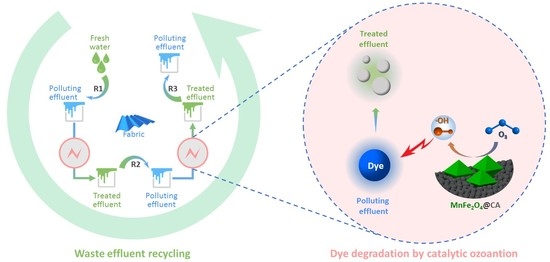
Removal of Reactive Dyes in Textile Effluents by Catalytic Ozonation Pursuing on-Site Effluent Recycling
Abstract
1. Introduction
2. Results and Discussion
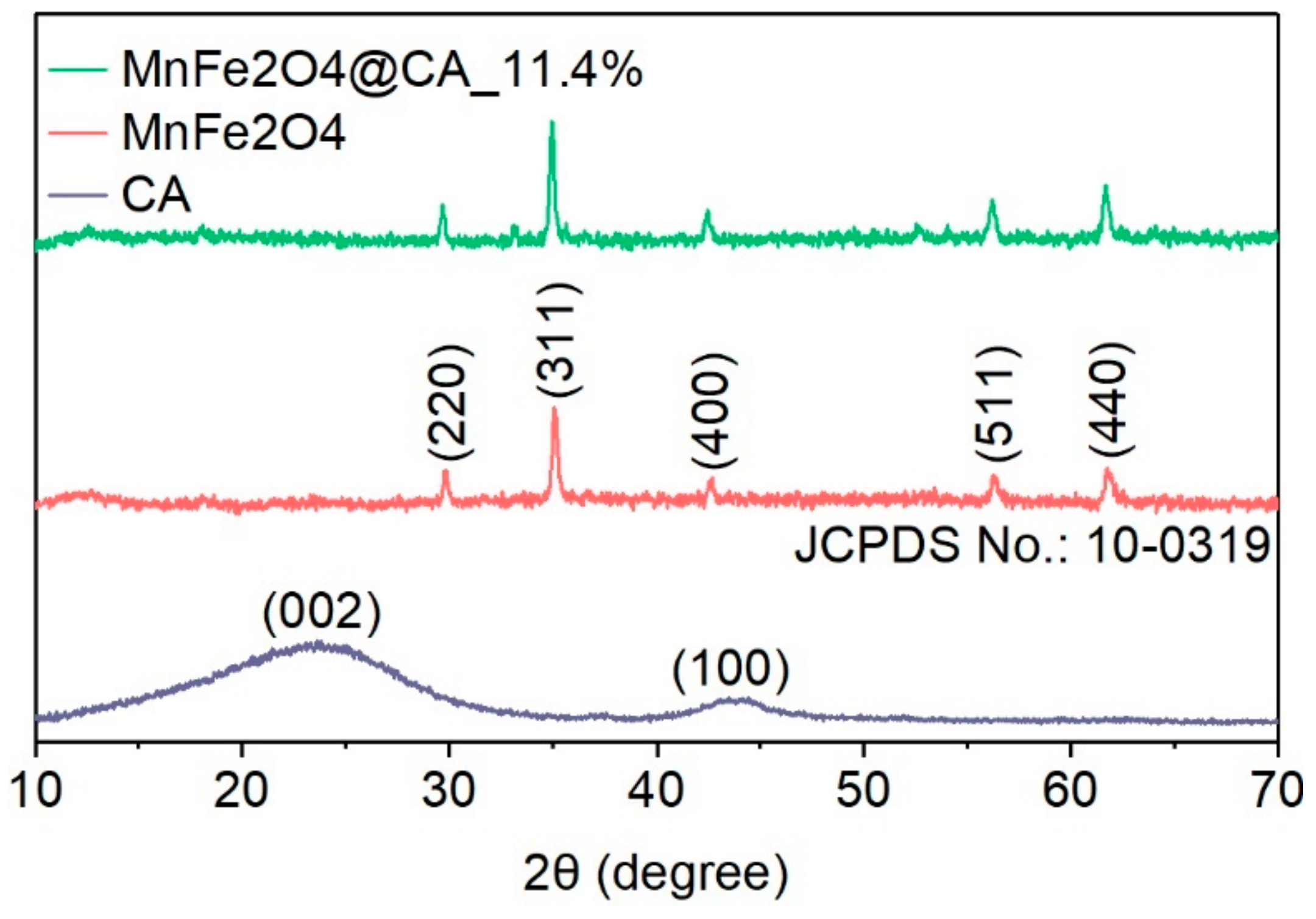
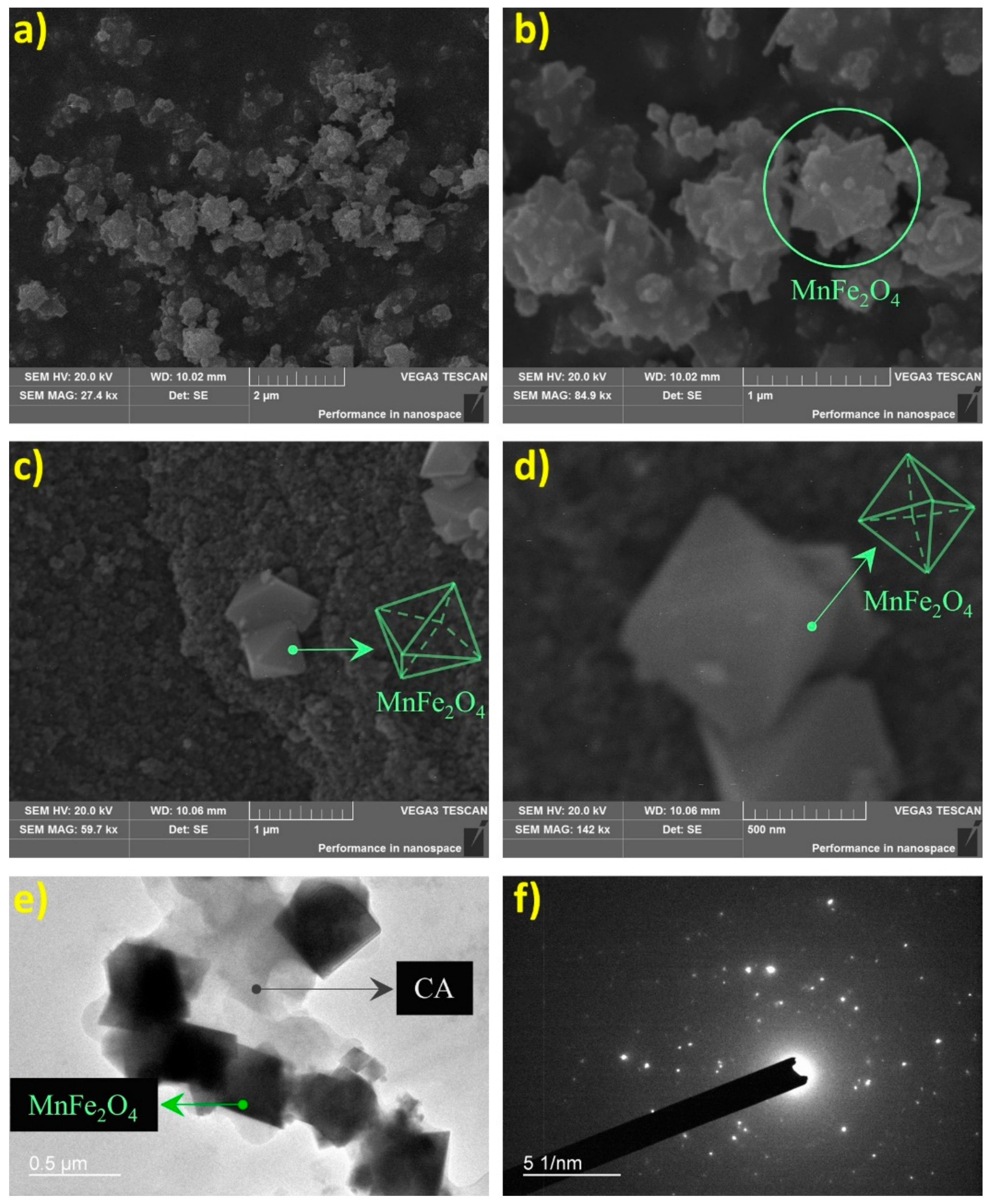
2.1. Characterization of Catalyst Materials
2.2. Degradation of Reactive Dyes in Spent Wash-Off Effluents
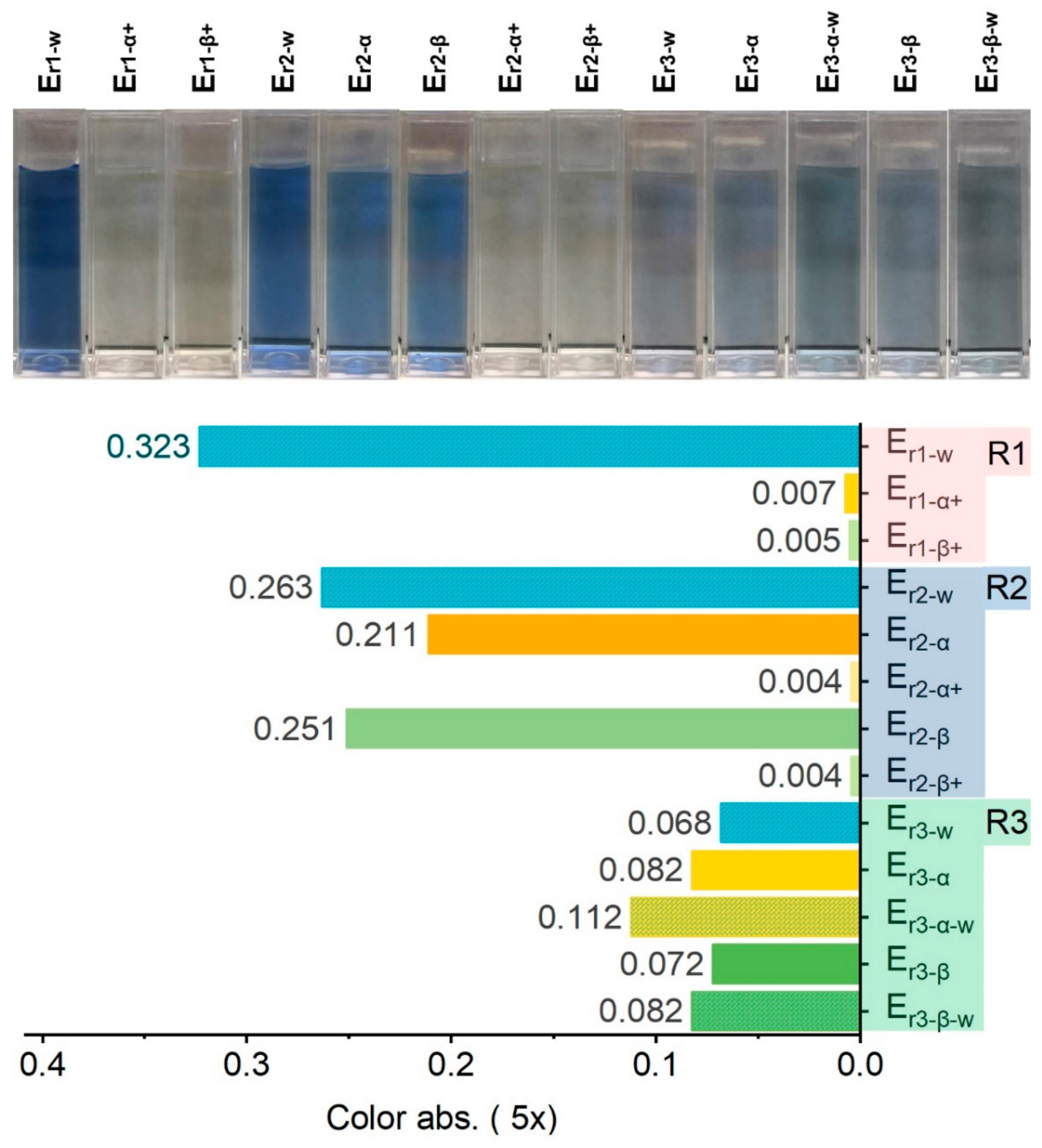
2.2.1. Color Removal in the Spent Rinsing Effluent (Er1)
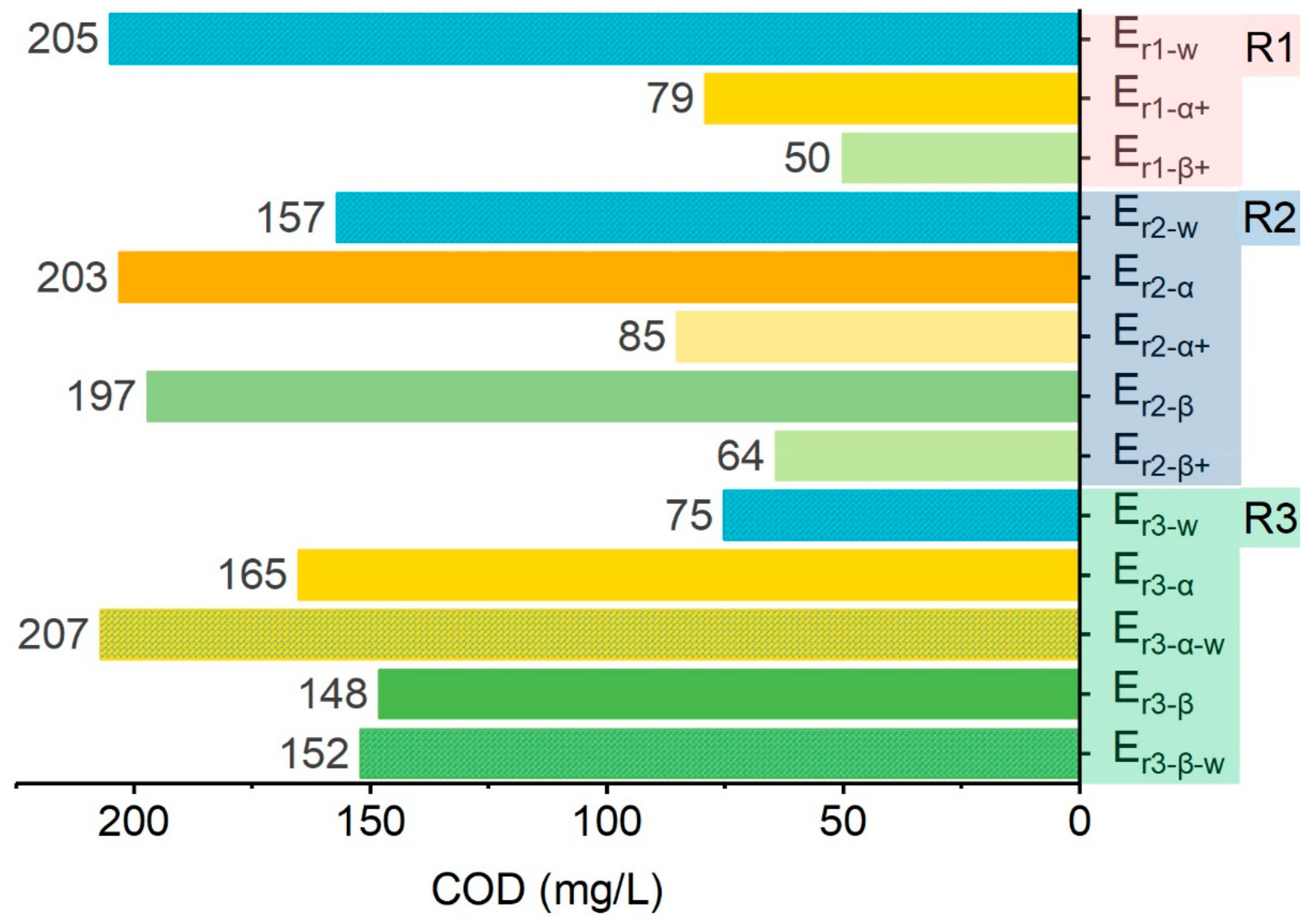
2.2.2. COD Elimination in Spent Rinsing Effluents (Er1)
2.2.3. Water Quality of Effluents in Recycling
2.3. Determination of Fabric Color Quality
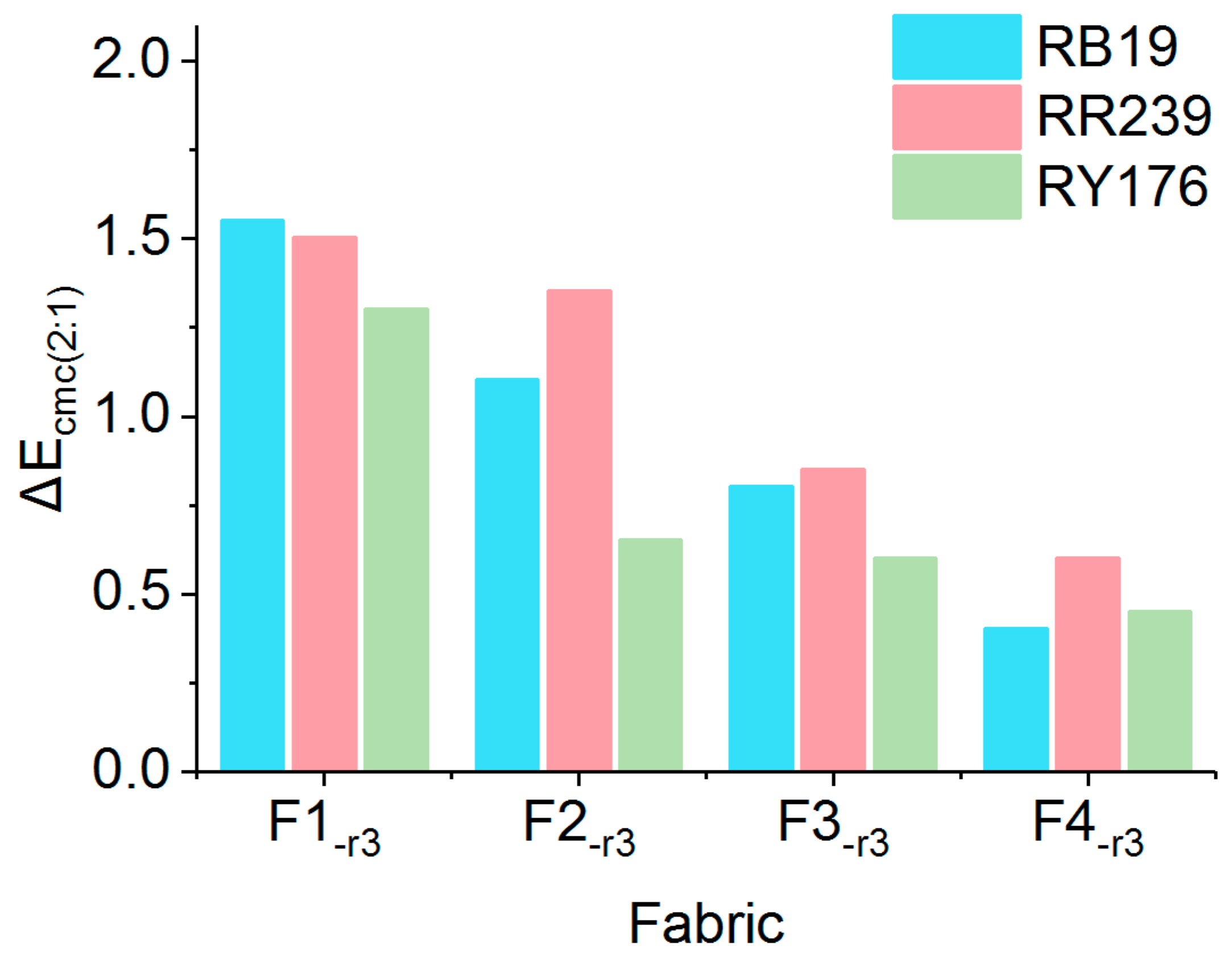
2.3.1. Color Reproducibility
2.3.2. Colorfastness
2.3.3. Color Evenness
3. Materials and Methods
3.1. Chemicals and Fabrics
3.2. Catalyst Preparation
3.3. Experimental Procedure
3.3.1. Fabric Dyeing
3.3.2. Effluent Recycling in Wash-Off Process
3.3.3. Ozonation Treatment of Rinsing Effluents
3.4. Analytical Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, A.; Xie, K.; Gao, A. Smart color-changing paper packaging sensors with pH sensitive chromophores based on azo-anthraquinone reactive dyes. Sens. Actuat. B Chem. 2019, 286, 362–369. [Google Scholar] [CrossRef]
- Shang, S.M. Process control in dyeing of textiles. In Process Control in Textile Manufacturing; Majumdar, A., Das, A., Alagirusamy, R., Kothari, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 300–338. [Google Scholar]
- Zhang, X.Q.; Fang, K.J.; Zhang, J.F.; Shu, D.W.; Gong, J.X.; Liu, X.M. A vacuum-dehydration aided pad-steam process for improving reactive dyeing of cotton fabric. J. Clean Prod. 2017, 168, 1193–1200. [Google Scholar] [CrossRef]
- Pisitsak, P.; Tungsombatvisit, N.; Singhanu, K. Utilization of waste protein from Antarctic krill oil production and natural dye to impart durable UV-properties to cotton textiles. J. Clean Prod. 2018, 174, 1215–1223. [Google Scholar] [CrossRef]
- Samanta, K.K.; Pandit, P.; Samanta, P.; Basak, S. Water consumption in textile processing and sustainable approaches for its conservation. In Water in Textiles and Fashion; Muthu, S.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 41–59. [Google Scholar]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.-Á. Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci. Total Environ. 2019, 651, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M. Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J. Clean Prod. 2019, 223, 837–848. [Google Scholar] [CrossRef]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Ozturk, E.; Koseoglu, H.; Karaboyaci, M.; Yigit, N.O.; Yetis, U.; Kitis, M. Minimization of water and chemical use in a cotton/polyester fabric dyeing textile mill. J. Clean Prod. 2016, 130, 92–102. [Google Scholar] [CrossRef]
- Alkaya, E.; Demirer, G.N. Sustainable textile production: a case study from a woven fabric manufacturing mill in Turkey. J. Clean Prod. 2014, 65, 595–603. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using Tectona grandis sawdust as a very low-cost adsorbent. Sci. Rep. UK 2018, 8, 8314. [Google Scholar] [CrossRef] [PubMed]
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 2019, 358, 992–1001. [Google Scholar] [CrossRef]
- Hu, E.; Shang, S.; Tao, X.; Jiang, S.; Chiu, K. Regeneration and reuse of highly polluting textile dyeing effluents through catalytic ozonation with carbon aerogel catalysts. J. Clean Prod. 2016, 137, 1055–1065. [Google Scholar] [CrossRef]
- Silva, L.G.M.; Moreira, F.C.; Souza, A.A.U.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Chemical and electrochemical advanced oxidation processes as a polishing step for textile wastewater treatment: A study regarding the discharge into the environment and the reuse in the textile industry. J. Clean Prod. 2018, 198, 430–442. [Google Scholar] [CrossRef]
- Morali, E.K.; Uzal, N.; Yetis, U. Ozonation pre and post-treatment of denim textile mill effluents: Effect of cleaner production measures. J. Clean Prod. 2016, 137, 1–9. [Google Scholar] [CrossRef]
- Afzal, S.; Quan, X.; Lu, S. Catalytic performance and an insight into the mechanism of CeO2 nanocrystals with different exposed facets in catalytic ozonation of p-nitrophenol. Appl. Catal. B-Environ. 2019, 248, 526–537. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Muthukumar, M. Studies on the possibility of recycling reactive dye bath effluent after decolouration using ozone. Dyes Pigment. 2007, 72, 251–255. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Vishnu, G.; Joseph, K. Ozonation of light-shaded exhausted reactive dye bath for reuse. Dyes Pigment. 2007, 75, 273–278. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, K.; Cheng, X.; Xu, Y.Y.; Jia, Q.B. Study on optimizing production scheduling for water-saving in textile dyeing industry. J. Clean Prod. 2017, 141, 721–727. [Google Scholar] [CrossRef]
- Amin, M.N.; Blackburn, R.S. Sustainable Chemistry Method to Improve the Wash-off Process of Reactive Dyes on Cotton. ACS Sustain. Chem. Eng. 2015, 3, 725–732. [Google Scholar] [CrossRef]
- He, B.Y.; Wang, X.C.; Xue, H.Y. The performance of chitosan/gelatin composite microspheres in the wash-off procedure of reactive dyeing. Color. Technol. 2016, 132, 353–360. [Google Scholar] [CrossRef]
- Liu, J.; Ke, L.; Liu, J.; Sun, L.; Yuan, X.; Li, Y.; Xia, D. Enhanced catalytic ozonation towards oxalic acid degradation over novel copper doped manganese oxide octahedral molecular sieves nanorods. J. Hazard. Mater. 2019, 371, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gu, W.; Li, G.; Wang, Q.; Liang, P.; Zhang, X.; Huang, X. Significant enhancement in catalytic ozonation efficacy: From granular to super-fine powdered activated carbon. Front Env. Sci. Eng. 2017, 12, 6. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Sheng, D.; Lian, H. Spinel-type manganese ferrite (MnFe2O4) microspheres: A novel affinity probe for selective and fast enrichment of phosphopeptides. Talanta 2017, 166, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.; Ren, Y.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.-H.; Kang, S.-H.; Choi, C.-J.; Rajesh, J.A.; Ahn, K.-S. Facile hydrothermal synthesis of cubic spinel AB2O4 type MnFe2O4 nanocrystallites and their electrochemical performance. Appl. Surf. Sci. 2017, 413, 83–91. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Q.; Wang, C.; Ma, Z.; Sun, Q.; Fan, B.; Jin, C.; Chen, Y. Hydrothermal synthesis of nanooctahedra MnFe2O4 onto the wood surface with soft magnetism, fire resistance and electromagnetic wave absorption. Nanomaterials (Basel) 2017, 7, 118. [Google Scholar] [CrossRef]
- Jin, R.C.; Jiang, Y.T.; Li, G.H.; Meng, Y.F. Amorphous carbon coated multiwalled carbon nanotubes@transition metal sulfides composites as high performance anode materials for lithium ion batteries. Electrochim. Acta 2017, 257, 20–30. [Google Scholar] [CrossRef]
- Li, Z.; Gao, K.; Han, G.; Wang, R.; Li, H.; Zhao, X.S.; Guo, P. Solvothermal synthesis of MnFe2O4 colloidal nanocrystal assemblies and their magnetic and electrocatalytic properties. New J. Chem. 2015, 39, 361–368. [Google Scholar] [CrossRef]
- Pang, H.; Sahu, R.P.; Duan, Y.; Puri, I.K. MnFe2O4-coated carbon nanotubes with enhanced microwave absorption: Effect of CNT content and hydrothermal reaction time. Diam. Relat. Mater 2019, 96, 31–43. [Google Scholar] [CrossRef]
- Yu, L.; Shang, X.; Chen, H.; Xiao, L.; Zhu, Y.; Fan, J. A tightly-bonded and flexible mesoporous zeolite-cotton hybrid hemostat. Nat. Commun. 2019, 10, 1932. [Google Scholar] [CrossRef]
- Jiao, H. Preparation and Magnetic Properties of MnFe2O4 Octahedral Microcrystals. J. Mater Eng. Perform. 2011, 20, 1638–1641. [Google Scholar] [CrossRef]
- Nikolic, A.S.; Boskovic, M.; Spasojevic, V.; Jancar, B.; Antic, B. Magnetite/Mn-ferrite nanocomposite with improved magnetic properties. Mater. Lett. 2014, 120, 86–89. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.J.; Tong, X.; Zhong, L.X.; Peng, X.W.; Sun, R.C. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crop. Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Ueda Yamaguchi, N.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4–graphene hybrid composite for efficient removal of glyphosate from water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Wang, M.; Shao, C.; Zhou, S.; Yang, J.; Xu, F. Preparation of carbon aerogels from TEMPO-oxidized cellulose nanofibers for organic solvents absorption. Rsc Adv. 2017, 7, 38220–38230. [Google Scholar] [CrossRef]
- Tuan Nguyen, D.H.; Nguyen, T.H.; Nguyen, T.T.; Le Thi, K.A.; Nguyen, D.T.; Phuong Bui, T.Q.; Bach, G.L. The Preparation and Characterization of MnFe2O4-Decorated Expanded Graphite for Removal of Heavy Oils from Water. Materials 2019, 12, 1913. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, M.; Gharabaghi, M.; Abdollahi, H.; Boroumand, Z.; Moradian, M. MnFe2O4-graphene oxide magnetic nanoparticles as a high-performance adsorbent for rare earth elements: Synthesis, isotherms, kinetics, thermodynamics and desorption. J. Hazard. Mater. 2018, 351, 308–316. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, B.; Liu, S.; Meng, Z.; Chen, Z.; Zou, C.; Liu, C.; Chen, F.; Zhou, X. Photo-Fenton degradation of ammonia via a manganese–iron double-active component catalyst of graphene–manganese ferrite under visible light. Chem. Eng. J. 2016, 283, 266–275. [Google Scholar] [CrossRef]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Wong, K.C.; Dravid, V.P. Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett. 2004, 4, 383–386. [Google Scholar] [CrossRef]
- Rajkumar, D.; Song, B.J.; Kim, J.G. Electrochemical degradation of Reactive Blue 19 in chloride medium for the treatment of textile dyeing wastewater with identification of intermediate compounds. Dyes Pigment. 2007, 72, 1–7. [Google Scholar] [CrossRef]
- Vittenet, J.; Aboussaoud, W.; Mendret, J.; Pic, J.S.; Debellefontaine, H.; Lesage, N.; Faucher, K.; Manero, M.H.; Thibault-Starzyk, F.; Leclerc, H.; et al. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal. A-Gen. 2015, 504, 519–532. [Google Scholar] [CrossRef]
- Oulton, R.; Haase, J.P.; Kaalberg, S.; Redmond, C.T.; Nalbandian, M.J.; Cwiertny, D.M. Hydroxyl radical formation during ozonation of multiwalled carbon nanotubes: performance optimization and demonstration of a reactive CNT filter. Environ. Sci. Technol. 2015, 49, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B-Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Hu, C.; Xing, S.T.; Qu, J.H.; He, H. Catalytic ozonation of herbicide 2,4-D over cobalt oxide supported on mesoporous zirconia. J. Phys. Chem. C 2008, 112, 5978–5983. [Google Scholar] [CrossRef]
- Rosa, J.M.; Tambourgi, E.B.; Curvelo Santana, J.C.; de Campos Araujo, M.; Ming, W.C.; Trindade, N. Development of colors with sustainability: A comparative study between dyeing of cotton with reactive and vat dyestuffs. Text Res. J. 2014, 84, 1009–1017. [Google Scholar] [CrossRef]
- Li, C.H.; He, J.X. Adsorption of a spent reactive dyebath by a chitosan bed: study of water reuse, bed regeneration, and UV/Fenton oxidation. Color. Technol. 2014, 130, 93–101. [Google Scholar] [CrossRef]
- Hu, E.; Wu, X.; Shang, S.; Tao, X.; Jiang, S.; Gan, L. Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J. Clean Prod. 2016, 112. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Evaluating analytical methods for the characterization of lipids, proteins and carbohydrates in organic substrates for anaerobic co-digestion. Bioresour Technol. 2018, 247, 697–704. [Google Scholar] [CrossRef]
- The American Association of Textile Chemists and Colorists. Test Method 173–2015: CMC: Calculation of small color differences for acceptability. In AATCC Technical Manual 2019; AATCC: Research Triangle Park, NC, USA, 2015; pp. 330–332. [Google Scholar]
- The American Association of Textile Chemists and Colorists. Test Method 61–2013: Colorfastness to laundering: Accelerated. In AATCC Technical Manual 2019; AATCC: Research Triangle Park, NC, USA, 2013; pp. 112–116. [Google Scholar]
- The American Association of Textile Chemists and Colorists. Test Method 8–2016: Colorfastness to laundering, home & commercial: Accelerated. In AATCC Technical Manual 2019; AATCC: Research Triangle Park, NC, USA, 2016; pp. 21–23. [Google Scholar]
- Kan, C.W.; Cheung, H.F.; Chan, Q. A study of plasma-induced ozone treatment on the colour fading of dyed cotton. J. Clean Prod. 2016, 112, 3514–3524. [Google Scholar] [CrossRef]
- Chong, C.L.; Li, S.Q.; Yeung, K.W. An objective method for the assessment of levelness of dyed materials. J. Soc. Dyers Colour. 1992, 108, 528–530. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
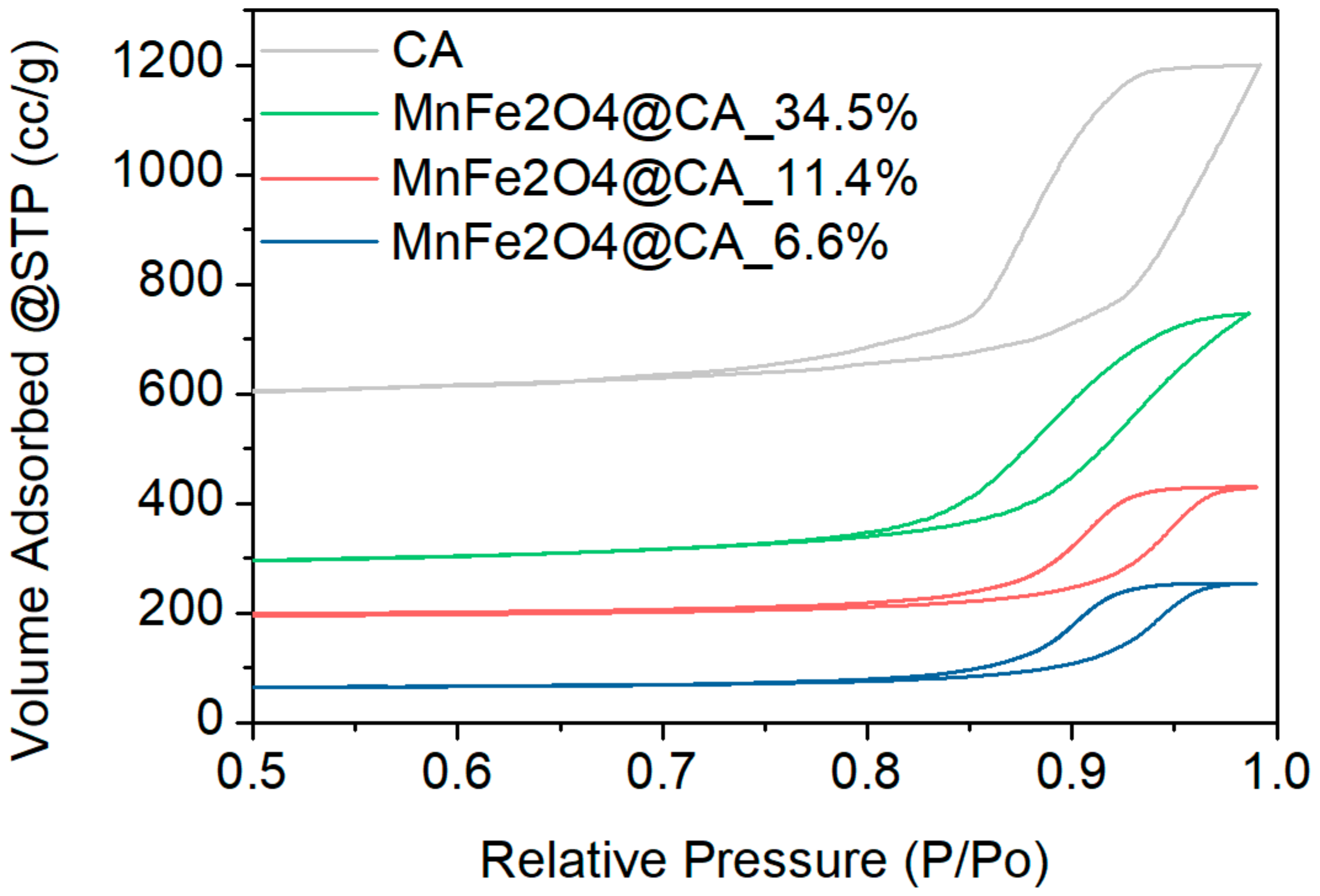

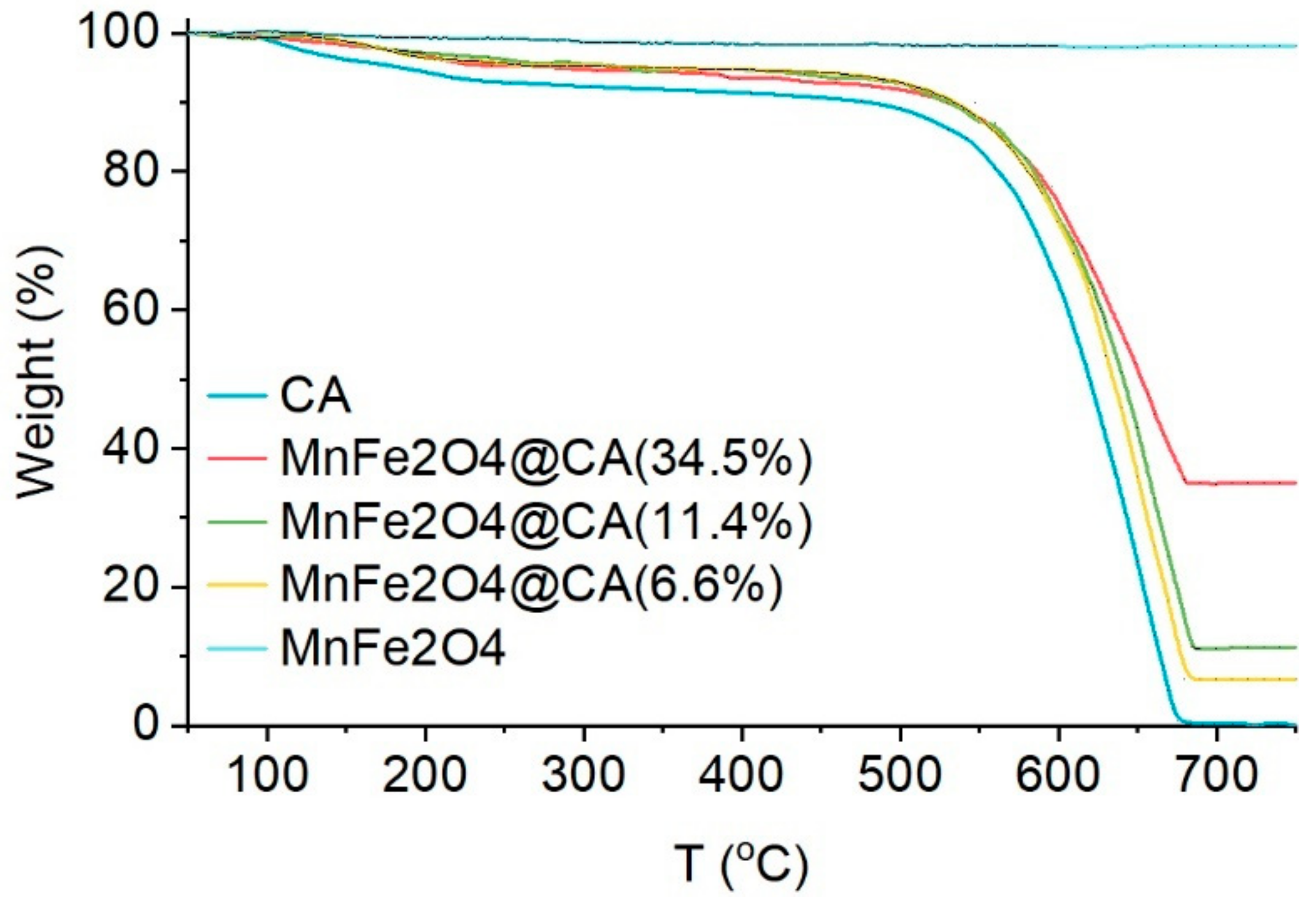

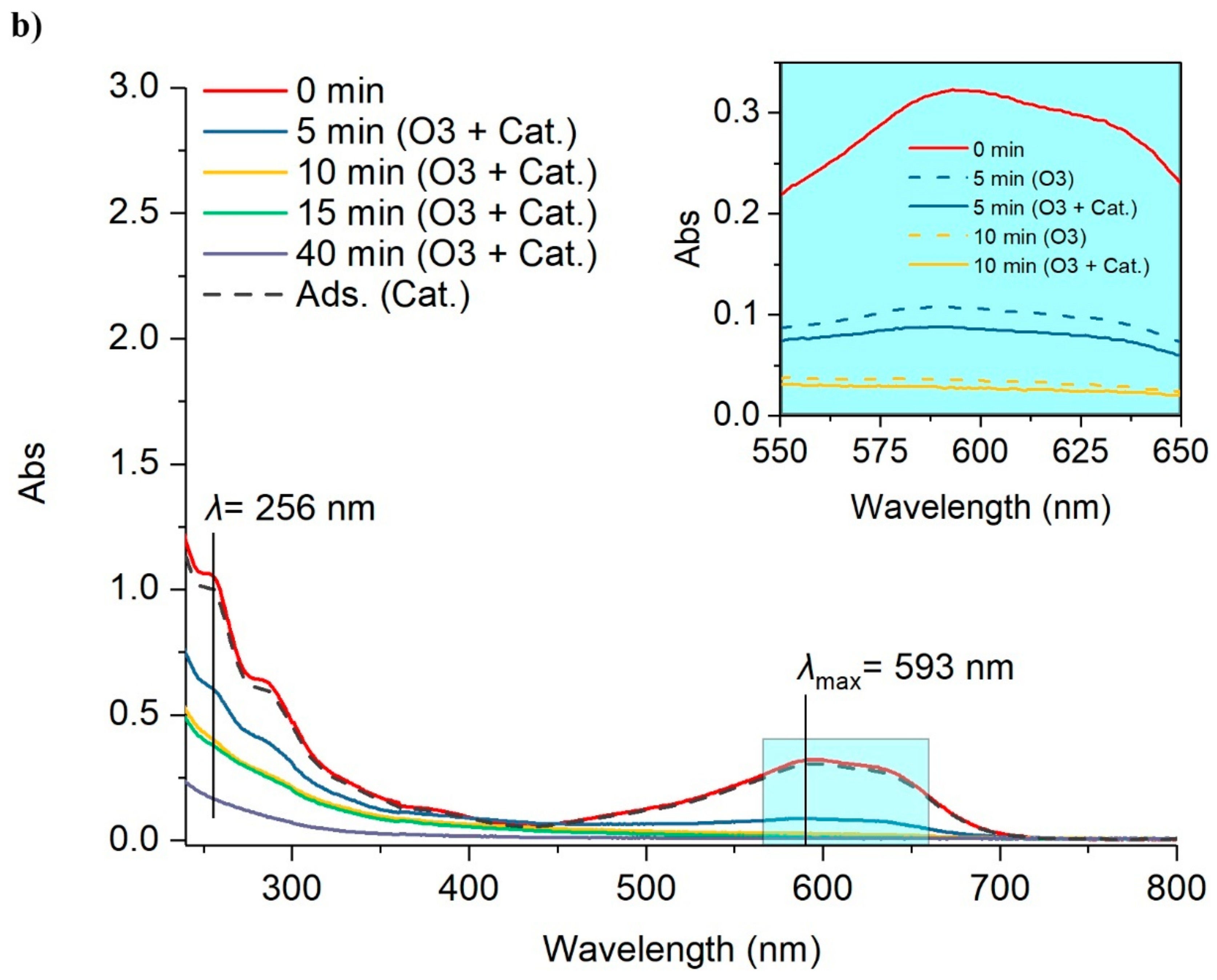
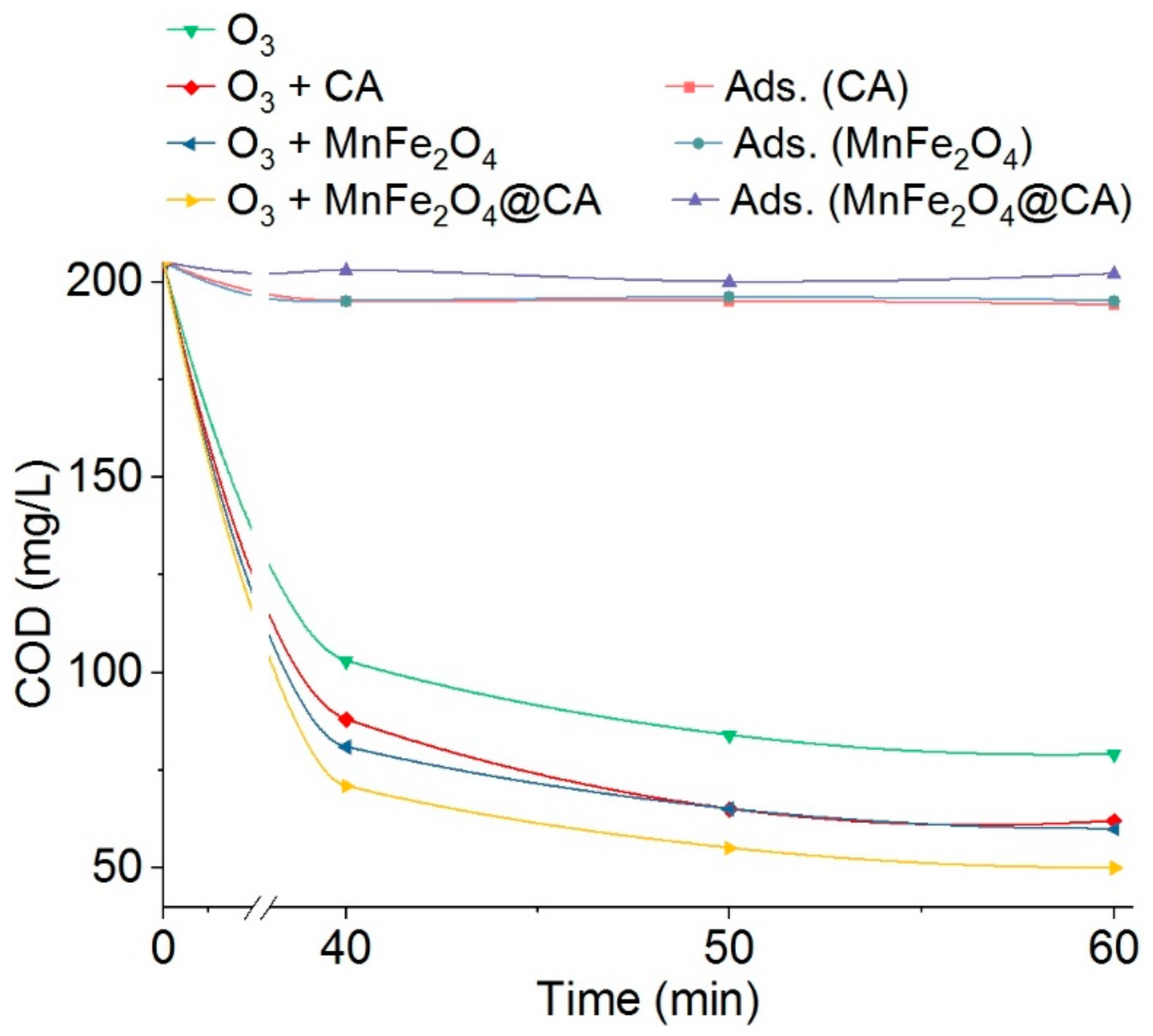


| Materials | Pore Volume (cc/g) | Pore Radius (nm) | BET (m2/g) |
|---|---|---|---|
| MnFe2O4@CA 34.5% | 0.81496 | 9.07018 | 378.565 |
| MnFe2O4@CA 11.4% | 0.99843 | 10.3588 | 467.092 |
| CA | 1.06902 | 8.76554 | 586.664 |
| Dyes | Fabric | Laundering | Crocking | ||
|---|---|---|---|---|---|
| Staining (Cotton) | Change | Dry | Wet | ||
| RB19 | F0 | 3.5 | 3 | 3 | 2 |
| F1−r3 | 4 | 3.5 | 3.5 | 3 | |
| F2−r3 | 4 | 4 | 4 | 3.5 | |
| F3−r3 | 4.5 | 4.5 | 4.5 | 4.5 | |
| F4−r3 | 5 | 4.5 | 5 | 4.5 | |
| F5−r3 | 4.5 | 5 | 5 | 4.5 | |
| RR239 | F0 | 3.5 | 3 | 3 | 2 |
| F1−r3 | 3.5 | 3.5 | 3.5 | 2.5 | |
| F2−r3 | 3.5 | 4 | 4 | 3 | |
| F3−r3 | 4 | 4 | 4 | 3.5 | |
| F4−r3 | 4.5 | 5 | 4.5 | 4 | |
| F5−r3 | 5 | 4.5 | 4.5 | 4 | |
| RY176 | F0 | 3.5 | 3 | 3 | 2.5 |
| F1−r3 | 4 | 3.5 | 3 | 3 | |
| F2−r3 | 4.5 | 4 | 3.5 | 3.5 | |
| F3−r3 | 4.5 | 4.5 | 4 | 4 | |
| F4−r3 | 4.5 | 5 | 4.5 | 4 | |
| F5−r3 | 5 | 4.5 | 4.5 | 4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, E.; Shang, S.; Chiu, K.-L. Removal of Reactive Dyes in Textile Effluents by Catalytic Ozonation Pursuing on-Site Effluent Recycling. Molecules 2019, 24, 2755. https://doi.org/10.3390/molecules24152755
Hu E, Shang S, Chiu K-L. Removal of Reactive Dyes in Textile Effluents by Catalytic Ozonation Pursuing on-Site Effluent Recycling. Molecules. 2019; 24(15):2755. https://doi.org/10.3390/molecules24152755
Chicago/Turabian StyleHu, Enling, Songmin Shang, and Ka-Lok Chiu. 2019. "Removal of Reactive Dyes in Textile Effluents by Catalytic Ozonation Pursuing on-Site Effluent Recycling" Molecules 24, no. 15: 2755. https://doi.org/10.3390/molecules24152755
APA StyleHu, E., Shang, S., & Chiu, K.-L. (2019). Removal of Reactive Dyes in Textile Effluents by Catalytic Ozonation Pursuing on-Site Effluent Recycling. Molecules, 24(15), 2755. https://doi.org/10.3390/molecules24152755