Study on Paramagnetic Interactions of (CH3NH3)2CoBr4 Hybrid Perovskites Based on Nuclear Magnetic Resonance (NMR) Relaxation Time
Abstract
:1. Introduction
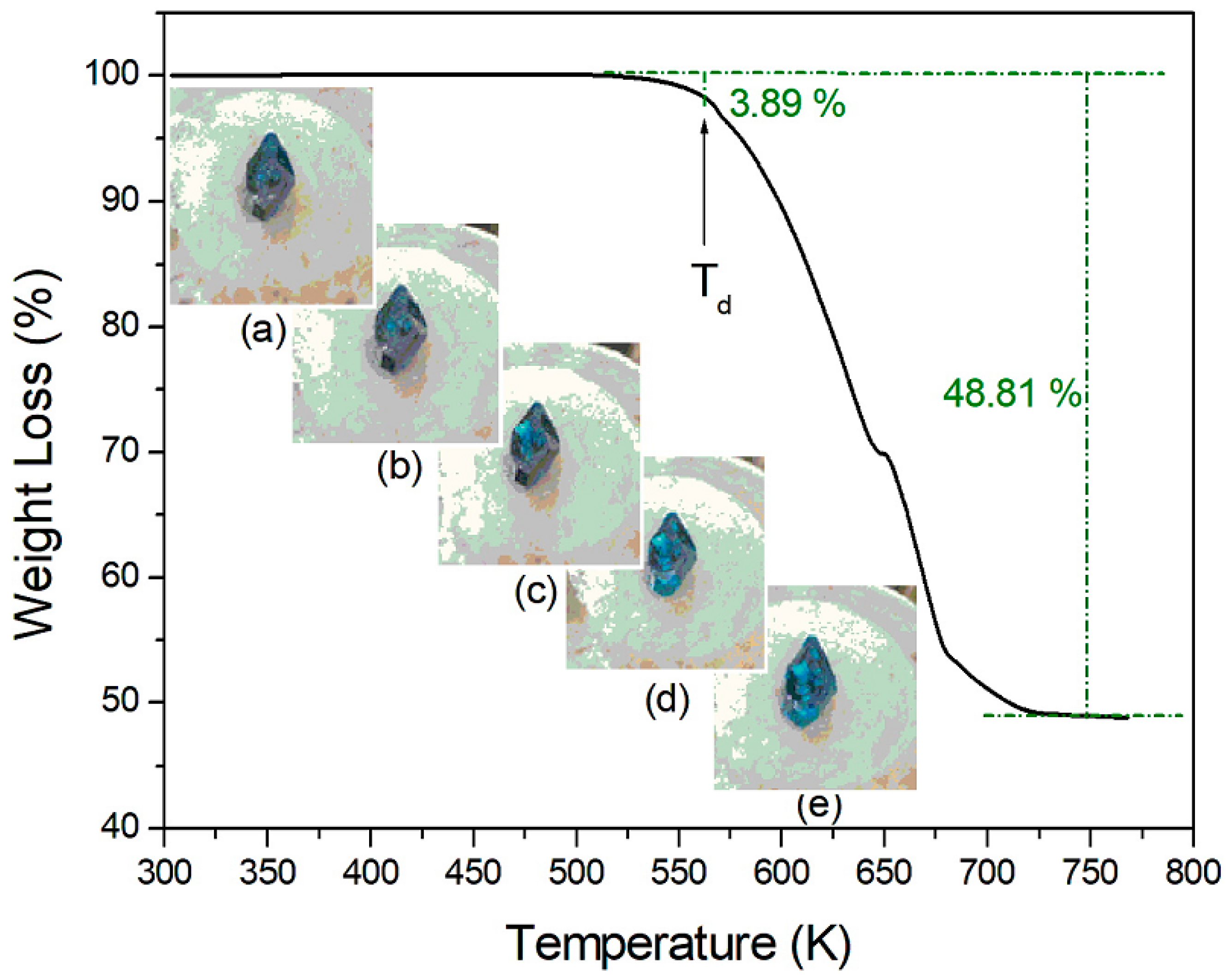
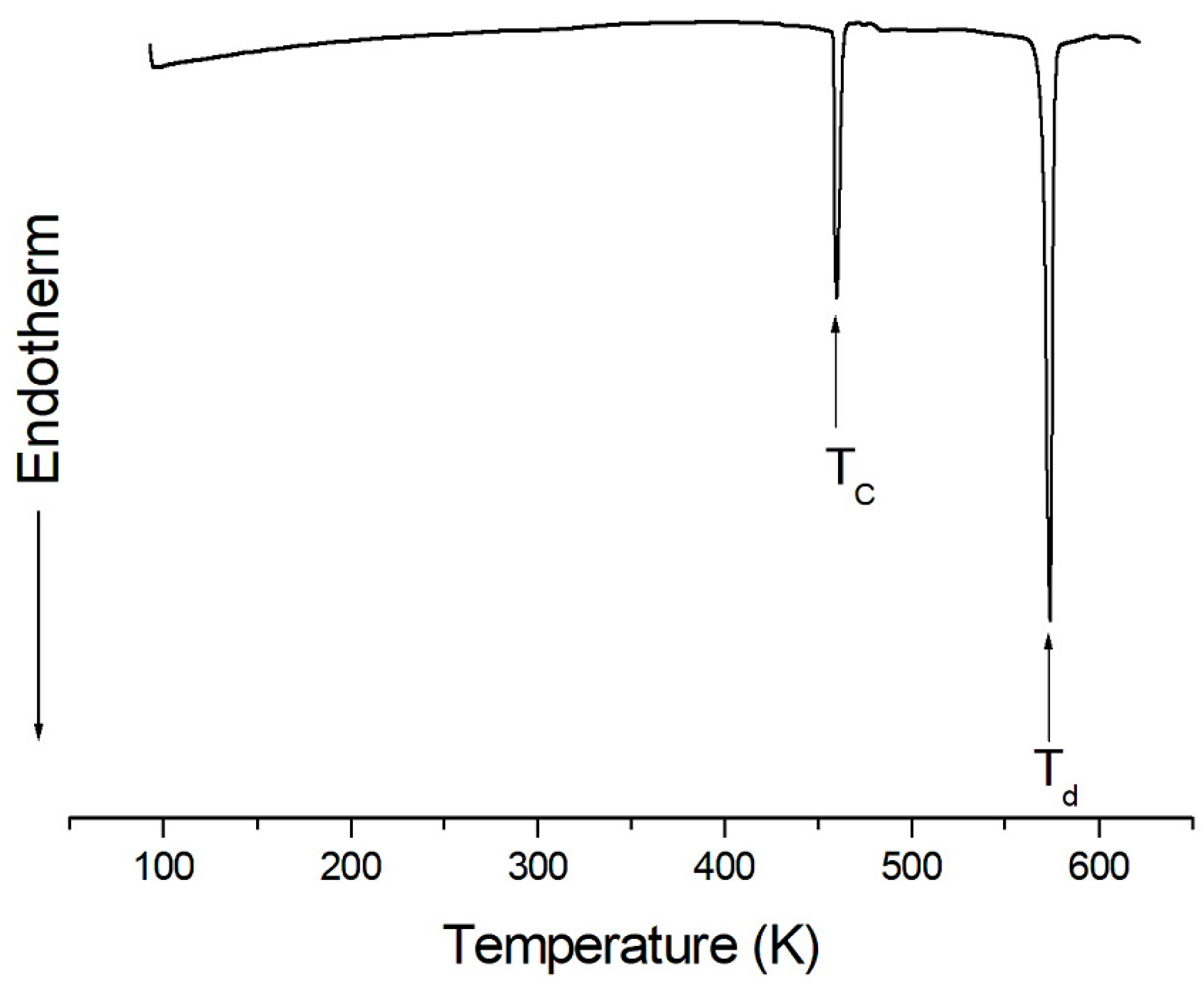
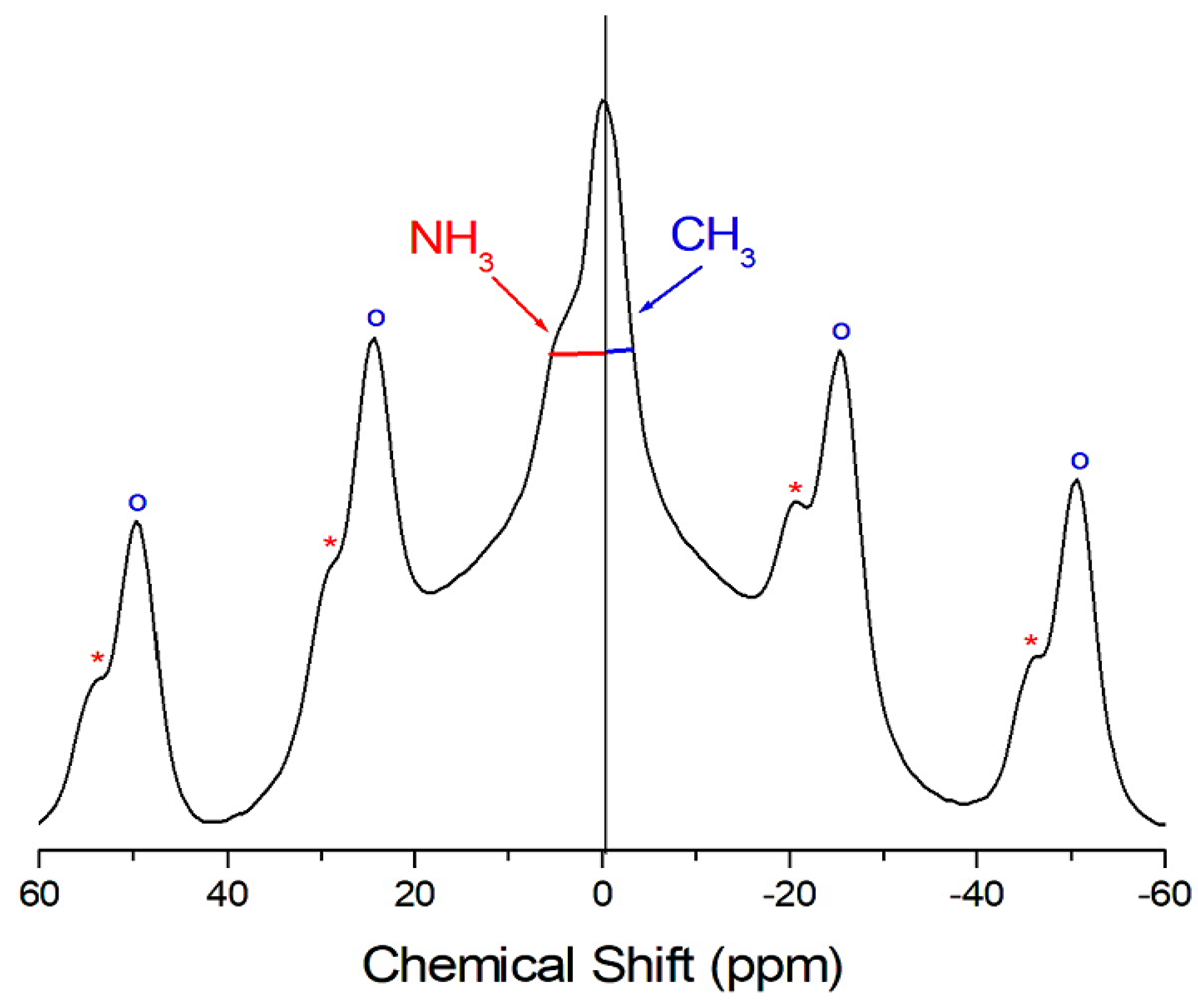
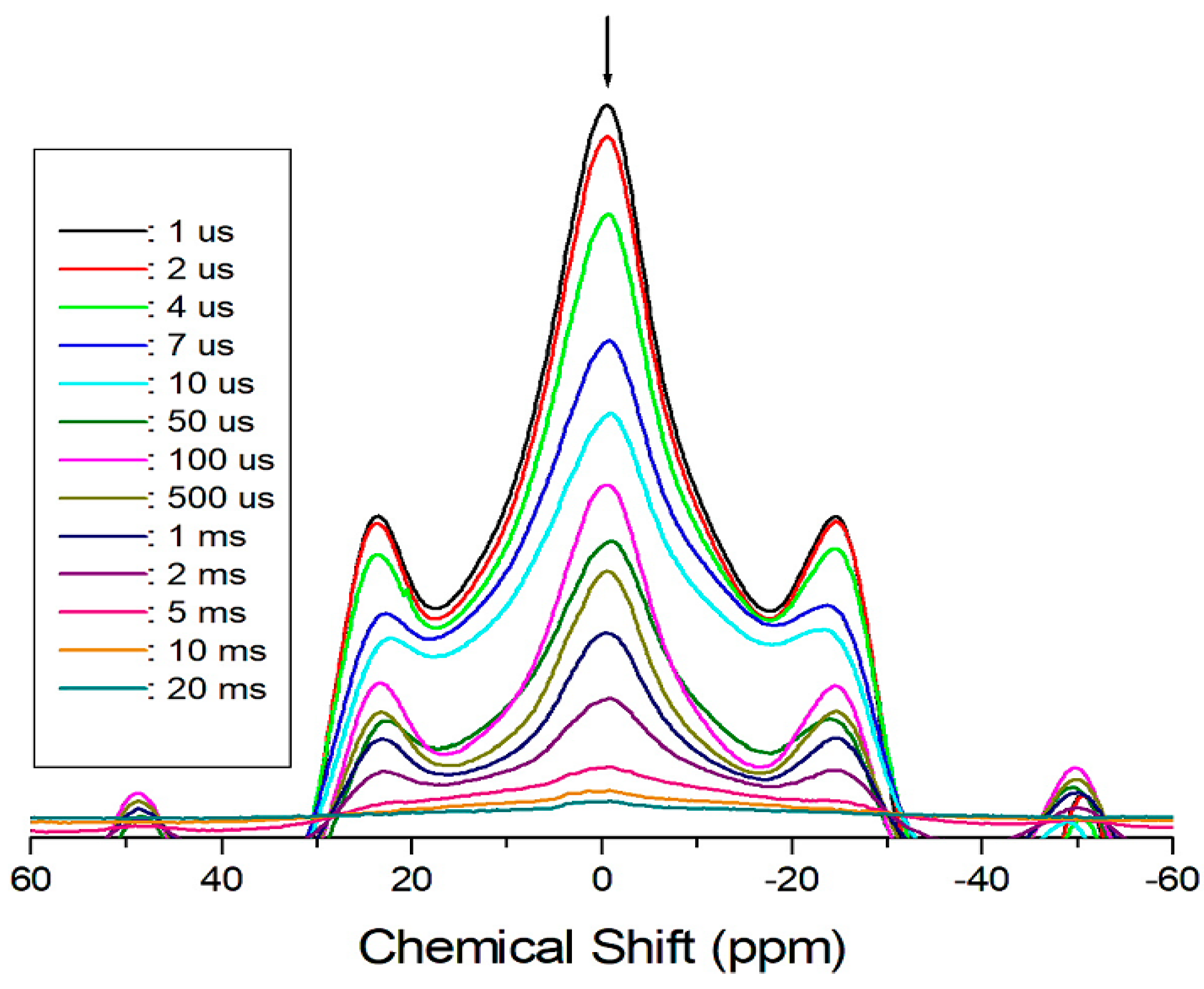
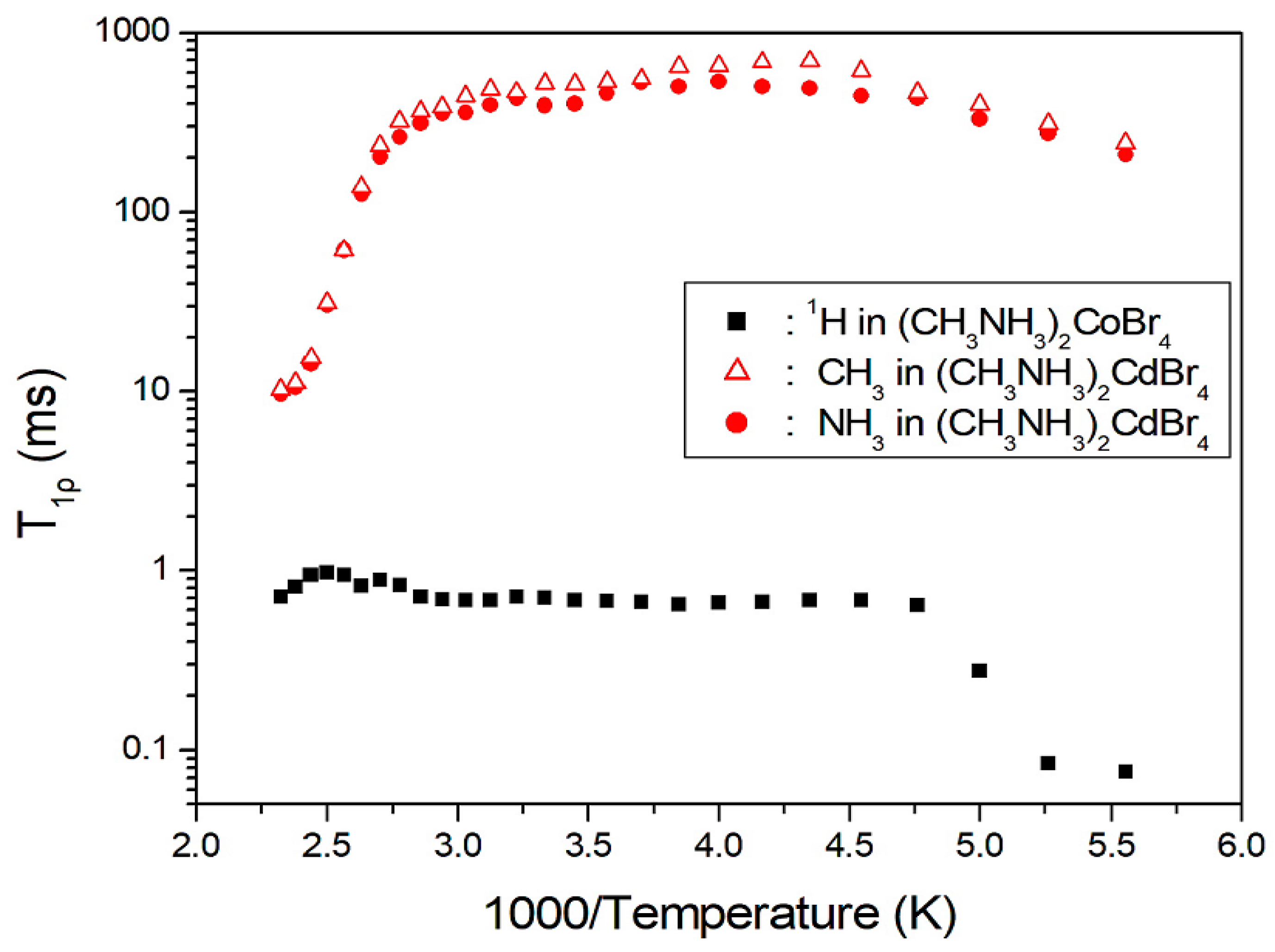
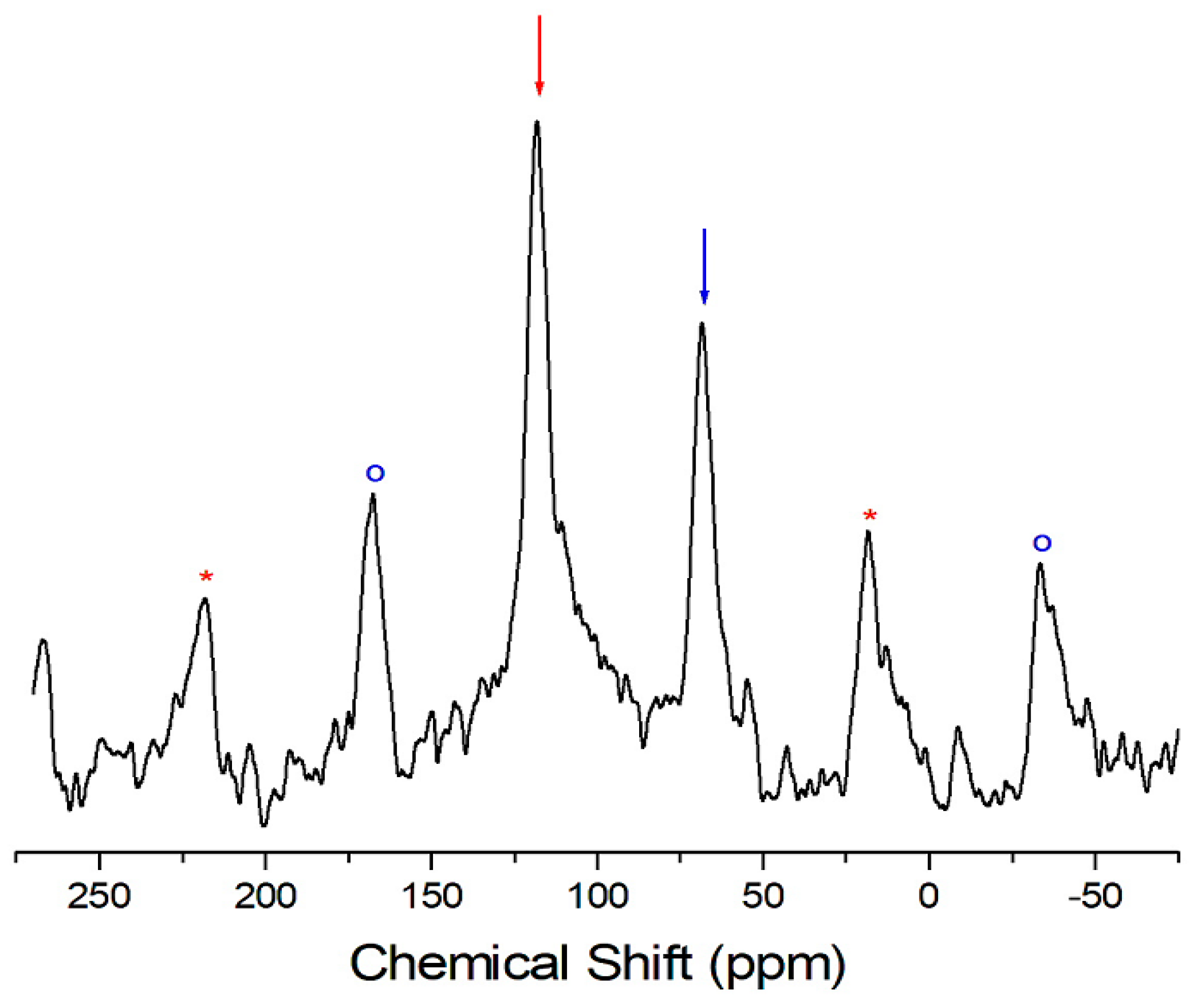
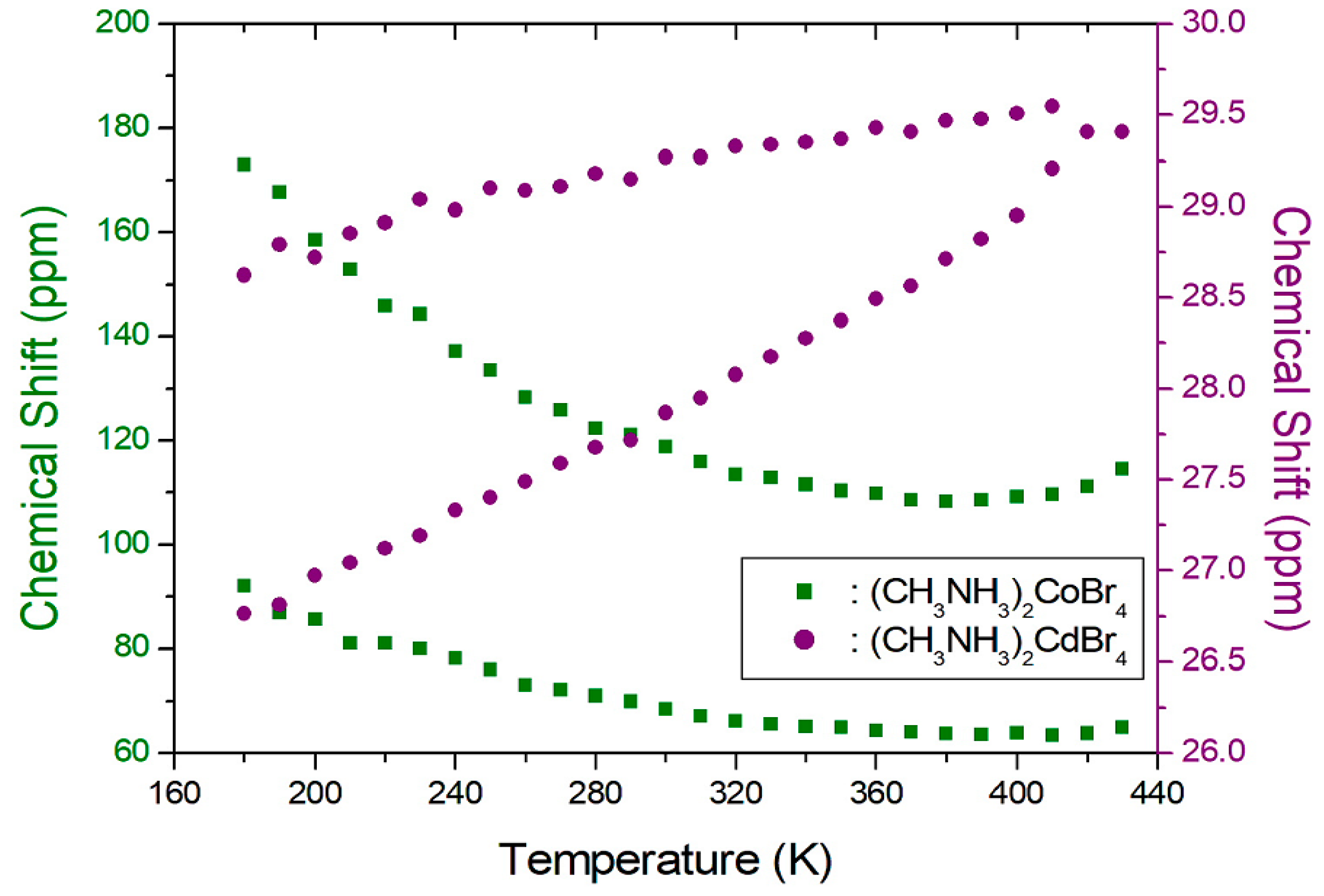
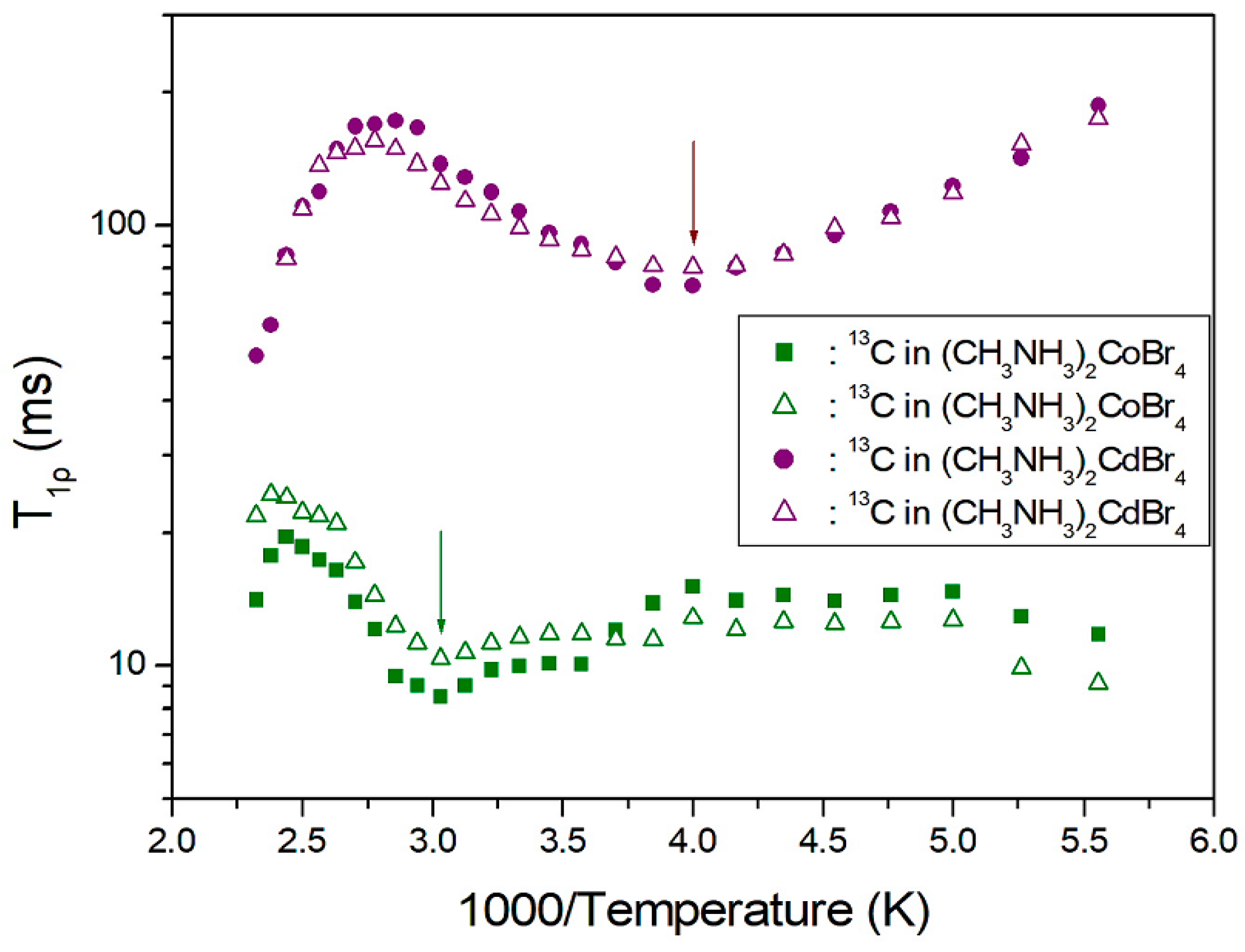
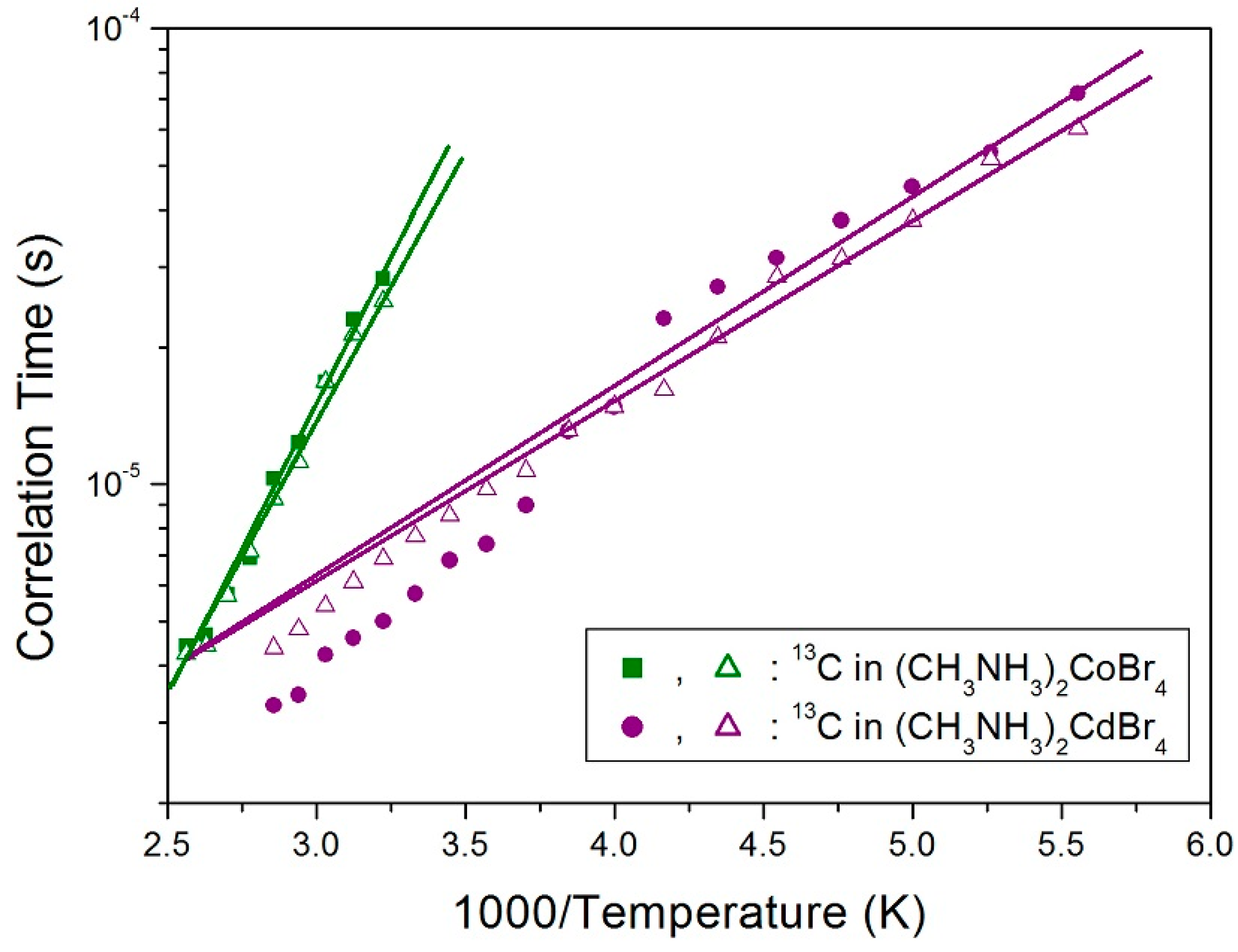
2. Results and Discussion
- Residue:[(CH3NH2)2CoBr2 (M = 280.857 g)]/[(CH3NH3)2CoBr4 (M = 442.681 g)] = 63.4%(CH3NH3)2CoBr4→(CH3NH2·HBr)2CoBr2→CoBr2 (s) + 2(CH3NH2·HBr) (g)
- Residue:[CoBr2 (M = 218.741 g)]/[(CH3NH3)2CoBr4 (M = 442.681 g)] = 49.4 %
- Fa = τC/[1 + ω12τC2]
- Fb = τC/[1 + (ωH ‒ ωC)2τC2]
- Fc = τC/[1 + ωC2τC2]
- Fd = τC/[1 + (ωH + ωC)2τC2]
- Fe = τC/[1 + ωH2τC2].
- Ga = τC/[1 + ω12τC2]
- Gb = τC/[1 + (ωC ‒ ωe)2τC2]
- Gc = τC/[1 + ωC2τC2]
- Gd = τC/[1 + (ωC + ωe)2τC2]
- Ge = τC/[1 + ωe2τC2].
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.; Li, L.; Yerramilli, A.S.; Qu, W.; Song, Y.; Li, N.; Shen, Y.; Alford, T.L. Introduction of nitrogen gas flow and precursor aging process to improve the efficiency of the lead acetate derived CH3NH3PbI3 perovskite solar cells. Sol. Energy Mater. Sol. Cells. 2019, 190, 49–56. [Google Scholar] [CrossRef]
- Elseman, A.M.; Shalan, A.E.; Sajid, S.; Rashad, M.M.; Hassan, A.M.; Li, M. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Interfaces. 2018, 10, 11699. [Google Scholar] [CrossRef] [PubMed]
- Aramburu, J.A.; Garcia-Fernandez, P.; Mathiesen, N.R.; Garcia-Lastra, J.M.; Moreno, M. Changing the usual interpretation of the structure and ground state of Cu2+-layered perovskites. J. Phys. Chem. C 2018, 122, 5071–5082. [Google Scholar] [CrossRef]
- Ahmad, K.; Ansari, S.N.; Natarajan, K.; Mobin, S.M. Design and synthesis of 1D-polymeric chain based [(CH3NH3)3Bi2Cl9]n perovskite: A new light absorber material for lead free perovskite solar cells. Solar Cell 2018, 1, 2405–2409. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Prochowicz, D.; Hofstetter, A.; Pechy, P.; Zakeeruddin, S.M.; Gratzel, M.; Emsley, L. Cation dynamics in mixed-cation (MA)x(FA)1-xPbI3 hybrid perovskites from solid-state NMR. J. Am. Chem. Soc. 2017, 139, 10055–10061. [Google Scholar] [CrossRef] [PubMed]
- Jahandar, M.; Heo, J.H.; Song, C.E.; Kong, K.J.; Shin, W.S.; Lee, J.C.; Im, S.H.; Moon, S.J. Highly efficient metal halide substituted CH3NH3I(PbI2)1-x(CuBr2)x planar perovskite solar cells. Nano Energy 2016, 27, 330–339. [Google Scholar] [CrossRef]
- Novotny, J.; Sojka, M.; Komorovsky, S.; Necas, M.; Marek, R. Interpreting the paramagnetiC-NMR spectra of potential Ru(III) metallodrugs: Synergy between experiment and relativistic DFT calculations. J. Am. Chem. Soc. 2016, 138, 8432–8445. [Google Scholar] [CrossRef]
- Cui, X.P.; Jiang, K.J.; Huang, J.H.; Zhang, Q.Q.; Su, M.J.; Yang, L.M.; Song, Y.L.; Zhou, X.Q. Cupric bromide hybrid perovskite heterojunction solar cells. Synthetic Metals 2015, 209, 247–250. [Google Scholar] [CrossRef]
- Chen, Q.; Marco, N.D.; Yang, Y.; Song, T.B.; Chen, C.C.; Zhau, M.; Hong, Z.; Zhou, H.; Yang, Y. Under the spotlight: The organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Cheetham, A.K.; Thirumurugan, A. Hybrid inorganic-organic materials: A new family in condensed matter physics. J. Phys. Condens. Matter 2008, 20, 159801. [Google Scholar]
- Yadav, R.; Swain, D.; Kundu, P.P.; Nair, H.S.; Narayana, C.; Elizabeth, S. Dielectric and Raman investigations of structural phase transitions in (C2H5NH3)2CdCl4. Phys. Chem. Chem. Phys. 2015, 17, 12207–12214. [Google Scholar] [CrossRef] [PubMed]
- Pabst, I.; Fuess, H.; Bats, J.W. Structure of monomethylammonium tetrachlorocuprate at 297 and 100 K. Acta crystallogr. C 1987, 43, 413–416. [Google Scholar] [CrossRef]
- Babu, R.; Vardhaman, A.K.; Dhavale, V.M.; Giribabu, L.; Singh, S.P. MA2CoBr4: Lead-free cobalt-based perovskite for electrochemical conversion of water to oxygen. Chem. Commun. 2019, 55, 6779–6782. [Google Scholar] [CrossRef] [PubMed]
- Daub, M.; Stroh, R.; Hillebrecht, H. Synthesis, Crystal structure, and optical properties of (CH3NH3)2CoX4 (X = Cl, Br, I, Cl0.5Br0.5, Cl0.5I0.5, Br0.5I0.5). Z. Anorg. Allg. Chem. 2016, 642, 268–274. [Google Scholar] [CrossRef]
- Roccanova, R.; Ming, W.; Whiteside, V.R.; McGuire, M.A.; Sellers, I.R.; Du, H.H.; Saparov, B. Synthesis, crystal and electronic structures, and optical properties of (CH3NH3)2CdX4 (X = Cl, Br, I). Inorg. Chem. 2017, 56, 13878–13888. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, D.; Arend, H.; Niggli, A.; Petter, W. New tetrahedrally coordinated A2CdBr4 compounds (A = Cs, CH2NH3). Mater. Res. Bull. 1979, 14, 1391–1396. [Google Scholar] [CrossRef]
- Lim, A.R. Molecular dynamics of cations and anions by 1H MAS NMR, 13C CP/MAS NMR, 14N NMR, and 113Cd NMR in hybrid organic-inorganic (CH3NH3)2CdBr4. J. Mol. Structure 2018, 1167, 255–260. [Google Scholar] [CrossRef]
- Koenig, J.L. Spectroscopy of Polymers; Elsevier: New York, NY, USA, 1999. [Google Scholar]
- Mcbrierty, V.J.; Packer, K.J. Nuclear Magnetic Resonance in Solid Polymers; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Abragam, A. The Principles of Nuclear Magnetism; Oxford University Press: Oxford, UK, 1961. [Google Scholar]
- Charles, J.P.; Jacqlynn, B. The Aldrich Library of 13C and 1H FT NMR Spectra; Aldrich Chemical Company: Saint Louis, MI, USA, 1993. [Google Scholar]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation effects in nuclear magnetic resonance absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Lim, A.R. Role of NH4 ions in successive phase transitions of perovskite type (NH4)2ZnX4 (X= Cl, Br) by 1H MAS NMR and 14N NMR. RSC Advances 2018, 8, 11316–11323. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation processes in a system of two spins. Phys. Rev. B 1955, 99, 559–565. [Google Scholar] [CrossRef]
- Aliev, A.E.; Harris, K.D.M. Simple technique for temperature calibration of a MAS probe for solid-state NMR spectroscopy. Mag. Reson. Chem. 1994, 32, 366–369. [Google Scholar] [CrossRef]
- Langer, B.; Schnell, I.; Spiess, H.W.; Gimmer, A.-R. Temperature calibration under ultrafast MAS conditions. J. Mag. Reson. 1999, 138, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, D.J.; Prochowicz, D.; Pinon, A.; Stevanato, G.; Hofstetter, A.; Zakeeruddin, S.M.; Gratzel, M.; Emsley, L. Doping and phase segregation in Mn2+- and Co2+- doped lead halide perovskite from 133Cs and 1H-NMR relaxation enhancement. J. Mater. Chem. A 2019, 7, 2326–2333. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (CH3NH3)2CoBr4 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.R.; Kim, S.H. Study on Paramagnetic Interactions of (CH3NH3)2CoBr4 Hybrid Perovskites Based on Nuclear Magnetic Resonance (NMR) Relaxation Time. Molecules 2019, 24, 2895. https://doi.org/10.3390/molecules24162895
Lim AR, Kim SH. Study on Paramagnetic Interactions of (CH3NH3)2CoBr4 Hybrid Perovskites Based on Nuclear Magnetic Resonance (NMR) Relaxation Time. Molecules. 2019; 24(16):2895. https://doi.org/10.3390/molecules24162895
Chicago/Turabian StyleLim, Ae Ran, and Sun Ha Kim. 2019. "Study on Paramagnetic Interactions of (CH3NH3)2CoBr4 Hybrid Perovskites Based on Nuclear Magnetic Resonance (NMR) Relaxation Time" Molecules 24, no. 16: 2895. https://doi.org/10.3390/molecules24162895
APA StyleLim, A. R., & Kim, S. H. (2019). Study on Paramagnetic Interactions of (CH3NH3)2CoBr4 Hybrid Perovskites Based on Nuclear Magnetic Resonance (NMR) Relaxation Time. Molecules, 24(16), 2895. https://doi.org/10.3390/molecules24162895




