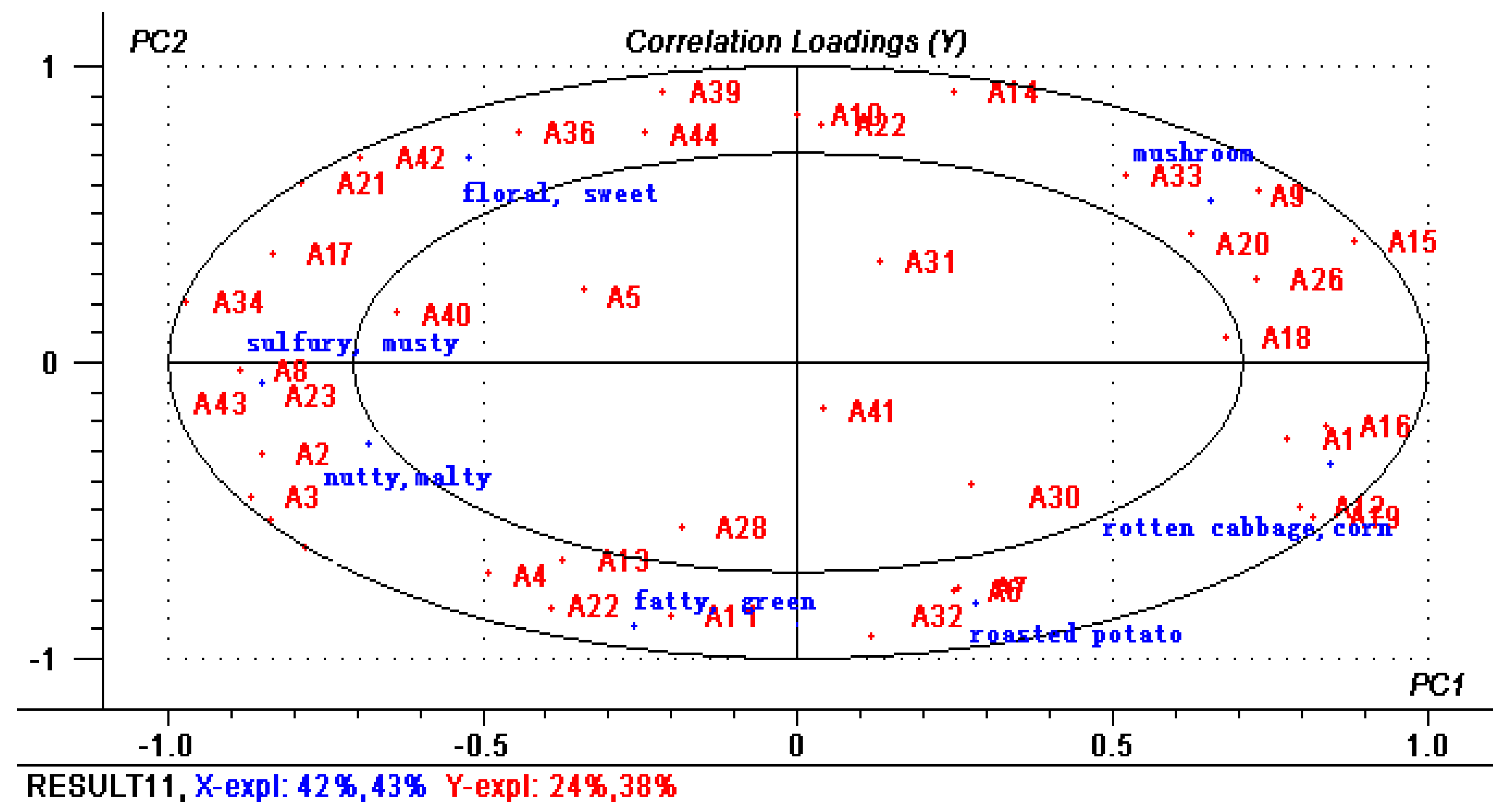
| No. | Compound A | RI (Calculate) | RI (Reference) B | Aroma | Identification D | Aroma Intensity | Frequency F | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DB-5 | HP-Innowax | DB-5 | HP-Innowax | Description | T1 | RSD (%) | T2 | RSD (%) | T3 | RSD (%) | T1 | T2 | T3 | |||
| A1 | Dimethyl sulfide | 515 | 721 | 515 | 716 | sulfuric, garlic, cabbage-like | AD, RI, Std | 8.7a E | 6.4 | 8.6a | 5.3 | 8.3a | 6.8 | 10 | 10 | 10 |
| A2 | 2-Methylbutanal | 641 | 923 | 640 | 925 | cocoa, almond-like | AD, RI, Std | 8.3a | 6.8 | 7.7b | 5.2 | 8.5a | 5.1 | 10 | 10 | 10 |
| A3 | 3-Methylbutanal | 650 | 913 | 650 | 910 | green, nutty, cocoa-like | AD, RI, Std | 7.6ab | 5.6 | 6.6b | 4.9 | 8.8a | 3.4 | 10 | 10 | 10 |
| A4 | Pentanal | 715 | 935 | 732 | 935 | vegetable, green | AD, RI, Std | 5.5a | 7.1 | 5.8a | 6.5 | 5.2a | 6.7 | 8 | 9 | 8 |
| A5 | Hexanal | 802 | 1084 | 801 | 1084 | grass, leafy, fruity, sweaty | AD, RI, Std | 5.2ab | 6.8 | 6.8a | 4.9 | 4.6b | 5.9 | 10 | 10 | 9 |
| A6 | Dimethyl disulfide | 785 | 1086 | 785 | 1086 | sulfuric, cabbage, onion-like | AD, RI, Std | 8.5a | 5.9 | 7.3b | 5.1 | 6.9b | 4.2 | 10 | 10 | 10 |
| A7 | 2-Methylpropanol | 608 | 1094 | 609 | 1098 | winey | AD, RI, Std | 2.2 | 15.2 | — | — | 1.8 | 27.9 | 8 | 0 | 7 |
| A8 | (E)-2-Methyl-2-butenal | 743 | 1089 | 739 | 1088 | fruity, green, almond, nutty | AD, RI, Std | 3.1b | 5.9 | 3.3b | 7.2 | 4.9a | 6.2 | 8 | 8 | 8 |
| A9 | Limonene | 1031 | 1192 | 1031 | 1198 | citrus, orange, fresh, sweet | AD, RI, Std | 4.8a | 6.7 | 3.5b | 7.3 | 3.2b | 5.8 | 9 | 9 | 9 |
| A10 | (E)-2-Heptenal | 960 | 1334 | 961 | 1336 | fresh, aldehydic, fatty, green | AD, RI, Std | 7.8a | 5.3 | 4.5b | 6.6 | 7.2a | 5.4 | 10 | 10 | 10 |
| A11 | (E)-2-Butenal | 646 | 1050 | 644 | 1047 | green, vegetable | AD, RI, Std | — | — | — | — | 3.3 | 8.1 | 0 | 0 | 8 |
| A12 | 2-Methylbutanol | 742 | 1206 | 742 | 1208 | malty | AD, RI, Std | 3.8b | 7.9 | 4.3b | 8.3 | 8.1a | 5.8 | 10 | 10 | 10 |
| A13 | 3-Methylbutanol | 736 | 1209 | 732 | 1209 | roasted, winey, onion-like | AD, RI, Std | 4.1b | 5.8 | 4.6ab | 5.2 | 4.8a | 6.9 | 9 | 8 | 7 |
| A14 | 2-Pentyl-furan | 994 | 1239 | 994 | 1235 | fruity, green, earthy | AD, RI, Std | 5.6a | 6.7 | 4.5b | 6.3 | 4.1b | 7.2 | 10 | 10 | 10 |
| A15 | 3-Octanone | 984 | 1243 | 984 | 1240 | herbal, lavender, mushroom | AD, RI, Std | 7.6b | 6.9 | 8.9a | 5.9 | 7.7b | 6.1 | 10 | 10 | 10 |
| A16 | 1-Pentanol | 763 | 1262 | 763 | 1256 | fusel | AD, RI, Std | 2.2a | 8.1 | 1.2b | 28.8 | 1.1b | 25.6 | 8 | 6 | 6 |
| A17 | Bis(methylthio) methane | 898 | 1280 | 896 | 1282 | sulfuric, garlic | AD, RI, Std | 8.3a | 5.3 | 8.4a | 4.1 | 7.1b | 9.6 | 10 | 10 | 10 |
| A18 | 2-Octanone | 965 | 1244 | 965 | 1244 | earthy, herbal | AD, RI, Std | 7.1a | 5.8 | 7.5a | 5.7 | 7.3a | 4.5 | 10 | 10 | 10 |
| A19 | Octanal | 1006 | 1282 | 1006 | 1280 | waxy, orange, peel | AD, RI, Std | 8.4a | 5.9 | 4.8c | 6.3 | 6.2b | 8.9 | 10 | 9 | 10 |
| A20 | 1-Octen-3-one | 975 | 1305 | 975 | 1305 | mushroom, earthy, musty | AD, RI, Std | 8.3a | 6.2 | 8.8a | 7.5 | 6.1b | 7.4 | 10 | 10 | 10 |
| A21 | Isobutyl hexanoate | 1143 | 1344 | 1145 | 1356 | fruity, pineapple, green | AD, RI, Std | 5.4 | 6.3 | — | — | — | — | 9 | 0 | 0 |
| A22 | Nonanal | 1104 | 1358 | 1104 | 1358 | waxy, aldehydic, fatty | AD, RI, Std | 6.5 | 7.7 | — | — | 5.8 | 6.5 | 10 | 0 | 9 |
| A23 | Heptanoic acid ethyl ester | 1089 | 1329 | 1093 | 1329 | fruity, pineapple, banana, strawberry | AD, RI, Std | 3.5a | 5.1 | 3.3ab | 8.1 | 2.8b | 15.3 | 8 | 7 | 8 |
| A24 | 1-Hexanol | 864 | 1364 | 851 | 1360 | alcoholic, pungent, green | AD, RI, Std | 1.9a | 5.8 | 1.8a | 26.5 | 2.2a | 17.4 | 9 | 9 | 9 |
| A25 | Unknown 1 | — C | 1396 | — | — | pungent | AD | 1.2 | 18.2 | — | — | 2.9 | 17.9 | 7 | 0 | 8 |
| A26 | 3-Octanol | 991 | 1386 | 995 | 1386 | earthy, mushroom, herbal | AD, RI, Std | 7.6b | 5.8 | 8.6a | 6.2 | 7.3b | 9.6 | 10 | 10 | 10 |
| A27 | Octanoic acid ethyl ester | 1196 | 1442 | 1196 | 1446 | fruity, creamy, mushroom | AD, RI, Std | — | — | 5.9 | 6.7 | — | — | 0 | 8 | 0 |
| A28 | (E)-2-Octenal | 1057 | 1432 | 1057 | 1434 | green, citrus, peel, fatty | AD, RI, Std | — | — | 5.2 | 7.2 | 6.1 | 3.2 | 0 | 9 | 10 |
| A29 | 2-Nonenal | 1161 | 1536 | 1161 | 1537 | green, cucumber, fatty | AD, RI, Std | 4.6a | 5.6 | 4.9a | 5.9 | 3.8b | 15.5 | 9 | 8 | 9 |
| A30 | Heptanol | 972 | 1457 | 972 | 1457 | musty, sweet, woody | AD, RI, Std | 2.1 | 17.5 | 1.9 | 25.2 | — | — | 8 | 8 | 0 |
| A31 | 1-Octen-3-ol | 982 | 1426 | 982 | 1426 | mushroom, earthy | AD, RI, Std | 7.8b | 6.3 | 9.2a | 6.4 | 8.1b | 9.6 | 10 | 10 | 10 |
| A32 | 3-(Methylthio)propanal | 907 | 1456 | 907 | 1456 | musty, potato, onion, beefy, | AD, RI, Std | 7.9a | 5.9 | 7.7a | 5.9 | 8.4a | 8.9 | 10 | 10 | 10 |
| A33 | 1-Octanol | 1068 | 1564 | 1068 | 1564 | waxy, green, citrus | AD, RI, Std | 3.8a | 7.9 | 3.7a | 8.5 | 3.3a | 6.4 | 8 | 8 | 6 |
| A34 | Dimethyl sulfoxide | 825 | 1560 | 820 | 1560 | cheesy, garlic, mushroom | AD, RI, Std | — | — | — | — | 5.5 | 9.6 | 0 | 0 | 10 |
| A35 | Unknown 2 | — | 1598 | — | — | smoky | AD | 3.9 | 6.7 | 4.8 | 7.6 | — | — | 8 | 9 | 0 |
| A36 | Benzaldehyde | 963 | 1528 | 963 | 1528 | sweet, bitter, almond, cherry | AD, RI, Std | 5.3a | 6.5 | 2.6b | 14.8 | 5.8a | 6.2 | 9 | 10 | 9 |
| A37 | 2-Methylbutanoic acid | 858 | 1650 | 858 | 1652 | acid, fatty | AD, RI, Std | 4.5a | 5.6 | 2.7b | 17.3 | 2.1b | 18.2 | 8 | 7 | 7 |
| A38 | 3-Methylbutanoic acid | 846 | 1684 | 875 | 1686 | pungent, acid, cheese | AD, RI, Std | 3.8ab | 8.4 | 3.2b | 6.3 | 4.2a | 6.9 | 9 | 8 | 9 |
| A39 | Benzeneacetaldehyde | 1051 | 1646 | 1051 | 1646 | honey, sweet, floral | AD, RI, Std | 5.2b | 6.9 | 4.8b | 6.5 | 8.3a | 4.8 | 10 | 10 | 10 |
| A40 | 3-(Methylthio)propanol | 998 | 1706 | 998 | 1706 | sulfuric, onion, garlic | AD, RI, Std | 5.8b | 7.8 | 6.2a | 7.8 | 6.5a | 7.4 | 9 | 8 | 9 |
| A41 | Unknown 3 | — | 1831 | — | — | sulfuric | AD | 4.8 | 6.2 | — | — | 3.7 | 7.9 | 8 | 0 | 8 |
| A42 | Benzyl alcohol | 1038 | 1890 | 1035 | 1886 | floral, rose, balsamic | AD, RI, Std | — | — | 2.1 | 14.9 | — | — | 0 | 8 | 0 |
| A43 | Dimethyl sulfone | 913 | 1912 | 925 | 1912 | sulfuric | AD, RI, Std | — | — | 7.3 | 5.6 | — | — | 0 | 10 | 0 |
| A44 | Phenylethyl alcohol | 1110 | 1923 | 1113 | 1923 | floral, rose | AD, RI, Std | 3.3c | 8.4 | 4.5b | 6.7 | 6.7a | 6.1 | 7 | 6 | 9 |
| No. | Compounds A | Identification B | RI (calculate) | RI (reference) C | Concentration (μg kg−1) | Threshold G | OAV H | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DB-5 | HP-Innowax | DB-5 | HP-Innowax | T1 | RSD (%) | T2 | RSD (%) | T3 | RSD (%) | (μg kg−1) | T1 | T2 | T3 | |||
| 1 | Methanethiol | FPD,RI,Std | 464 | 696 | 464 | 690 | 7.52 | 1.84 | 4.65 | 2.68 | — | — | 4 | 2 | 1 | — |
| 2 | Dimethyl sulfide | FPD,RI,Std | 515 | 721 | 515 | 716 | 1260a D | 87.12 | 1156a | 66.29 | 1089a | 59.73 | 0.3 | 4200 | 3853 | 3630 |
| 3 | 2-Methylbutanal | MS,RI,Std | 641 | 923 | 640 | 925 | 335ab | 35.45 | 189c | 17.4 | 580a | 43.28 | 12.5 | 27 | 15 | 46 |
| 4 | 3-Methylbutanal | MS,RI,Std | 650 | 913 | 650 | 910 | 2781b | 44.92 | 135c | 1.58 | 4573a | 72.38 | 9 | 309 | 15 | 508 |
| 5 | Pentanal | MS,RI,Std | 715 | 935 | 732 | 935 | 19.25a | 2.79 | 16.38a | 1.74 | 9.81b | 2.073 | 22 | <1 | <1 | <1 |
| 6 | Isopropyl alcohol | MS,RI,Std | 510 | 884 | 510 | 884 | 7.8 | 1.63 | — E | — | — | — | 6400 | <1 | — | — |
| 7 | 1-Propanol | MS,RI,Std | 536 | 1037 | 536 | 1037 | 137ab | 11.42 | 116b | 13.26 | 266a | 23.48 | 200 | <1 | <1 | <1 |
| 8 | 1-Butanol | MS,RI,Std | 654 | 1142 | 654 | 1150 | 4.38a | 1.56 | 2.54a | 2.19 | 9.47a | 2.73 | 130 | <1 | <1 | <1 |
| 9 | Hexanal | MS,RI,Std | 802 | 1084 | 802 | 1084 | 47.28a | 9.72 | 53.63a | 8.89 | 33.56b | 7.23 | 9 | 5 | 6 | 4 |
| 10 | Dimethyl disulfide | FPD,RI,Std | 785 | 1086 | 785 | 1086 | 1139a | 48.9 | 56.32b | 4.83 | 23.94b | 2.04 | 7 | 163 | 8 | 3 |
| 11 | 2-Methyl-1-propanol | MS,RI,Std | 628 | 1098 | 628 | 1094 | 489 | 49.13 | — | — | 1.59 | 3.048 | 640 | <1 | — | <1 |
| 12 | (E)-2-Methyl-2-butenal | MS,RI,Std | 743 | 1088 | 745 | 1088 | 19.32b | 1.98 | 21.21b | 1.89 | 231a | 29.9 | 380 | <1 | <1 | <1 |
| 13 | Limonene | MS,RI,Std | 1031 | 1192 | 1031 | 1198 | 45.79a | 5.34 | 3.61b | 1.34 | 1.98b | 2.16 | 5.9 | 8 | 1 | <1 |
| 14 | (E)-2-Heptenal | MS,RI,Std | 960 | 1334 | 961 | 1336 | 271a | 25.27 | 2.84c | 1.19 | 108b | 9.94 | 550 | <1 | <1 | <1 |
| 15 | 2-Butenal | MS,RI,Std | 646 | 1050 | 644 | 1047 | — | — | — | — | 118 | 10.03 | 420 | — | — | <1 |
| 16 | 2-Methylbutanol | MS,RI,Std | 742 | 1206 | 742 | 1208 | 734c | 49.8 | 1123b | 32.3 | 3879a | 54.73 | 140 | 5 | 8 | 28 |
| 17 | 3-Methylbutanol | MS,RI,Std | 736 | 1209 | 732 | 1209 | 15.12b | 1.23 | 26.54ab | 2.89 | 38.95a | 3.44 | 250 | <1 | <1 | <1 |
| 18 | 2-Pentylfuran | MS,RI,Std | 994 | 1239 | 994 | 1235 | 68.41a | 7.99 | 10.34b | 2.88 | 7.96b | 2.66 | 270 | <1 | <1 | <1 |
| 19 | 3-Octanone | MS,RI,Std | 984 | 1243 | 984 | 1240 | 863b | 21.36 | 6300a | 78.29 | 950b | 33.73 | 1.3 | 672 | 4846 | 731 |
| 20 | 1-Pentanol | MS,RI,Std | 763 | 1262 | 763 | 1256 | 7.02a | 1.82 | 6.84a | 2.47 | 5.41a | 2.37 | 4000 | <1 | <1 | <1 |
| 21 | Bis(methylthio) methane | FPD,RI,Std | 898 | 1280 | 896 | 1282 | 5.76ab | 1.63 | 7.89a | 2.51 | 3.28b | 3.29 | 0.012 | 480 | 658 | 273 |
| 22 | 2-Octanone | MS,RI,Std | 965 | 1244 | 965 | 1244 | 6.73a | 1.49 | 11.92a | 1.38 | 9.64ab | 3.79 | 50 | <1 | <1 | <1 |
| 23 | p-cymene | MS,RI,Std | 1027 | 1295 | 1027 | 1295 | 2.73a | 9.85 | 4.81a | 2.39 | 0.18b | 2.62 | 4 | <1 | 1 | <1 |
| 24 | Octanal | MS,RI,Std | 1006 | 1282 | 1006 | 1280 | 873a | 19.1 | 42.9c | 4.52 | 436b | 33.29 | 0.7 | 1247 | 61 | 623 |
| 25 | 1-Octen-3-one | MS,RI,Std | 975 | 1305 | 975 | 1305 | 719b | 31.48 | 1034a | 85.5 | 81.73c | 7.48 | 0.8 | 899 | 1293 | 102 |
| 26 | Isobutyl hexanoate | MS,RI,Std | 1143 | 1356 | 1145 | 1356 | 2.14 | 1.19 | — | — | — | — | 3 | <1 | — | — |
| 27 | Nonanal | MS,RI,Std | 1104 | 1358 | 1104 | 1358 | 65.38 | 7.36 | — | — | 38.75 | 3.16 | 40 | 2 | — | <1 |
| 28 | Heptanoic acid ethyl ester | MS,RI,Std | 1089 | 1329 | 1093 | 1329 | 11.59ab | 1.2 | 13.92a | 1.22 | 7.83b | 3.62 | 39 | <1 | <1 | <1 |
| 29 | 1-Hexanol | MS,RI,Std | 854 | 1364 | 851 | 1360 | 3.89ab | 1.41 | 1.29b | 1.23 | 4.38a | 2.39 | 100 | <1 | <1 | <1 |
| 30 | 3-Octanol | MS,RI,Std | 991 | 1386 | 995 | 1386 | 322.76b | 20.19 | 2157a | 49.8 | 248.19b | 21.29 | 22 | 15 | 98 | 11 |
| 31 | Octanoic acid ethyl ester | MS,RI,Std | 1196 | 1442 | 1196 | 1446 | — | — | 17.53 | 1.44 | — | — | 22 | — | <1 | — |
| 32 | (E)-2-Octenal | MS,RI,Std | 1057 | 1432 | 1057 | 1434 | — | — | 7.68 | 1.63 | 2.19 | 2.19 | 3 | — | 3 | <1 |
| 33 | (E)- 2-Nonenal | MS,RI,Std | 1161 | 1536 | 1161 | 1537 | 14.72ab | 1.76 | 23.59a | 2.48 | 8.76b | 3.83 | 0.4 | 37 | 59 | 22 |
| 34 | P-cresyl methyl ether | MS,RI,Std | 1018 | 1445 | 1018 | 1445 | 870.84 | 20.73 | — | — | — | — | 560 | 2 | — | — |
| 35 | 1-Heptanol | MS,RI,Std | 972 | 1457 | 972 | 1457 | 3.54a | 1.66 | 1.47a | 1.25 | 3.25a | 2.26 | 200 | <1 | <1 | <1 |
| 36 | 1-Octen-3-ol | MS,RI,Std | 982 | 1426 | 982 | 1426 | 437b | 42.8 | 5849a | 57.69 | 566b | 44.32 | 1 | 437 | 5849 | 566 |
| 37 | 3-(Methylthio)propanal | FPD,RI,Std | 907 | 1456 | 907 | 1456 | 2.89ab | 1.098 | 1.28a | 1.42 | 4.93a | 1.097 | 0.1 | 29 | 13 | 49 |
| 38 | 1-Octanol | MS,RI,Std | 1068 | 1564 | 1068 | 1564 | 5.73a | 1.48 | 3.66ab | 1.38 | 2.14b | 1.19 | 37 | <1 | <1 | <1 |
| 39 | Dimethyl sulfoxide | FPD,RI,Std | 825 | 1560 | 820 | 1560 | — | — | — | — | 5.93 | 2.42 | — | — | — | — |
| 40 | Benzaldehyde | MS,RI,Std | 963 | 1528 | 963 | 1528 | 6.82a | 3.53 | 0.97b | 1.069 | 8.95a | 2.74 | 320 | <1 | <1 | <1 |
| 41 | 2-Methylbutanoic acid | MS,RI,Std | 858 | 1650 | 858 | 1652 | 15.13ab | 2.39 | 16.85a | 1.77 | 11.72b | 2.98 | 20 | <1 | <1 | <1 |
| 42 | 3-Methylbutanoic acid | MS,RI,Std | 846 | 1684 | 875 | 1686 | 8.87ab | 2.65 | 9.45a | 2.82 | 6.32b | 3.51 | 1 | 9 | 9 | 6 |
| 43 | 2-Acetylthiazole | FPD,RI,Std | 1015 | 1652 | 1015 | 1652 | 5.84a | 2.47 | 4.71a | 3.58 | 3.43a | 1.27 | 4 | 1 | 1 | <1 |
| 44 | Benzeneacetaldehyde | MS,RI,Std | 1051 | 1646 | 1051 | 1646 | 26.77b | 2.74 | 23.94b | 3.3 | 403a | 45.39 | 0.7 | 38 | 34 | 576 |
| 45 | Dipropyl trisulfide | FPD,RI,Std | 1326 | 1659 | 1326 | 1659 | 7.52a | 1.88 | 6.18a | 2.67 | 5.57a | 2.45 | 4.3 | 2 | 1 | 1 |
| 46 | 3-(Methylthio)propanol | FPD,RI,Std | 998 | 1706 | 998 | 1706 | 18.19ab | 2.56 | 11.84b | 1.25 | 23.19a | 1.79 | 500 | <1 | <1 | <1 |
| 47 | Benzyl alcohol | MS,RI,Std | 1038 | 1890 | 1035 | 1886 | — | — | 0.97 | 0.088 | — | — | 100 | — | <1 | — |
| 48 | Dimethyl sulfone | FPD,RI,Std | 923 | 1912 | 925 | 1912 | — | — | 5.88 | 1.43 | — | — | — | — | — | — |
| 49 | Phenylethyl alcohol | MS,RI,Std | 1110 | 1923 | 1113 | 1923 | 2.25c | 1.29 | 21.67b | 2.35 | 211a | 37.89 | 80 | <1 | <1 | 3 |
| 50 | gamma-nonalactone | MS,RI,Std | 1370 | 2012 | 1370 | 2012 | 11.32a | 1.43 | 9.54ab | 2.93 | 8.73b | 2.63 | 25 | <1 | <1 | <1 |
| 51 | [Bis(2-methyl-3-furyl)disulfide] | FPD,RI,Std | 1425 | 2156 | 1425 | 2156 | — | — | — | — | 1.81 | 2.15 | 0.014 | — | — | 129 |
| No | Compound | Standard Curve | r2 | Validation Range (μg kg−1) |
|---|---|---|---|---|
| 1 | Methanethiol | y = 0.065x − 0.0037 | 0.986 | 1–10 |
| 2 | Dimethyl trisulfide | y = 1.7x + 0.0373 | 0.973 | 500–5000 |
| 3 | 2-Methylbutanal | y = 4.53x − 0.00591 | 0.981 | 50–500 |
| 4 | 3-Methylbutanal | y = 0.84x + 0.109 | 0.971 | 10–5000 |
| 5 | Pentanal | y = 1.27x + 0.054 | 0.982 | 1–20 |
| 6 | Isopropyl alcohol | y = 2.13x + 0.0016 | 0.993 | 1–10 |
| 7 | 1-Propanol | y = 3.39x − 0.0303 | 0.942 | 50–500 |
| 8 | 1-Butanol | y = 1.16x − 0.0239 | 0.996 | 1–10 |
| 9 | Hexanal | y = 0.70x − 0.0531 | 0.971 | 10–100 |
| 10 | Dimethyl disulfide | y = 4.53x − 0.591 | 0.968 | 10–2000 |
| 11 | 2-Methylpropanol | y = 0.99x − 0.0477 | 0.987 | 1–500 |
| 12 | (E)-2-Methyl-2-butenal | y = 1.51x + 0.019 | 0.979 | 10–500 |
| 13 | Limonene | y = 3.40x − 0.041 | 0.988 | 1–50 |
| 14 | Heptenal | y = 1.56x − 0.0193 | 0.984 | 1–500 |
| 15 | 2-Butenal | y = 4.73x − 0.025 | 0.983 | 5–200 |
| 16 | 2-Methylbutanol | y = 0.91x + 0.031 | 0.986 | 500–5000 |
| 17 | 3-Methylbutanol | y = 1.05x + 0.076 | 0.985 | 5–50 |
| 18 | 2-Pentylfuran | y = 0.80x + 0.0492 | 0.971 | 5–100 |
| 19 | 3-Octanone | y = 0.38x + 0.0852 | 0.997 | 500–10000 |
| 20 | 1-Pentanol | y = 0.88x − 0.017 | 0.992 | 1–10 |
| 21 | Bis(methylthio) mathane | y = 0.9x + 0.145 | 0.977 | 2–20 |
| 22 | 2-Octanone | y = 0.26 − 0.0138 | 0.993 | 2–20 |
| 23 | 4-Isopropyltoluene | y = 0.1277x + 0.00985 | 0.987 | 0.1–5 |
| 24 | Octanal | y = 1.654x − 0.0235 | 0.973 | 20–1000 |
| 25 | 1-Octen-3-one | y = 1.977x + 0.0713 | 0.986 | 50–2000 |
| 26 | Isobutyl hexanoate | y = 1.488x − 0.0790 | 0.982 | 0.1–5 |
| 27 | Nonanal | y = 1.61x − 0.0233 | 0.985 | 10–100 |
| 28 | Heptanoic acid ethyl ester | y = 2.87x − 0.0188 | 0.976 | 1–20 |
| 29 | 1-Hexanol | y = 1.70 − 0.0918 | 0.992 | 1–10 |
| 30 | 3-Octanol | y = 0.93x + 0.029 | 0.969 | 200–5000 |
| 31 | Octanoic acid ethyl ester | y = 1.07 + 0.068 | 0.983 | 5–50 |
| 32 | (E)-2-Octenal | y = 1.50x − 0.0218 | 0.979 | 1–10 |
| 33 | (E)-2-Nonenal | y = 2.59x − 0.0376 | 0.986 | 2–50 |
| 34 | P-cresyl methyl ether | y = 0.79 − 0.0034 | 0.967 | 20–200 |
| 35 | 1-Heptanol | y = 1.49x − 0.00208 | 0.983 | 1–10 |
| 36 | 1-Octen-3-ol | y = 2.13x − 0.0289 | 0.995 | 200–10000 |
| 37 | 3-(Methylthio)propanal | y = 2.48x + 0.0102 | 0.992 | 0.5–5 |
| 38 | 1-Octanol | y = 3.51x − 0.0472 | 0.992 | 1–10 |
| 39 | Dimethyl sulfoxide | y = 0.30x − 0.0446 | 0.989 | 1–10 |
| 40 | Benzaldehyde | y = 0.40x − 0.0595 | 0.976 | 0.5–10 |
| 41 | 2-Methylbutanoic acid | y = 0.78x − 0.0421 | 0.971 | 2–20 |
| 42 | 3-Methylbutanoic acid | y = 0.89x − 0.0086 | 0.975 | 1–10 |
| 43 | 2-Acetylthiazole | y = 1.25x + 0.41 | 0.965 | 1–10 |
| 44 | Benzeneacetaldehyde | y = 1.77x − 0.0142 | 0.982 | 20–500 |
| 45 | Dipropyl trisulfide | y = 2.40x − 0.0320 | 0.982 | 2–10 |
| 46 | 3-(Methylthio)propanol | y = 2.072x − 0.0282 | 0.979 | 2–20 |
| 47 | Benzyl alcohol | y = 2.47x + 0.0079 | 0.987 | 0.05–1 |
| 48 | Dimethyl sulfone | y = 1.97x + 0.031 | 0.981 | 1–10 |
| 49 | Phenylethyl alcohol | y = 4.95x − 0.0356 | 0.973 | 2–500 |
| 50 | Γ-Nonalactone | y = 0.78x + 0.0512 | 0.988 | 2–20 |
| 51 | [Bis(2-methyl-3-furyl) disulfide] | y = 2.97x + 0.0053 | 0.976 | 0.2–2 |
| Sensory Attributes | Mean Score | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| mushroom | 6.27b A | 9.39a | 6.03b |
| nutty, malty | 3.14b | 2.37c | 4.37a |
| fatty, green | 5.89a | 4.19b | 4.98ab |
| floral, sweet | 0.98b | 0.76b | 3.39a |
| sulfury, musty | 4.12a | 4.23a | 4.18a |
| roasted potato | 2.07ab | 1.34b | 2.89a |
| rotten cabbage, corn | 8.13a | 7.96a | 7.89a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Shui, M.; Song, S.; Zhuang, H.; Sun, M.; Yao, L. Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach. Molecules 2019, 24, 3305. https://doi.org/10.3390/molecules24183305
Feng T, Shui M, Song S, Zhuang H, Sun M, Yao L. Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach. Molecules. 2019; 24(18):3305. https://doi.org/10.3390/molecules24183305
Chicago/Turabian StyleFeng, Tao, Mengzhu Shui, Shiqing Song, Haining Zhuang, Min Sun, and Lingyun Yao. 2019. "Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach" Molecules 24, no. 18: 3305. https://doi.org/10.3390/molecules24183305
APA StyleFeng, T., Shui, M., Song, S., Zhuang, H., Sun, M., & Yao, L. (2019). Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach. Molecules, 24(18), 3305. https://doi.org/10.3390/molecules24183305







