Complementary Untargeted and Targeted Metabolomics for Differentiation of Extra Virgin Olive Oils of Different Origin of Purchase Based on Volatile and Phenolic Composition and Sensory Quality
Abstract
1. Introduction
2. Results and Discussion
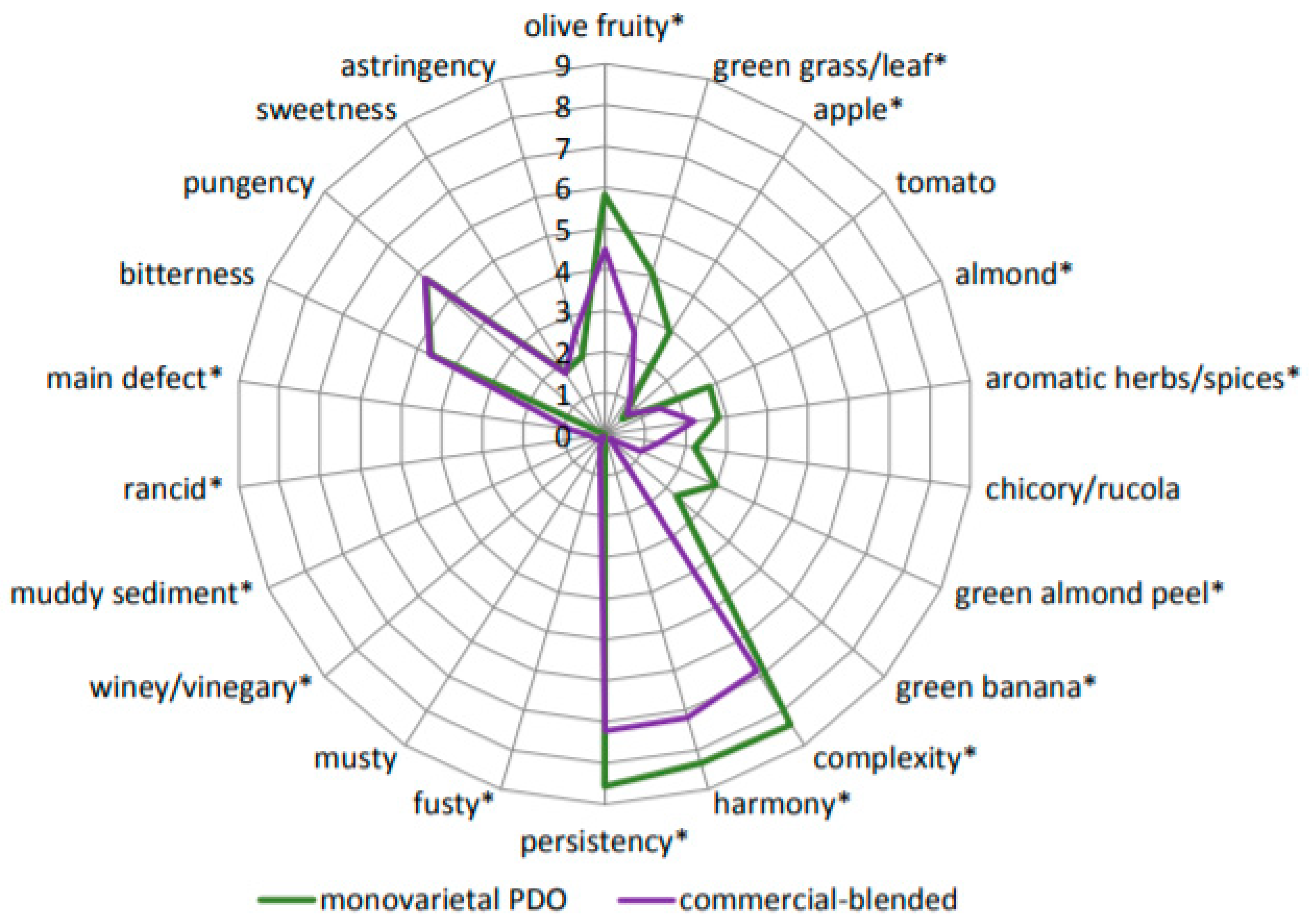
2.1. Sensory Attributes
2.2. Volatile Compounds (VOCs) and Sensory Attributes
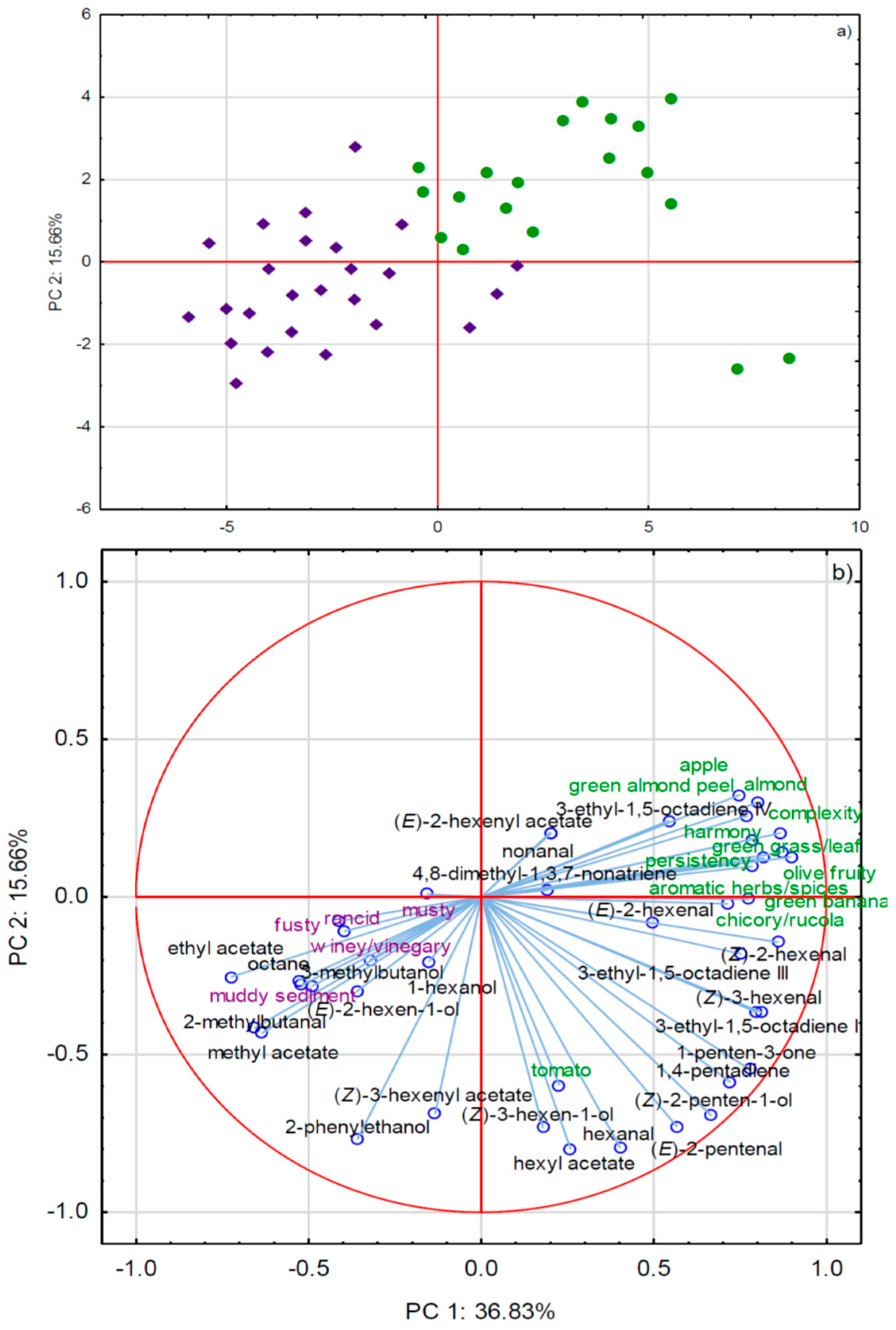
2.2.1. GC-MS and Sensory Attributes
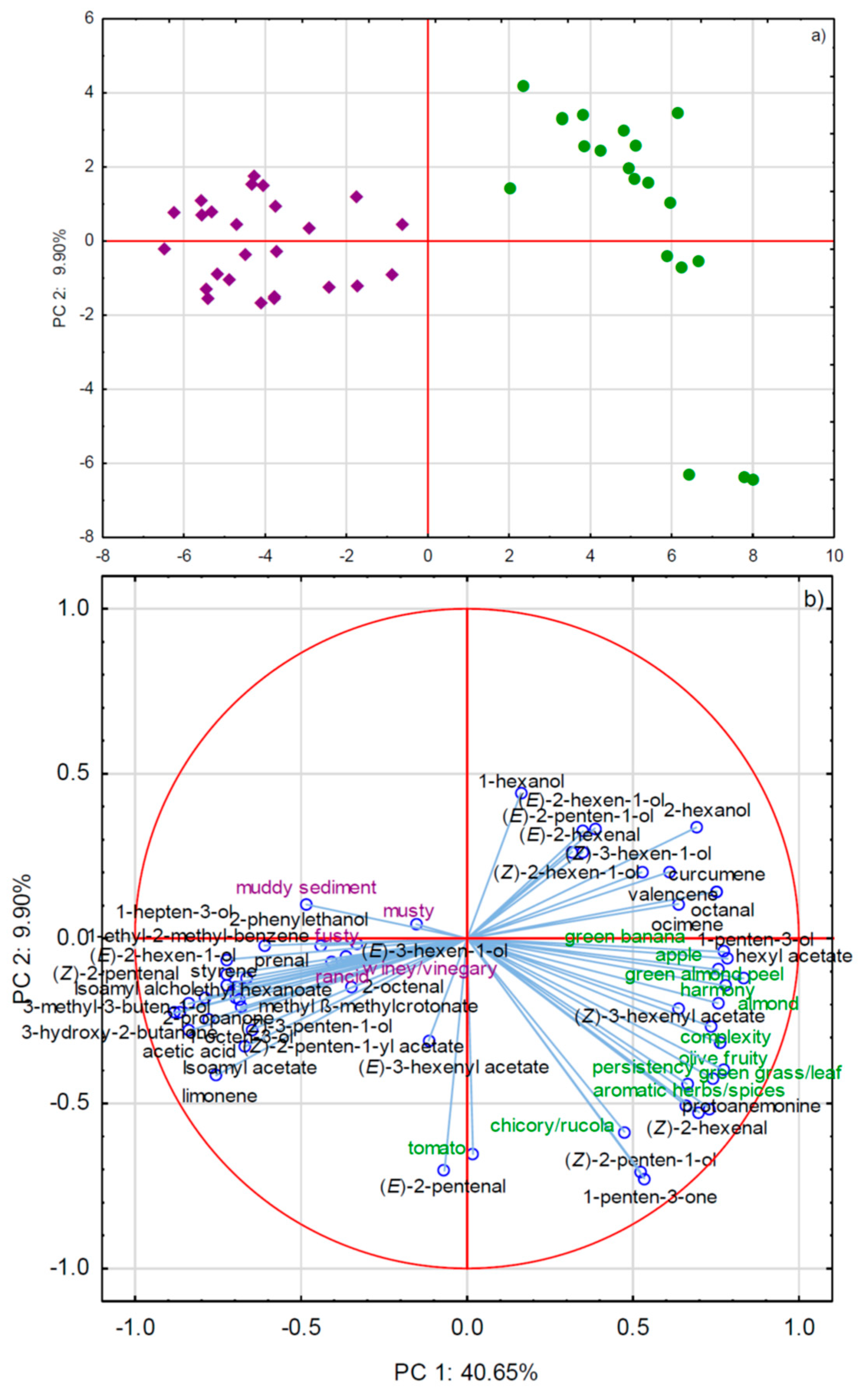
2.2.2. GC×GC-TOF-MS and Sensory Attributes
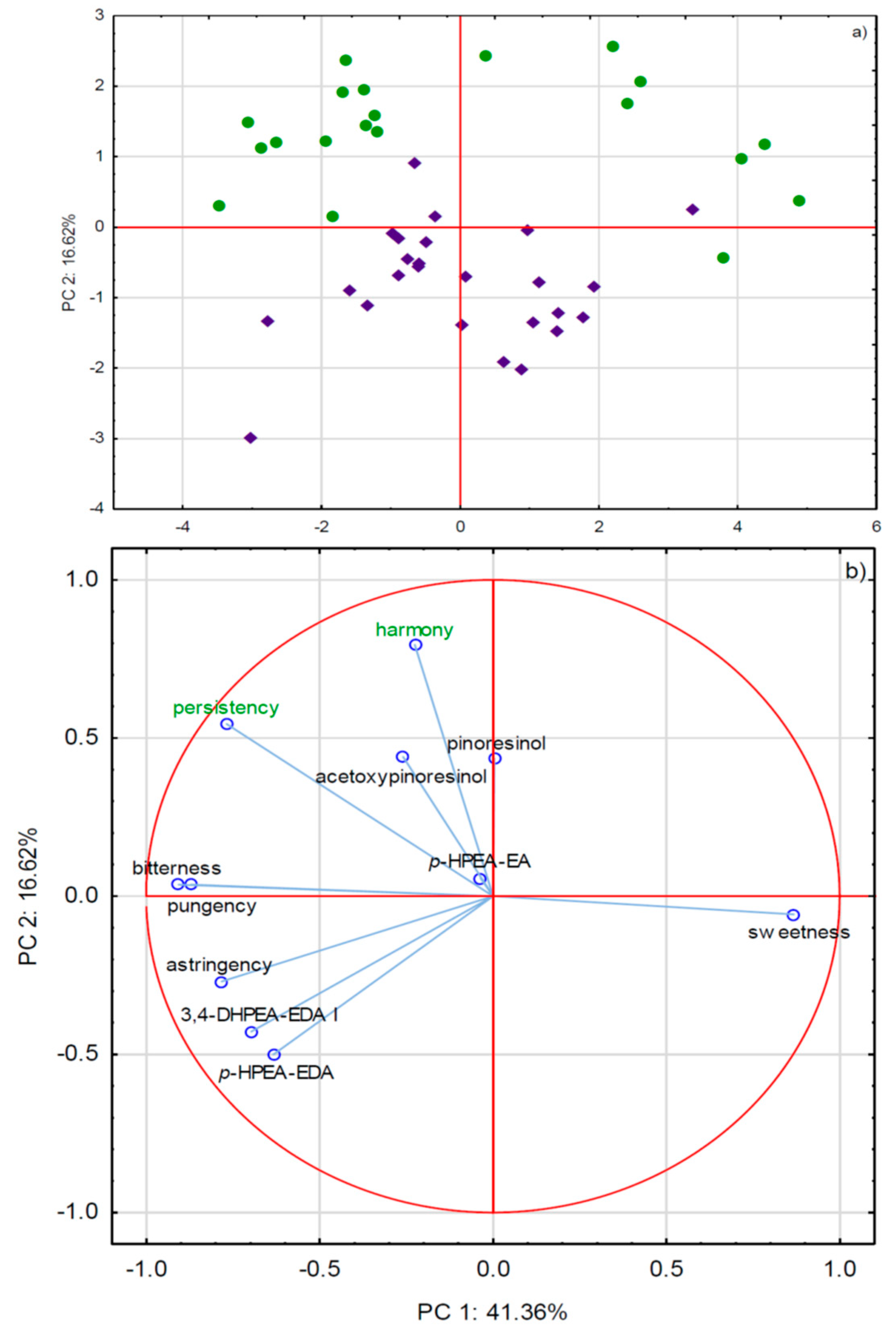
2.3. Phenols and Sensory Attributes
3. Materials and Methods
3.1. EVOO Samples
3.2. Standards and Solvents
3.3. GC-MS Analysis of Volatile Aroma Compounds
3.4. GC×GC-TOF-MS Analysis of Volatile Aroma Compounds
3.5. HPLC-DAD-MS Analysis Of Phenols
3.6. Sensory Analysis
3.7. Statistical Data Elaboration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiorini, D.; Boarelli, M.C.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; Gabbianelli, R.; Pacetti, D. Chemical and sensory differences between high price and low price extra virgin olive oils. Food Res. Int. 2018, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, E.R.; Arriaza, M.; Rodríguez-Entrena, M.; Rodríguez-Entrena, M. Is the extra virgin olive oil market facing a process of differentiation? A hedonic approach to disentangle the effect of quality attributes. Grasas Y Aceites 2015, 66, e105. [Google Scholar] [CrossRef]
- Bongartz, A.; Popp, M.; Schneller, R.; Oberg, D. Evaluation of the ‘Harmony Value’: A Sensory Method to Discriminate the Quality Range within the Category of EVOO. Prod. Olive Tree 2016. [Google Scholar] [CrossRef]
- López-Feria, S.; Cárdenas, S.; García-Mesa, J.A.; Valcárcel, M. Usefulness of the direct coupling headspace–mass spectrometry for sensory quality characterization of virgin olive oil samples. Anal. Chim. Acta 2007, 583, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Sestili, S.; Di Vincenzo, D. Influence of olive processing on virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 587–601. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Angerosa, F. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Angerosa, F.; Basti, C.; Vito, R. Virgin Olive Oil Volatile Compounds from Lipoxygenase Pathway and Characterization of Some Italian Cultivars. J. Agric. Food Chem. 1999, 47, 836–839. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio, R.; Rios, J.J. Dynamic headspace gas chromatographic method for determining volatiles in virgin olive oil. J. Chromatogr. A 1994, 668, 455–462. [Google Scholar] [CrossRef]
- Vichi, S.; Castellote, A.I.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J. Chromatogr. A 2003, 983, 19–33. [Google Scholar] [CrossRef]
- De Medina, V.S.; Priego-Capote, F.; de Castro, M.D.L. Characterization of monovarietal virgin olive oils by phenols profiling. Talanta 2015, 132, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rosales, F.; Rios, J.J.; Gomez-Rey, M.L. Main Polyphenols in the Bitter Taste of Virgin Olive Oil. Structural Confirmation by On-Line High-Performance Liquid Chromatography Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6021–6025. [Google Scholar] [CrossRef] [PubMed]
- Marsilio, V.; Lanza, B. Characterisation of an Oleuropein Degrading Strain of Lactobacillus plantarum. Combined Eþects of Compounds Present in Olive Fermenting Brines (Phenols, Glucose and NaCl) on Bacterial Activity. J. Sci. Food Agric. 1998, 76, 520–524. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Lukić, I.; Žanetić, M.; Špika, M.J.; Lukić, M.; Koprivnjak, O.; Bubola, K.B. Complex interactive effects of ripening degree, malaxation duration and temperature on Oblica cv. virgin olive oil phenols, volatiles and sensory quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of malaxation temperature and time on the quality of virgin olive oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Horvat, I.; Vrhovsek, U. Combined targeted and untargeted profiling of volatile aroma compounds with comprehensive two-dimensional gas chromatography for differentiation of virgin olive oils according to variety and geographical origin. Food Chem. 2019, 270, 403–414. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; López-Tamames, E. Solid-Phase Microextraction in the Analysis of Virgin Olive Oil Volatile Fraction: Characterization of Virgin Olive Oils from Two Distinct Geographical Areas of Northern Italy. J. Agric. Food Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Coca, R.B.; Fernandes, G.D.; del Carmen Pérez-Camino, M.; Moreda, W. Fatty acid ethyl esters (FAEE) in extra virgin olive oil: A case study of a quality parameter. LWT—Food Sci. Technol. 2016, 66, 378–383. [Google Scholar] [CrossRef]
- Purcaro, G.; Cordero, C.; Liberto, E.; Bicchi, C.; Conte, L.S. Toward a definition of blueprint of virgin olive oil by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2014, 1334, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sonia, E.; GianFrancesco, M.; Roberto, S.; Ibanez, R.; Agnese, T.; Stefania, U.; Maurizio, S. Monitoring of virgin olive oil volatile compounds evolution during olive malaxation by an array of metal oxide sensors. Food Chem. 2009, 113, 345–350. [Google Scholar] [CrossRef]
- Alves, E.; Melo, T.; Rey, F.; Moreira, A.S.; Domingues, P.; Domingues, M.R. Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT 2016, 74, 371–377. [Google Scholar] [CrossRef]
- Fregapane, G.; Salvador, M.D. Production of superior quality extra virgin olive oil modulating the content and profile of its minor components. Food Res. Int. 2013, 54, 1907–1914. [Google Scholar] [CrossRef]
- Polari, J.J.; Garcí-Aguirre, D.; Olmo-García, L.; Carrasco-Pancorbo, A.; Wang, S.C. Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chem. 2018, 242, 362–368. [Google Scholar] [CrossRef]
- Jabeur, H.; Zribi, A.; Abdelhedi, R.; Bouaziz, M. Effect of olive storage conditions on Chemlali olive oil quality and the effective role of fatty acids alkyl esters in checking olive oils authenticity. Food Chem. 2015, 169, 289–296. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef]
- EEC. Regulation EEC/2568/91 and later modifications on the characteristics of olive oil and olive-residue oil and the relevant methods of analysis. Off. J. Eur. Community 1991, 24, 1–83. [Google Scholar]
Sample Availability: Not available. |
| Compounds | Class | Confirmed by | F-Ratio | Class | |
|---|---|---|---|---|---|
| Monovarietal | Commercial/Blended | ||||
| Ethyl acetate | Ester | Std; MS | 95.76 | 0.638 | 5.465 * |
| Methyl acetate | Ester | Std; MS | 80.37 | 0.436 | 2.994 * |
| 3-Ethyl-1,5-octadiene III | Hydrocarbon | MS | 64.32 | 4.260 * | 1.853 |
| 2-Methylbutanal | Aldehyde | Std; MS | 38.87 | 0.181 | 0.715 * |
| 3-Ethyl-1,5-octadiene I | Hydrocarbon | MS | 37.64 | 0.957 * | 0.511 |
| (Z)-2-Hexenal | Aldehyde | Std; MS | 27.14 | 1.696 * | 0.591 |
| 3-Methylbutanol | Alcohol | Std; MS | 22.79 | 0.477 | 1.229 * |
| 2-Phenylethanol | Alcohol | Std; MS | 18.50 | 0.181 | 0.376 * |
| 3-Ethyl-1,5-octadiene IV | Hydrocarbon | MS | 17.12 | 1.434 * | 0.473 |
| (Z)-3-Hexenal | Aldehyde | Std; MS | 15.79 | 0.844 * | 0.320 |
| Octane | Alkane | Std; MS | 14.06 | 1.197 | 2.912 * |
| 1,4-Pentadiene | Hydrocarbon | MS | 12.88 | 2.889 * | 1.582 |
| 4,8-Dimethyl-1,3,7-nonatriene | Hydrocarbon | Std; MS | 11.60 | 0.677 * | 0.235 |
| 1-Penten-3-one | Ketone | Std; MS | 11.26 | 6.508 * | 1.772 |
| (E)-2-Hexen-1-ol | Alcohol | Std; MS | 7.79 | 4.145 | 6.825 * |
| (E)-2-Hexenal | Aldehyde | Std; MS | 7.79 | 72.229 * | 46.644 |
| (Z)-2-Penten-1-ol | Alcohol | RI | 4.78 | 2.413 * | 1.570 |
| (E)-2-Hexenyl acetate | Ester | Std; MS | n.s | 0.249 | 0.100 |
| (E)-2-Pentenal | Aldehyde | Std; MS | n.s | 0.708 | 0.547 |
| (Z)-3-Hexen-1-ol | Alcohol | Std; MS | n.s | 9.490 | 8.718 |
| (Z)-3-Hexenyl acetate | Ester | Std; MS | n.s | 3.184 | 4.829 |
| 1-Hexanol | Alcohol | Std; MS | n.s | 6.859 | 7.009 |
| Hexanal | Aldehyde | Std; MS | n.s | 6.535 | 6.086 |
| Hexyl acetate | Ester | Std; MS | n.s | 0.812 | 0.814 |
| Nonanal | Aldehyde | RI | n.s | 0.414 | 0.149 |
| Compounds | Class | LRIlit | LRIcal | F-Ratio | Class | |
|---|---|---|---|---|---|---|
| Monovarietal | Commercial/Blended | |||||
| Acetic acid | Acid | 1430 | 1422 | 131.84 | 41,378.57 | 608,832.20 * |
| 3-Methyl-3-buten-1-ol | Alcohol | 1250 | 1240 | 106.60 | 1688.17 | 23,649.01 * |
| 3-Hydroxy-2-butanone | Ketone | 1282 | 1285 | 90.63 | 83,167.97 | 708,294.36 * |
| 1-Octen-3-ol | Alcohol | 1412 | 1422 | 88.30 | 2574.32 | 39,706.39 * |
| Curcumene | (Sesqui)terpene | 1288 | 1280 | 77.95 | 898,857.67 * | 5177.70 |
| Octanal | Aldehyde | 1284 | 1288 | 77.95 | 998,857.69 * | 5458.86 |
| Limonene | (Sesqui)terpene | 1185 | 1181 | 75.85 | 129,158.57 | 608,832.20 * |
| Hexyl acetate | Ester | 1264 | 1259 | 70.83 | 6,599,680.79 * | 6310.99 |
| 1-Penten-3-ol | Ketone | 1308 | 1306 | 69.95 | 3,577,557.14 * | 484,727.96 |
| Amine n.i. # | 1363 | 67.39 | 22,153.18 | 251,515.59 * | ||
| Isoamyl alchol | Alcohol | 1205 | 1198 | 66.96 | 318,293.21 | 2,312,851.79 * |
| 2-Methyl-4-cyclohexene # | 1734 | 58.61 | 1803.80 | 45,944.97 * | ||
| 2-Propanone | Ketone | 1284 | 1294 | 58.11 | 12,487.26 | 523,313.68 * |
| Ester n.i.# | 1509 | 57.56 | 6219.40 | 228,814.46 * | ||
| 1-Hepten-3-ol | Alcohol | 1433 | 1421 | 55.66 | 563,027.24 | 4,629,899.09 * |
| 1-Ethyl-2-methyl-benzene | Benzeoid | 1270 | 1258 | 50.71 | 4110.12 | 1,119,876.52 * |
| 2-Hexanol | Alcohol | 1226 | 1223 | 45.46 | 5,769,336.31 * | 9376.97 |
| Ethyl hexanoate | Ester | 1236 | 1234 | 41.26 | 29,284.40 | 344,293.49 * |
| Isoamyl alcohol | Alcohol | 1209 | 1213 | 40.93 | 10,846.43 | 70,256.03 * |
| Ocimene | (Sesqui)terpene | 1245 | 1240 | 39.75 | 690,396.59 * | 15,647.62 |
| Prenal | Aldehyde | 1199 | 1191 | 37.43 | 20,683.38 | 115,270.66 * |
| Methyl β-methylcrotonate | Ester | 1148 | 1154 | 35.84 | 2395.85 | 78,223.49 * |
| Styrene | Hydrocarbon | 1250 | 1247 | 35.70 | 1869.71 | 41,640.74 * |
| (Z)-3-Penten-1-ol | Alcohol | 1307 | 1297 | 35.40 | 2788.20 | 13,671.26 * |
| (Z)-2-Pentenal | Aldehyde | 1115 | 1109 | 35.24 | 6181.93 | 229,444.57 * |
| Protoanemonine | Lacton | 1560 | 1570 | 32.50 | 122,469.33 * | 7190.78 |
| Valencene | (Sesqui)terpene | 1689 | 1699 | 32.23 | 144,338.84 * | 4953.13 |
| 1,3,6-Heptatriene # | 1899 | 31.30 | 13,879.77 | 70,256.03 * | ||
| (Z)-3-Hexenyl acetate | Ester | 1312 | 1303 | 30.24 | 5,114,574.62 * | 95,741.68 |
| 4-Cyclononen-1-ol # | 2486 | 28.89 | 78,002.49 | 293,135.33 * | ||
| Carboxaldehyde # | 1712 | 28.84 | 14,287.60 | 162,555.65 * | ||
| 3,7-Dimethyl-1-octanol | Alcohol | 1245 | 1238 | 28.84 | 332,962.19 | 8,020,636.63 * |
| 4-Methyl-phenol | Phenol | 2079 | 2076 | 28.73 | 19,100.40 | 61,672.58 * |
| Bicyclo [4.2.0]octa-1,3,5-triene # | 1337 | 28.63 | 0.00 | 183,495.37 * | ||
| 3-Hexen-1-ol | Alcohol | 1384 | 1369 | 28.60 | 14,977.65 | 162,555.65 * |
| (Z)-2-Penten-1-yl acetate # | 1210 | 27.60 | 10,072.53 | 83,072.51 * | ||
| Ester n.i.# | 1335 | 27.50 | 1353.68 | 14,751.55 * | ||
| 3-Methylpentanoate | Ester | 1489 | 1500 | 26.78 | 1078.36 | 13,195.71 * |
| (Z)-2-Hexenal | Aldehyde | 1120 | 1117 | 26.28 | 17,580,448.97 * | 79,490.84 |
| 2-Methyl-1-penten-3-ol # | 1240 | 26.16 | 5507.31 | 21,808.06 * | ||
| Methylecyclooctene-3,4-diol # | 1896 | 26.14 | 61.05 | 3562.17 * | ||
| Ocimene | (Sesqui)terpene | 1291 | 1284 | 25.98 | 10,321.31 | 85,200.46 * |
| Pentyl isobutyrate | Ester | 1237 | 1254 | 25.74 | 38,917.41 * | 26,987.41 |
| Isoamyl acetate | Ester | 1108 | 1107 | 25.37 | 25,476.74 | 108,410.01 * |
| 3-Ethyl-1,5-octadiene | Hydrocarbon | 1027 | 1094 | 24.52 | 288,269.98 * | 63,403.01 |
| 2-Phenylethanol | Alcohol | 1919 | 1923 | 24.42 | 2,356,534.13 | 4,436,340.01 * |
| (Z)-3-Hexen-1-ol | Alcohol | 1387 | 1380 | 24.21 | 8,419,114.15 * | 1,690,846.47 |
| 2-Methylbutyl acetate | Ester | 1114 | 1109 | 23.80 | 123,593.44 | 678,518.54 * |
| 5-Hexen-2-one # | 1520 | 23.80 | 152.64 | 188,184.38 * | ||
| m-Xylene | Hydrocarbon | 1120 | 1116 | 23.16 | 271,361.98 | 801,814.21 * |
| 2-Heptanone | Ketone | 1160 | 1161 | 22.68 | 44,632.84 | 229,444.57 * |
| Dimethyl-1,3,5,7-octatetraene # | 1421 | 21.70 | 431,163.75 * | 17,677.81 | ||
| 3-octen-2-one | Ketone | 1388 | 1382 | 20.34 | 11,311.04 | 51,531.63 * |
| Methylsulfonylmethane | Sulfur | 1890 | 1890 | 20.03 | 76,235.32 | 392,322.15 * |
| 2-Furanmethanol | Alcohol | 1659 | 1653 | 19.55 | 12,092.65 * | 1737.38 |
| Propylhydrazonealdehyde # | 1191 | 19.45 | 25,103.83 | 153,832.39 * | ||
| 2-Methylenecyclohexanol # | 2450 | 19.23 | 19,701.77 * | 405.28 | ||
| Aldehyde n.i.# | 1150 | 18.48 | 80,847.43 * | 5697.35 | ||
| [S-(R*,R*)]-2,3-butanediol | Alcohol | 1548 | 1546 | 18.47 | 834.54 | 31,576.19 * |
| 1-Heptanol | Alcohol | 1440 | 1424 | 18.32 | 229,133.39 | 489,320.13* |
| p-Ethyltoluene | Benzeoid | 1208 | 1208 | 18.30 | 604,528.11 * | 3697.81 |
| (Z)-2-Hexen-1-yl acetate | Ester | 1321 | 1319 | 18.08 | 3572.24 | 87,445.13 * |
| Amyl acetate | Ester | 1169 | 1158 | 17.44 | 9659.86 | 37,325.80 * |
| 4-Hydroxy 2-pentenoic acid | Lactone | 1663 | 17.22 | 19,924.12 * | 1578.65 | |
| Monoterpene n.i.# | 1330 | 16.44 | 80,557.56 * | 6891.90 | ||
| 5-Hexenoic acid # | 1761 | 16.40 | 5732.67 | 41,172.27 * | ||
| Methyl pyruvate | Ester | 1217 | 1237 | 16.14 | 47,523.10 | 112,112.44 * |
| Benzenoid n.i.# | 1329 | 15.27 | 71,087.15 | 195,957.32 * | ||
| Ethyl benzoate | Benzeoid | 1660 | 1658 | 14.77 | 156,018.91 | 488,215.74 * |
| (E)-Epoxy-ocimene | (Sesqui)terpene | 1476 | 1465 | 14.44 | 27,390.24 * | 6442.21 |
| 2-Methyl-4-pentenal | Aldehyde | 1141 | 1120 | 14.15 | 1908.99 | 104,431.18 * |
| Copaene | (Sesqui)terpene | 1460 | 1468 | 14.10 | 1,117,994.55 * | 80,281.16 |
| 2-Methoxy-phenol | Phenol | 1830 | 1835 | 14.00 | 83,229.22 | 225,226.46 * |
| Dodecane | Alkan | 1200 | 1193 | 14.00 | 1,762,590.66 * | 26,611.62 |
| 3-Methyl-2-pentanone | Ketone | 1012 | 1016 | 13.76 | 29,918.16 * | 257.94 |
| Prenyl acetate | Ester | 1251 | 1243 | 13.58 | 1337.15 | 14,792.50 * |
| Neo-allo-ocimene | (Sesqui)terpene | 1369 | 1376 | 13.49 | 638,240.82 * | 202,839.74 |
| 2-(acetylmethyl)-(+)-3-carene # | 1890 | 13.41 | 5272.02 * | 166.82 | ||
| α-Pyronene | Hydrocarbon | 1365 | 1366 | 13.40 | 367,598.19 * | 108,884.71 |
| 5-Methylfurfural | Furan | 1550 | 1558 | 12.95 | 8333.39 * | 0.00 |
| Methyl 3-hydroxybutanoate | Ester | 1461 | 1461 | 12.69 | 6077.46 | 21,076.30 * |
| Propanoic acid | Acid | 1525 | 1517 | 12.52 | 953,828.13 | 1,898,014.73 * |
| 2-Methyl-2-butenoic acid# | 1812 | 11.72 | 4331.16 * | 216.27 | ||
| 2-Ethyl-1-hexanol | Alcohol | 1489 | 1484 | 11.64 | 293,625.77 * | 5390.86 |
| (Z)-2-Heptenal | Aldehyde | 1319 | 1324 | 11.38 | 431,923.73 | 800,135.47 * |
| Isocumene | (Sesqui)terpene | 1196 | 1197 | 11.26 | 25,262.49 | 84,791.28 * |
| Ester n.i.# | 1293 | 10.65 | 18,124.76 | 150,379.31 * | ||
| Heptanal | Aldehyde | 1180 | 1163 | 10.20 | 37,747.26 | 93,699.89 * |
| Cyclopropylbenzene | Benzeoid | 1377 | 1361 | 10.04 | 34,705.29 | 71,891.03 * |
| Dodecanoic acid | Acid | 2509 | 2508 | 9.74 | 986.62 | 140,612.14 * |
| Nonanoic acid | Acid | 2192 | 2211 | 9.74 | 975.23 | 14,462.14 * |
| (E,E)-2,4-Heptadienal | Aldehyde | 1480 | 1452 | 9.59 | 1,438,596.07 | 3,620,983.61 * |
| α-Ocimene | (Sesqui)terpene | 1245 | 1235 | 9.37 | 431,193.48 * | 160,245.15 |
| Nonanal | Aldehyde | 1374 | 1375 | 9.28 | 5,890,979.35 | 9,656,470.33 * |
| (Z)-2-Penten-1-ol | Alcohol | 1296 | 1306 | 9.27 | 39,568.33 | 132,475.15 * |
| 1-Octanol | Alcohol | 1559 | 1554 | 9.23 | 10,512.66 | 121,637.59 * |
| (E)-2-Hexen-1-ol | Alcohol | 1388 | 1379 | 9.10 | 398,863.62 * | 16,242.32 |
| Hexanoic acid | Acid | 1880 | 1881 | 8.93 | 29,882.90 * | 1,798.32 |
| (Z)-2-Penten-1-ol | Alcohol | 1320 | 1316 | 8.82 | 6,095,917.32 * | 24,624.06 |
| 1-Penten-3-one | Ketone | 1038 | 1096 | 8.73 | 54,514.73 * | 1,594.20 |
| 2-Octanol | Alcohol | 1398 | 1402 | 8.62 | 61,420.45 | 221,737.82 * |
| Prunolide | (Sesqui)terpene | 2048 | 2052 | 8.61 | 103,712.87 * | 21,671.55 |
| Allo-ocimene | (Sesqui)terpene | 1369 | 1370 | 8.58 | 61,726.03 * | 2328.21 |
| 3,7-Dimethyl-1,6-octadiene | Hydrocarbon | 1050 | 1049 | 8.51 | 4922.38 * | 879.32 |
| Decanoic acid | Acid | 2278 | 2267 | 8.33 | 7316.34 * | 2015.68 |
| 2-Ethyl-furan | Furan | 960 | 950 | 8.22 | 6844.34 | 31,477.56 * |
| 3-Pentanol | Alcohol | 1106 | 1099 | 8.17 | 48,766.06 | 529,880.55 * |
| Ethyl tiglate | Ester | 1232 | 1236 | 8.00 | 136,021.23 * | 7618.67 |
| (E)-2-Hepten-1-ol | Alcohol | 1507 | 1499 | 7.99 | 1801.99 | 94,353.20 * |
| 4-Penten-1-ol | Alcohol | 1290 | 1295 | 7.73 | 5902.15 | 1048.98 * |
| Ethyl 3-furoate # | 1609 | 7.71 | 0.00 | 3666.65 * | ||
| 2-Pentanol | Alcohol | 1117 | 1104 | 7.43 | 30,503.88 | 249,804.69 * |
| Acetophenone | Ketone | 1627 | 1645 | 7.42 | 65,342.62 * | 0.00 |
| Farnesene | (Sesqui)terpene | 1755 | 1745 | 7.24 | 110,230,278.27 * | 160,673.90 |
| Toluene | Hydrocarbon | 1055 | 1049 | 7.22 | 136,021.23 * | 7618.67 |
| 4-Ethyl-benzaldehyde | Benzeoid | 1728 | 1726 | 6.87 | 13,952.89 * | 4618.86 |
| Methyl hexanoate | Ketone | 1177 | 1165 | 6.62 | 35,131.15 | 89,231.51 * |
| α-Muurolene | (Sesqui)terpene | 1728 | 1708 | 6.38 | 578,172.46 * | 257,509.06 |
| 2,4-Hexadien-1-ol | Alcohol | 1523 | 1502 | 6.36 | 13,201.54 * | 928.53 |
| (E)-2-Penten-1-ol | Alcohol | 1321 | 1320 | 6.22 | 103,370,445.14 * | 3991.97 |
| (Z)-Bergamotene | (Sesqui)terpene | 1909 | 1899 | 6.19 | 13,104.79 * | 766.76 |
| (E)-2-hexenal | Aldehyde | 1196 | 1195 | 6.18 | 26,108,383.11 * | 510,524.92 |
| 2-Ethyl-1,3-dimethyl-benzene | Benzeoid | 1347 | 1344 | 6.14 | 52,113.37 | 114,494.73 * |
| 5-Ethyl-2(5H)furanone | Furan | 1733 | 1734 | 6.10 | 7,784,864.23 * | 3779,623.65 |
| 3-Penten-2-one | Ketone | 1111 | 1109 | 5.94 | 11,013.36 | 19,977.38 * |
| 2-Pentyl-furan | Furan | 1215 | 1213 | 5.71 | 118,791.62 * | 15,014.39 |
| 1-Butanol | Alcohol | 1139 | 1139 | 5.54 | 595,215.31 * | 130,420.18 |
| 2-Octenal | Aldehyde | 1416 | 1410 | 5.42 | 52,163.93 | 97,028.48 * |
| (Z)-2-Hexen-1-ol | Alcohol | 1436 | 1421 | 5.41 | 246,873.49 * | 2877.40 |
| Cumene | (Sesqui)terpene | 1288 | 1280 | 5.40 | 248,241.74 | 447,051.04 * |
| Citronellol | (Sesqui)terpene | 1757 | 1754 | 5.24 | 24,356.34 * | 14,352.34 |
| 4-Ethyl-m-xylene | Hydrocarbon | 1319 | 1323 | 5.22 | 63,423.72 | 121,964.53 * |
| m-Ethylmethylbenzene | Benzeoid | 1246 | 1247 | 5.13 | 0.00 | 14,536.13 * |
| Methyl (Z)-3-hexenoate | Ester | 1948 | 1941 | 5.05 | 3259.45 | 10,258.45 * |
| Hexyl butanoate | Ester | 1388 | 1401 | 4.91 | 40,413.52 * | 16,743.01 |
| 5-Methyl-2(3H)-furanone | Furan | 1416 | 1412 | 4.84 | 47,851.94 | 21,192.70 * |
| Ethyl 2-butenoate | Ester | 1161 | 1151 | 4.74 | 0.00 | 4134.40 * |
| Sulcatone | Hydrocarbon | 1338 | 1329 | 4.74 | 4060.19 | 408,627.09 * |
| Isobutanoic acid | Acid | 1565 | 1554 | 4.55 | 4937.86 | 412,112.44 * |
| 4,8-Dimethyl-1,3,7-nonatriene | Hydrocarbon | 1304 | 1289 | 4.38 | 12,734,050.10 * | 6165,191.22 |
| Benzaldehyde | Aldehyde | 1518 | 1508 | 4.32 | 560,180.99 * | 25,052.60 |
| 2-Methyl-3-penten-1-ol | Alcohol | 1354 | 1350 | 4.24 | 69,968.019 * | 21,918.81 |
| (E)-3-Hexen-1-ol | Alcohol | 1378 | 1370 | 4.16 | 812,8013.46 | 11,029,551.14 * |
| (E)-2-Hexenoic acid | Acid | 1962 | 1955 | n.s | 2,467,127.31 | 2,652,972.27 |
| (E)-3-Hexenyl acetate | Ester | 1333 | 1329 | n.s | 5,281,303.89 | 6227,652.94 |
| α-Copaene | (Sesqui)terpene | 1460 | 1468 | n.s | 32,609.69 | 14,152.46 |
| β-Ocimene | (Sesqui)terpene | 1255 | 1245 | n.s | 6512.82 | 10,863.14 |
| 1-Hexanol | Alcohol | 1336 | 1337 | n.s | 128,095.59 | 104,703.26 |
| 1-Hexen-3-ol | Alcohol | 1230 | 1225 | n.s | 25,697.59 | 16,238.11 |
| 1-Pentanol | Alcohol | 1245 | 1243 | n.s | 715,152.52 | 560,511.12 |
| 2,4-Pentadienal | Aldehyde | 1197 | 1207 | n.s | 332,491.61 | 273,823.24 |
| (E)-2-Pentenal | Aldehyde | 1121 | 1111 | n.s | 661,208.23 | 841,871.57 |
| 3-Hexanal | Aldehyde | 1146 | 1120 | n.s | 517,211.48 | 594,193.33 |
| (E)-3-Hexenyl butanoate | Ester | 1451 | 1449 | n.s | 99,944.21 | 54,576.52 |
| δ-Cadinene | (Sesqui)terpene | 1729 | 1726 | n.s | 26,908.50 | 16,020.85 |
| Octanoic acid | Acid | 2071 | 2057 | n.s | 21,213.91 | 34,572.98 |
| Pentanoic acid | Acid | 1720 | 1716 | n.s | 212,384.72 | 325,869.83 |
| Pentanol | Alcohol | 1271 | 1261 | n.s | 1734.76 | 1069.51 |
| Compounds | F-Ratio | Class | |
|---|---|---|---|
| Monovarietal | Commercial-Blanded | ||
| Acetoxypinoresinol | 6.06 | 16.437 * | 10.537 |
| Pinoresinol | 5.89 | 5.964 * | 3.850 |
| p-HPEA-EDA | 5.31 | 28.816 | 45.282 * |
| Hydroxytyrosol | n.s | 13.253 | 12.906 |
| Tyrosol | n.s | 17.873 | 16.497 |
| Vanillic acid | n.s | 0.735 | 0.726 |
| 3,4-DHPEA-EDA I | n.s | 15.846 | 22.012 |
| Oleuropein | n.s | 10.912 | 11.736 |
| 3,4-DHPEA-EDA II | n.s | 1.087 | 1.448 |
| 3,4-DHPEA-EA | n.s | 11.759 | 15.871 |
| p-HPEA-EA | n.s | 16.451 | 11.943 |
| Vanillin | n.s | 0.374 | 0.339 |
| p-Coumaric acid | n.s | 0.437 | 0.377 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Ros, A.; Masuero, D.; Riccadonna, S.; Brkić Bubola, K.; Mulinacci, N.; Mattivi, F.; Lukić, I.; Vrhovsek, U. Complementary Untargeted and Targeted Metabolomics for Differentiation of Extra Virgin Olive Oils of Different Origin of Purchase Based on Volatile and Phenolic Composition and Sensory Quality. Molecules 2019, 24, 2896. https://doi.org/10.3390/molecules24162896
Da Ros A, Masuero D, Riccadonna S, Brkić Bubola K, Mulinacci N, Mattivi F, Lukić I, Vrhovsek U. Complementary Untargeted and Targeted Metabolomics for Differentiation of Extra Virgin Olive Oils of Different Origin of Purchase Based on Volatile and Phenolic Composition and Sensory Quality. Molecules. 2019; 24(16):2896. https://doi.org/10.3390/molecules24162896
Chicago/Turabian StyleDa Ros, Alessio, Domenico Masuero, Samantha Riccadonna, Karolina Brkić Bubola, Nadia Mulinacci, Fulvio Mattivi, Igor Lukić, and Urska Vrhovsek. 2019. "Complementary Untargeted and Targeted Metabolomics for Differentiation of Extra Virgin Olive Oils of Different Origin of Purchase Based on Volatile and Phenolic Composition and Sensory Quality" Molecules 24, no. 16: 2896. https://doi.org/10.3390/molecules24162896
APA StyleDa Ros, A., Masuero, D., Riccadonna, S., Brkić Bubola, K., Mulinacci, N., Mattivi, F., Lukić, I., & Vrhovsek, U. (2019). Complementary Untargeted and Targeted Metabolomics for Differentiation of Extra Virgin Olive Oils of Different Origin of Purchase Based on Volatile and Phenolic Composition and Sensory Quality. Molecules, 24(16), 2896. https://doi.org/10.3390/molecules24162896










