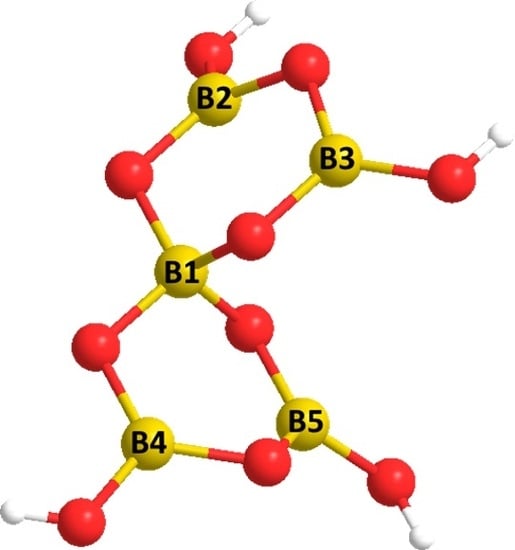
Novel Non-Metal Cation (NMC) Pentaborate Salts of Some Amino Acids
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
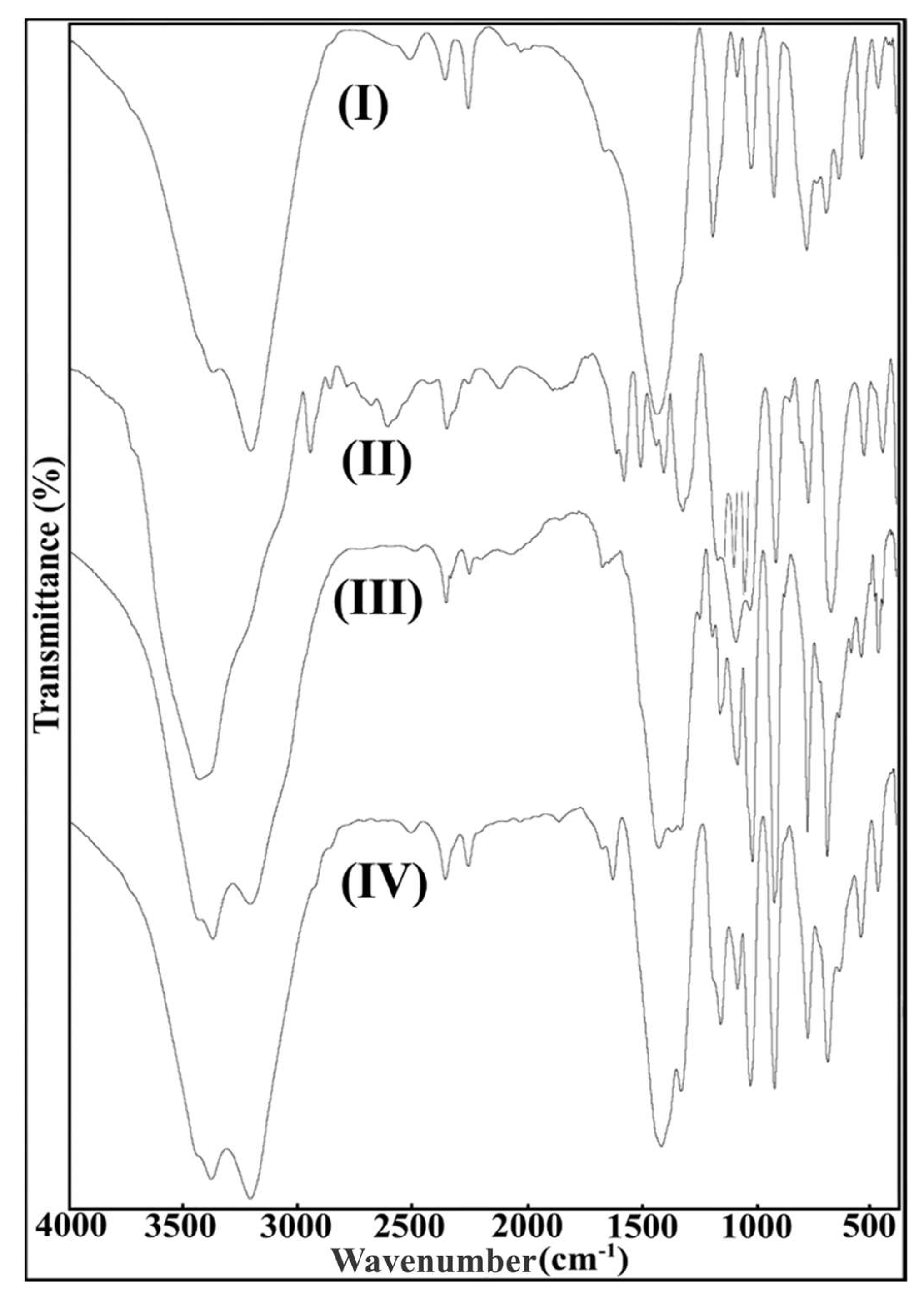
2.2. FT-IR Spectroscopy
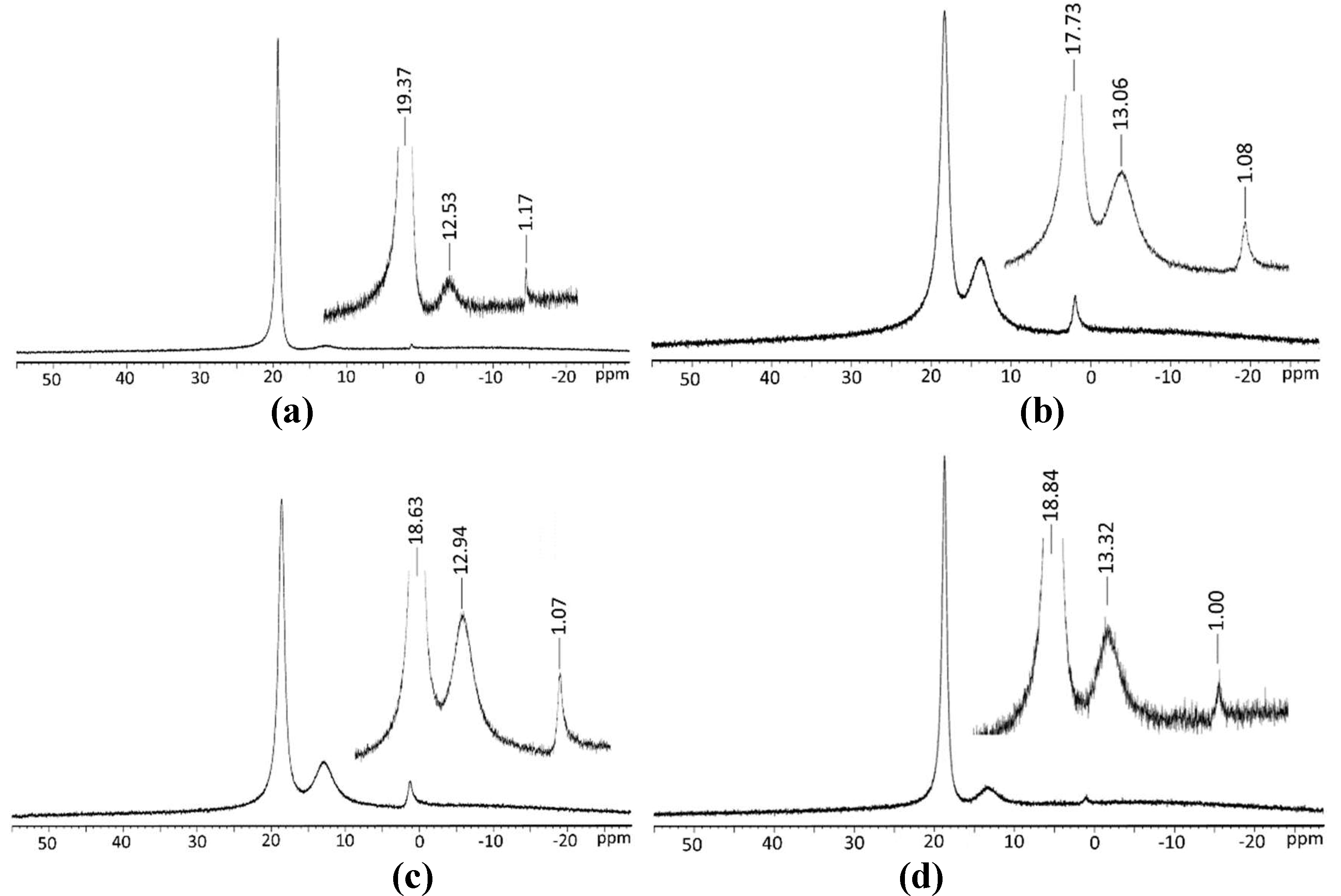
2.3. 11B-NMR Spectroscopy
2.4. Thermal Analysis
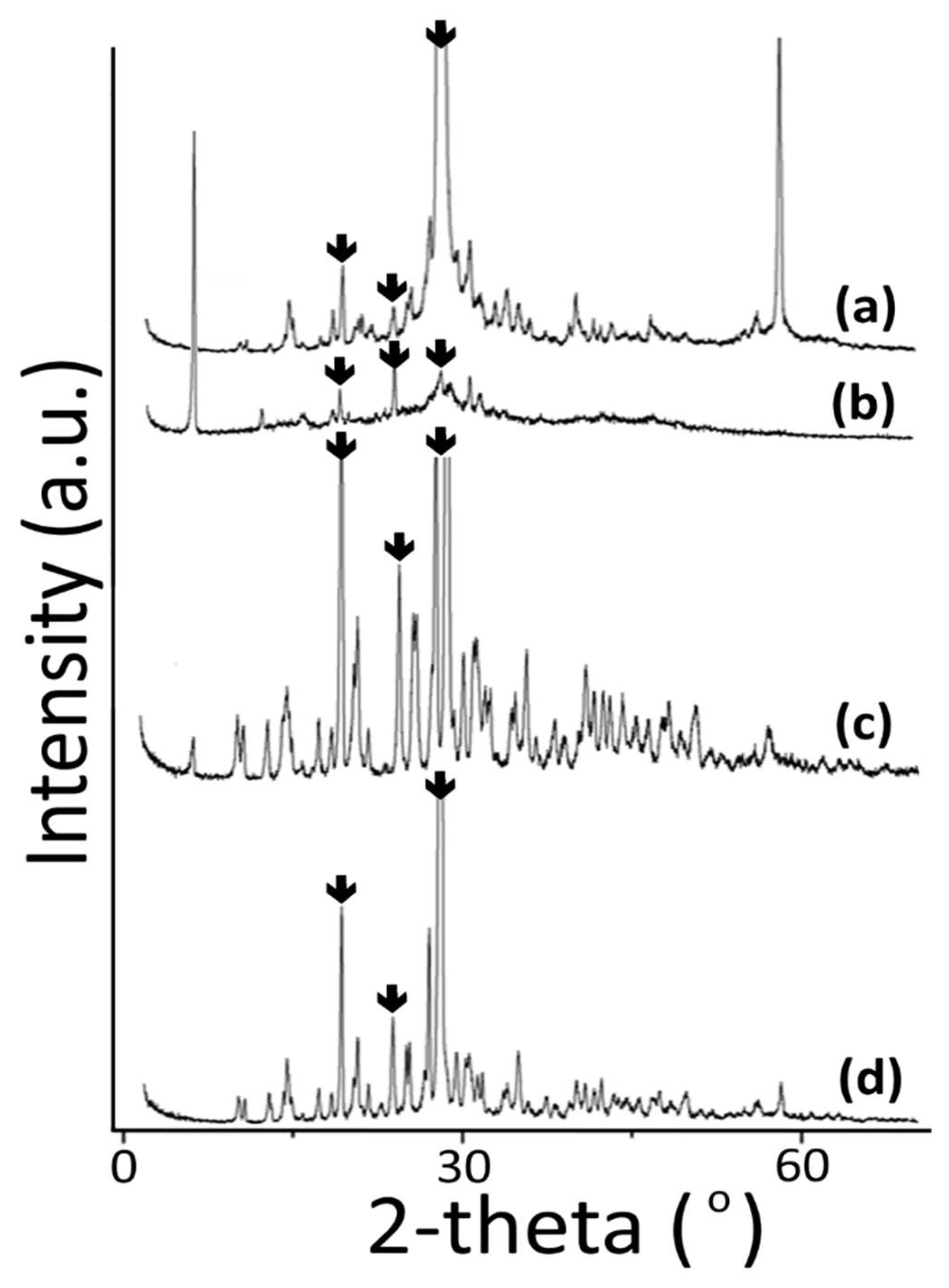
2.5. Powder X-ray Diffraction Analysis
2.6. Mass Spectrometry Analysis
2.7. BET Analysis
2.8. Determination of Hydrogen Storage Capacity
3. Material and Methods
Synthesis of Aminoacid-Pentaborates Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schubert, D.M. Borates in Industrial Use. Structure and Bonding. In Group 13 Chemistry III, Industrial Applications; Roesky, H.W., Atwood, D.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 105, pp. 1–40. [Google Scholar]
- Beckett, M.A.; Bland, C.C.; Horton, P.N.; Hursthouse, M.B.; Varma, K.S.J. Supramolecular structures containing ‘isolated’ pentaborate anions and non-metal cations: Crystal structures of [Me3NCH2CH2OH][B5O6(OH)4] and [4-MepyH, 4-Mepy] [B5O6(OH)4]. J. Organomet. Chem. 2007, 692, 2832–2838. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Bazarov, B.G.; Gavrilova, T.A.; Grossman, V.G.; Molokeev, M.S.; Bazarova, Z.G. Preparation and structural properties of nonlinear optical borates K2(1−x)Rb2xAl2B2O7, 0<x<0.75. J. Alloy. Compd. 2012, 515, 119–122. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Zaitsev, A.I.; Molokeev, M.S.; Aleksandrovsky, A.S.; Kuzubov, A.A.; Ignatova, N.Y. Electronic structure of α-SrB4O7: Experiment and theory. J. Phys. Condens. Matter. 2013, 25, 085503. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Bazarov, B.G.; Grossman, V.G.; Molokeev, M.S.; Bazarova, Z.G. Structural field of K2Al2B2O7-family crystals. Proc. SPIE 2013, 8772, 87721O. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Dorzhieva, S.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; et al. Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv. Powder Technol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Brown, I.D. The Bond-Valence Method: An Empirical Approach to Chemical Structure and Bonding. In Structure and Bonding in Crystals; O’Keeffe, M., Navrotsky, A., Eds.; Academic Press: New York, NY, USA, 1981; Volume 2, pp. 1–30. [Google Scholar]
- Schindler, M.; Hawthorne, F.C. A bond-valence approach to the structure, chemistry, and paragenesis of hydroxy-hydrated oxysalt minerals. I. Theory. Can. Mineral. 2001, 39, 1225–1242. [Google Scholar] [CrossRef]
- Schindler, M.; Hawthorne, F.C. A bond-valence approach to the structure, chemistry, and paragenesis of hydroxy-hydrated oxysalt minerals. II. Crystal structure and chemical composition of borate minerals. Can. Mineral. 2001, 39, 1243–1256. [Google Scholar] [CrossRef]
- Schindler, M.; Hawthorne, F.C. A bond-valence approach to the structure, chemistry, and paragenesis of hydroxy-hydrated oxysalt minerals. III. Paragenesis of borate minerals. Can. Mineral. 2001, 39, 1257–1274. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Aleksandrovsky, A.S.; Kozhukhov, A.S.; Pokrovsky, L.D.; Atuchin, V.V. Growth, optical and microstructural properties of PbB4O7 plate crystals. Opt. Mater. 2014, 37, 298–301. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S. Structural and spectroscopic properties of new noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater. Des. 2018, 140, 488–494. [Google Scholar] [CrossRef]
- Cook, W.R., Jr.; Jaffe, H. The crystallographic, elastic and piezoelectric properties of ammonium pentaborate and potassium pentaborate. Acta Crystallogr. 1957, 10, 705–707. [Google Scholar] [CrossRef]
- Touboul, M.; Betourne, E. LiB2O3(OH)·H2O as precursor of lithium boron oxide LiB2O3,5: Synthesis and dehydration process. Solid State Ion. 1957, 63, 340–345. [Google Scholar] [CrossRef]
- Akella, A.; Keszler, D.A. Structure and Eu+2 luminescence of dibarium magnesium orthoborate. Mater. Res. Bull. 1995, 30, 105–111. [Google Scholar] [CrossRef]
- Dotsenko, V.P.; Efryshina, N.P.; Berezovskaya, I.V. Luminescence properties of GaBO3: Bi3+. Mater. Lett. 1996, 28, 517–520. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Tian, S.; Liao, F.; Lin, J. Synthesis, and Structure of [C2H10N2][B5O8(OH)]: A Nonmetal Pentaborate with Nonlinear Optical Properties. Cryst. Growth Des. 2007, 7, 1246–1250. [Google Scholar] [CrossRef]
- Liu, H.X.; Liang, Y.X.; Jiang, X. Synthesis, crystal structure, and NLO property of a nonmetal pentaborate [C6H13N2][B5O6(OH)4]. J. Solid State Chem. 2008, 181, 3243–3247. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.D.A.; Sanchez, C.; Lebeau, B.; Patarin, J. Chemical Strategies to Design Textured Materials: From Microporous and Mesoporous Oxides to Nanonetworks and Hierarchical Structures. Chem. Rev. 2002, 102, 4093–4138. [Google Scholar] [CrossRef] [PubMed]
- Merlino, S.; Sartori, F. Ammonioborite: New borate polyion and its structure. Science 1971, 17, 377–379. [Google Scholar] [CrossRef]
- Schubert, D.M.; Visi, M.Z.; Knobler, C.B.; Khan, S. Synthesis and Structure of a New Heptaborate Oxoanion Isomer: B7O9(OH)52−. Inorgonic Chem. 2008, 47, 4740–4745. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Horton, P.N.; Coles, S.J.; Kose, D.A.; Kreuziger, A.M. Structural and thermal studies of non-metal cation pentaborate salts with cations derived from 1,5-diazobicyclo [4.3.0]non-5-ene, 1,8-diazobicyclo[5.4.0]undec-7-ene and 1,8-bis(dimethylamino)naphthalene. Polyhedron 2012, 38, 157–161. [Google Scholar] [CrossRef]
- Schubert, D.M.; Smith, R.A.; Visi, M.Z. Studies of crystalline nonmetal borates. Soc. Glass Technol. 2003, 44, 63–70. [Google Scholar]
- Köse, D.A.; Beckett, M.A.; Çolak, N. Synthesis, Spectroscopic and Thermal Characterization of Non-Metal Cation (NMC) Pentaborates Salts Containing Cations Derived from Histidine and Arginine. Hacet. J. Biol. Chem. 2012, 40, 219–225. [Google Scholar]
- Schubert, D.M.; Visi, M.Z.; Knobler, C.B. Guanidinium and Imidazolium Borates Containing the First Examples of an Isolated Nonaborate Oxoanion: [B9O12(OH)6]3−. Inorg. Chem. 2000, 39, 2250–2251. [Google Scholar] [CrossRef] [PubMed]
- Vineyard, B.D.; Godt, H.C., Jr. A Study of the Reaction of Boric Acid with Amines: Hydroxyboroxin-Amine Salts. Inorgonic Chem. 1964, 3, 1144–1147. [Google Scholar] [CrossRef]
- Wiebcke, M.; Freyhardt, C.C.; Felsche, J.; Englehardt, G. Clathrates with three-dimensional host structures of hydrogen-bonded pentaborate [B5O6(OH)4]− ions: Pentaborates with the cations NMe4+, NEt4+, NPhMe3+ and pipH+ (pipH+ = piperidinium). Verl. Der Z. Für Nat. Sect. B 1993, 48, 978–985. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zheng, S.T.; Yang, G.Y. Tetramethylammonium pentaborate 0,25-hydrate. Acta Crystallogr. 2004, C60, o545–o546. [Google Scholar] [CrossRef]
- Baber, R.A.; Charmant, J.P.H.; Norman, N.C.; Orpen, A.G.; Rossi, J. Dimethyl ammonium tetrahydro pentaborate. Acta Crystallogr. 2004, E60, 1086–1088. [Google Scholar] [CrossRef]
- Wang, G.M.; Sun, Y.Q.; Yang, G.Y. Syntheses and crystal structures of three new borates template by transition-metal complexes in situ. J. Solid State Chem. 2006, 179, 1545–1553. [Google Scholar] [CrossRef]
- Visi, M.Z.; Knobler, C.B.; Owen, J.J.; Khan, M.I.; Schubert, D.M. Structures of Self-Assembled Nonmetal Borates Derived from α, ω-Diaminoalkanes. Cryst. Growth Des. 2006, 6, 538–545. [Google Scholar] [CrossRef]
- Peterson, R.C.; Finkelstein, M.; Ross, S.D. Quaternary Ammonium Polyborates. J. Am. Chem. Soc. 1959, 81, 3264–3267. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogels Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific and Technical: Exes, UK, 1989; pp. 971–973. [Google Scholar]
- Bland, C.C. Standard Methodology Using Dowex 550A Anion (Cl−/OH−) Exchange Resin, Experience of Using Ion Exchange in the Applicants Laboratories. Ph.D. Thesis, University of Wales, Bangor, UK, 12 May 2007. [Google Scholar]
- Heller, G. Die Hydrolyse von Borsäuretrimethylester in Gegenwart organischer Basen. J. Inorg. Nucl. Chem. 1968, 30, 2743–2754. [Google Scholar] [CrossRef]
- Beckett, M.A.; Bland, C.C.; Horton, P.N.; Hursthouse, M.B.; Varma, K.S.J. An Unprecedented Polyborate Ester Anion: X-ray Diffraction Studies on [1,8-C10H6(NMe2)2H][B5O6(OMe)4]. Inorg. Chem. 2007, 46, 3801–3803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Zheng, S.T.; Yang, G.Y. Pentaethylenehexamine manganese(II) pentaborate. Acta Crystallogr. 2004, C60, 545–546. [Google Scholar] [CrossRef]
- Hosmane, N.S. Boron Science. New Technologies and Applications; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2012; p. 422. [Google Scholar]
- Weir, C.E.; Schroeder, R. Infrared Spectra of Crystalline Inorganic Borates. J. Res. Natl. Bur. Stand. 1964, 68, 465–487. [Google Scholar] [CrossRef]
- Weir, C.E. Infrared spectra of the hydrated borates. J. Res. Natl. Bur. Stand. 1966, 70, 153–164. [Google Scholar] [CrossRef]
- Krough-Moe, J. Interpretation of the Infrared Spectra of Boron. Oxide and Alkali Borate Glasses. Phys. Chem. Glasses 1965, 6, 46–54. [Google Scholar]
- Yang, Y.; Wang, Y.; Sun, J.; Cui, M.; Meng, C. Synthesis, Crystal Structure, and Characterization of a Novel Metallo Organically-Templated Pentaborate with Mixed Ligands. Z. Anorg. Allg. Chem. 2011, 637, 729–734. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhang, J.J.; Zhang, W.J. Synthesis, crystal structure and vibrational spectroscopy of a novel mixed ligands Ni(II) pentaborate: [Ni(C4H10N2)(C2H8N2)2] [B5O6(OH)4]2. Inorg. Chim. Acta 2006, 359, 519–524. [Google Scholar] [CrossRef]
- Salentine, C.G. High-field boron-11 NMR of alkali borates. Aqueous polyborate equilibria. Inorg. Chem. 1983, 22, 3920–3924. [Google Scholar] [CrossRef]
- Beckett, M.A. Recent advances in crystalline hydrated borates with non-metal or transition-metal complex cations. Coord. Chem. Rev. 2016, 323, 2–14. [Google Scholar] [CrossRef]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Knox, D.A.; Timmis, J.L. Structural (XRD) and thermal (DSC, TGA) and BET analysis of materials derived from non-metal cation pentaborate salts. Dalton Trans. 2010, 39, 3944–3951. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Coles, S.J.; Davies, R.A.; Horton, P.N.; Jones, C.L. Pentaborate(1−) salts templated by substituted pyrrolidinium cations: Synthesis, structural characterization, and modelling of solid-state H-bond interactions by DFT calculations. Dalton Trans. 2015, 44, 7032–7040. [Google Scholar] [CrossRef] [PubMed]
- Munirathnam, B.; Madhavan, J. Investigations on the Structural, Mechanical and Photoconductive Studies of Pure and Lanthanum Doped Potassium Pentaborate Single Crystals. Indian J. Sci. Technol. 2009, 2, 44–45. [Google Scholar] [CrossRef]
- Bilcen, S. Investigation of the Kinetics of Reactions with Some Group B Metals of dl-Tryptophan and l-Phenylalanine from Amino Acids Containing Aromatic Rings and Clarifying the Structure of the Products Obtained. Master’s Thesis, Trakya University Institute of Science and Technology, Edirne, Turkey, 25 April 2008. [Google Scholar]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis fo a metal organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Cho, J.H.; Nahm, K.S.; Park, C.R. Enhanced hydrogen storage capacity of Pt-loaded CNT@MOF-5 hybrid composites. Int. J. Hydrogen Energy 2010, 35, 13062–13067. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

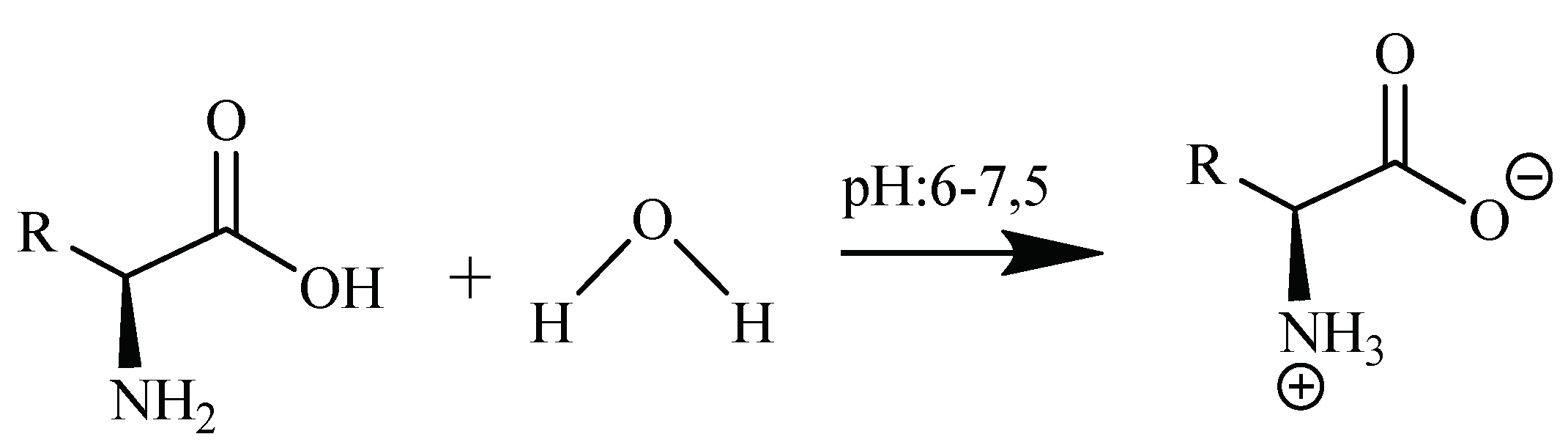
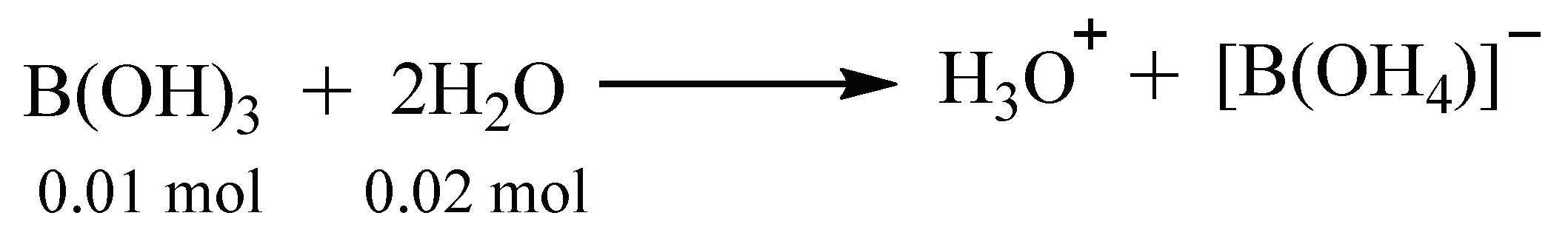
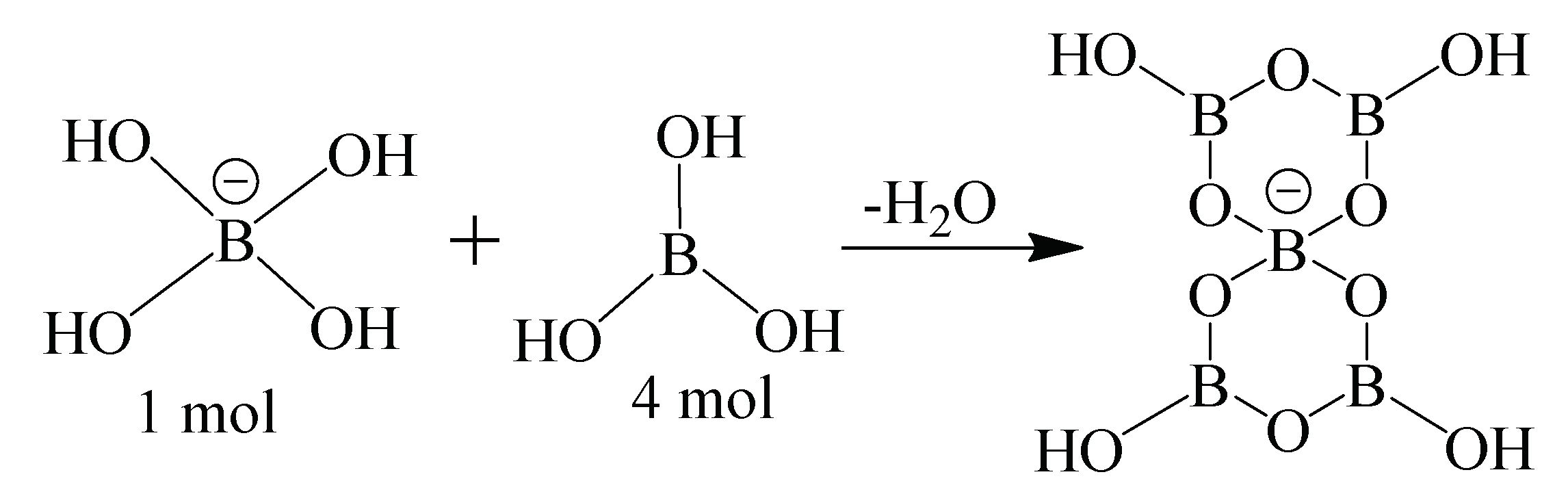

| Compound | Color | MP (°C) | MW g/mol | Yield (%) | Chemical Composition Exp. (Calc.) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| [C5H11NO2][B5O6(OH)4]H2O C5H17B5NO13 (I) | White | 134 | 345.24 | 65 | 18.97 (17.39) | 5.41 (4.96) | 3.89 (4.06) |
| [C6H13NO2][B5O6(OH)4]1/2H2O C6H18B5NO12,5 (II) | White | 122 | 368.28 | 71 | 19.91 (19.57) | 5.62 (5.47) | 3.87 (3.80) |
| [C6H13NO2][B5O6(OH)4]H2O C6H19B5NO13 (III) | White | 121 | 368.28 | 79 | 19.69 (19.57) | 5.81 (5.47) | 3.92 (3.80) |
| [C4H9NO3][B5O6(OH)4]H2O C4H15B5NO14 (IV) | White | 123 | 356.22 | 69 | 13.01 (13.49) | 4.88 (4.53) | 3.77 (3.93) |
| Groups | Valine Pentaborate (I) | Leucine Pentaborate (II) | Isoleucine Pentaborate (III) | Threonine Pentaborate (IV) |
|---|---|---|---|---|
| ν(-OH)H2O | 3378 | 3439 | 3383 | 3346 |
| ν(-NH) | 3214 | - | 3216 | 3213 |
| ν(-NH2) | 1666 | - | 1680 | 1678 |
| ν(-B-O)BO3 | 698 | 682 | 696 | 694 |
| ν(-B-O)BO3(asym) | 1439 | 1444 | 1432 | 1420 |
| ν(-B-O)BO3(sym) | - | 1329 | 1338 | 1334 |
| ν(-B-O-H) | 1199/1166 | 1194/1167 | 1193/1163 | 1195/1167 |
| ν(-B-O)BO4(asym) | 1026 | 1035 | 1024 | 1032 |
| ν(-B-O)BO4(sym) | 927 | 923 | 929 | 927 |
| Complex | Temp. Range (°C) | DTAmax. (°C) | Removed Group | Mass Loss (%) | Remaining Product (%) | Decomp. Product | Color | ||
|---|---|---|---|---|---|---|---|---|---|
| Found | Calc. | Found | Clac. | ||||||
| C5H17B5NO13 | 35–112 | 82 | -H2O | 2.03 | 2.61 | White | |||
| 345.24 g/mol | 113–156 | 144 | -2H2O, NH3 | 16.72 | 15.35 | ||||
| (I) | 157–580 | 172, 295, 460 | -C5H8O2,5 | 28.92 | 29.58 | 48.97 | 50.69 | 5/2 B2O3 | Black |
| C6H18B5NO12,5 | 37–97 | 64 | -H2O | 3.96 | 4.89 | White | |||
| 368.28 g/mol | 98–219 | 117, 144 | -2H2O, NH3 | 14.88 | 14.39 | ||||
| (II) | 220–720 | 256, 582 | -C6H9O2 | 30.17 | 31.54 | 45.97 | 47.52 | 5/2 B2O3 | Black |
| C6H19B5NO13 | 31–144 | 77 | -H2O | 4.02 | 4.89 | White | |||
| 368.28 g/mol | 102–145 | 130 | -2H2O, NH3 | 15.92 | 14.39 | ||||
| (III) | 146–712 | 181, 235, 317, 601 | -C6H10O2,5 | 30.82 | 31.54 | 46.19 | 47.52 | 5/2 B2O3 | Black |
| C4H15B5NO14 | 33–90 | 75 | -H2O | 3.27 | 4.08 | White | |||
| 356.22 g/mol | 91–150 | 131 | -2H2O, NH3 | 16.11 | 15.86 | ||||
| (IV) | 152–655 | 170, 325, 510 | -C4H6O3,5 | 39.07 | 39.93 | 38.69 | 39.66 | 5/2 B2O3 | Black |
| Molecules | Surface Area (m2/g) |
|---|---|
| [C5H11NO2][B5O6(OH)4]H2O C5H17B5NO13 (I) | 3.286 |
| [C6H13NO2][B5O6(OH)4]1/2H2O C6H18B5NO12.5 (II) | 1.873 |
| [C6H13NO2][B5O6(OH)4]H2O C6H29B5NO13 (III) | 2.309 |
| [C4H9NO3][B5O6(OH)4]H2O C4H15B5NO14 (IV) | 1.860 |
| Molecules | Mass Storage (%) | Volumetric Storage (mL/g) |
|---|---|---|
| [C5H11NO2][B5O6(OH)4]H2O C5H17B5NO13 (I) | 0.039 | 0.091 |
| [C6H13NO2][B5O6(OH)4]1/2H2O C6H18B5NO12.5 (II) | 0.047 | 0.115 |
| [C6H13NO2][B5O6(OH)4]H2O C6H29B5NO13 (III) | 0.055 | 0.133 |
| [C4H9NO3][B5O6(OH)4]H2O C4H15B5NO14 (IV) | 0.063 | 0.155 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sızır, Ü.; Yurdakul, Ö.; Köse, D.A.; Akkurt, F. Novel Non-Metal Cation (NMC) Pentaborate Salts of Some Amino Acids. Molecules 2019, 24, 2790. https://doi.org/10.3390/molecules24152790
Sızır Ü, Yurdakul Ö, Köse DA, Akkurt F. Novel Non-Metal Cation (NMC) Pentaborate Salts of Some Amino Acids. Molecules. 2019; 24(15):2790. https://doi.org/10.3390/molecules24152790
Chicago/Turabian StyleSızır, Ümit, Ömer Yurdakul, Dursun Ali Köse, and Fatih Akkurt. 2019. "Novel Non-Metal Cation (NMC) Pentaborate Salts of Some Amino Acids" Molecules 24, no. 15: 2790. https://doi.org/10.3390/molecules24152790
APA StyleSızır, Ü., Yurdakul, Ö., Köse, D. A., & Akkurt, F. (2019). Novel Non-Metal Cation (NMC) Pentaborate Salts of Some Amino Acids. Molecules, 24(15), 2790. https://doi.org/10.3390/molecules24152790