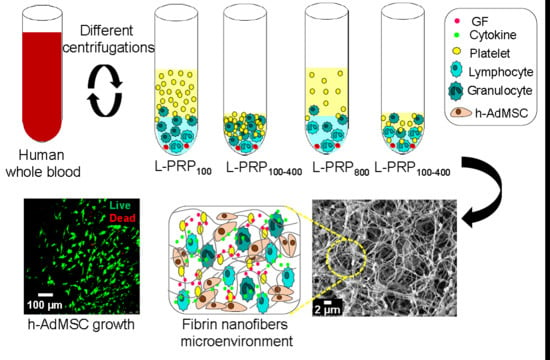
Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers
Abstract
1. Introduction
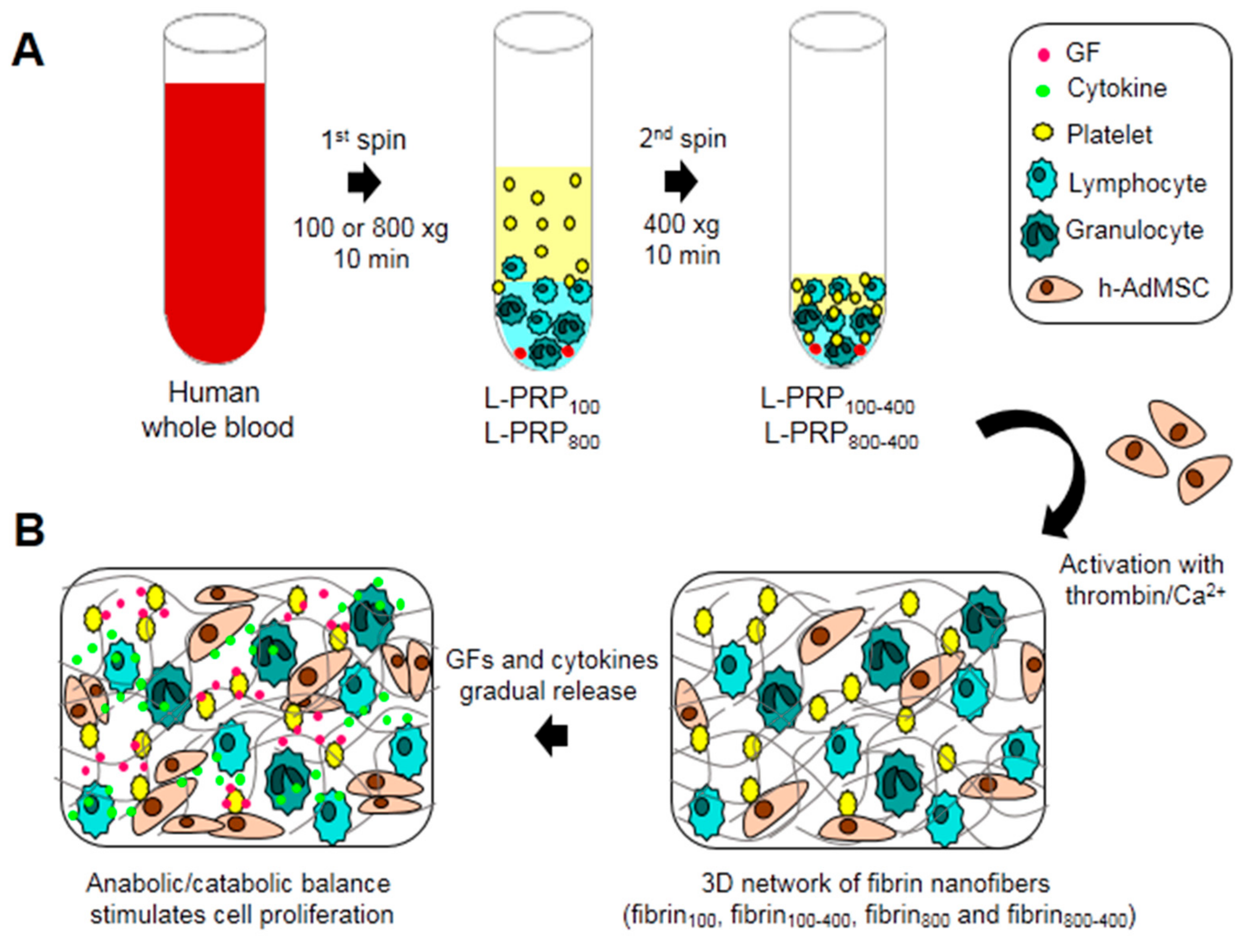
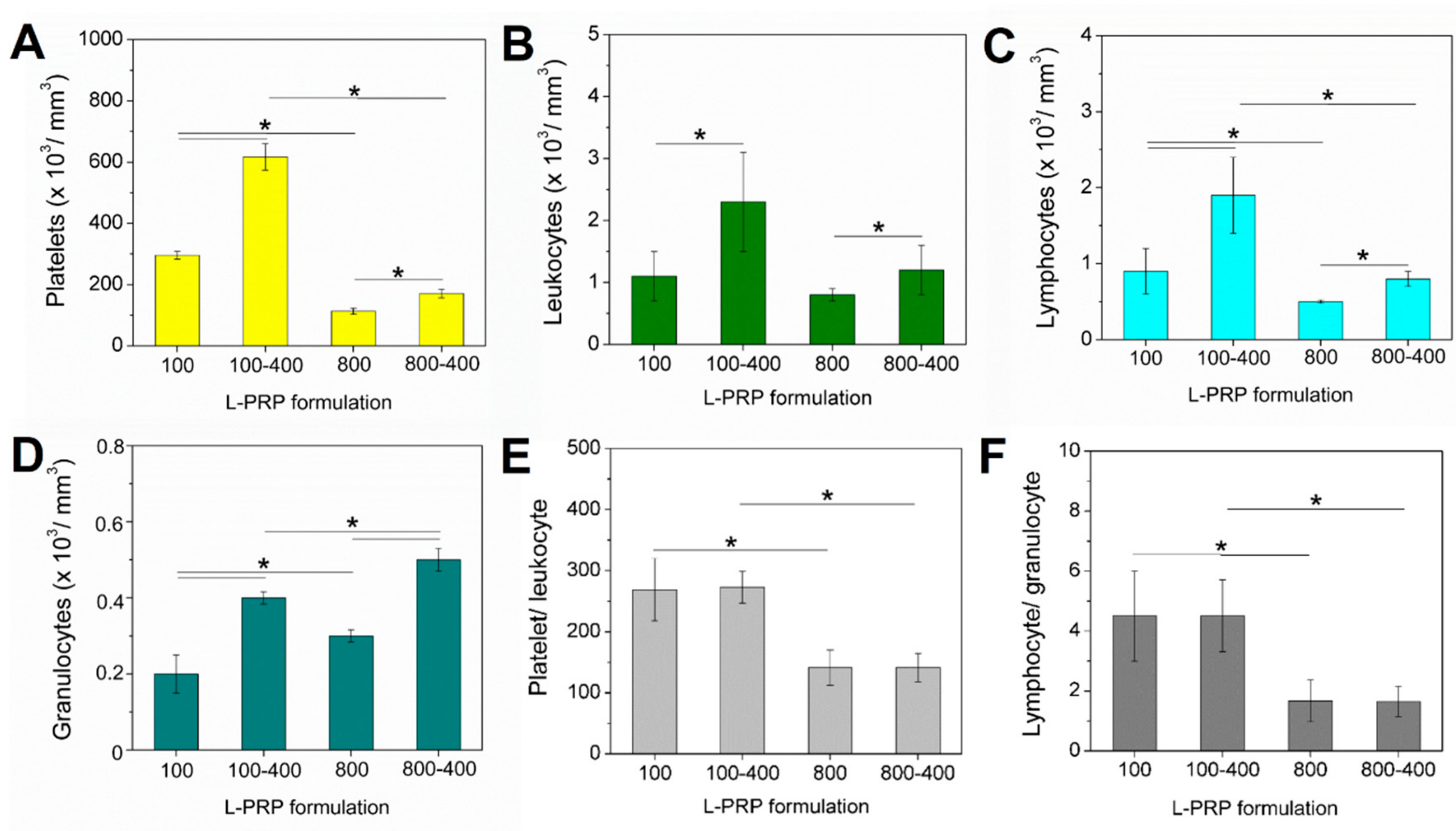
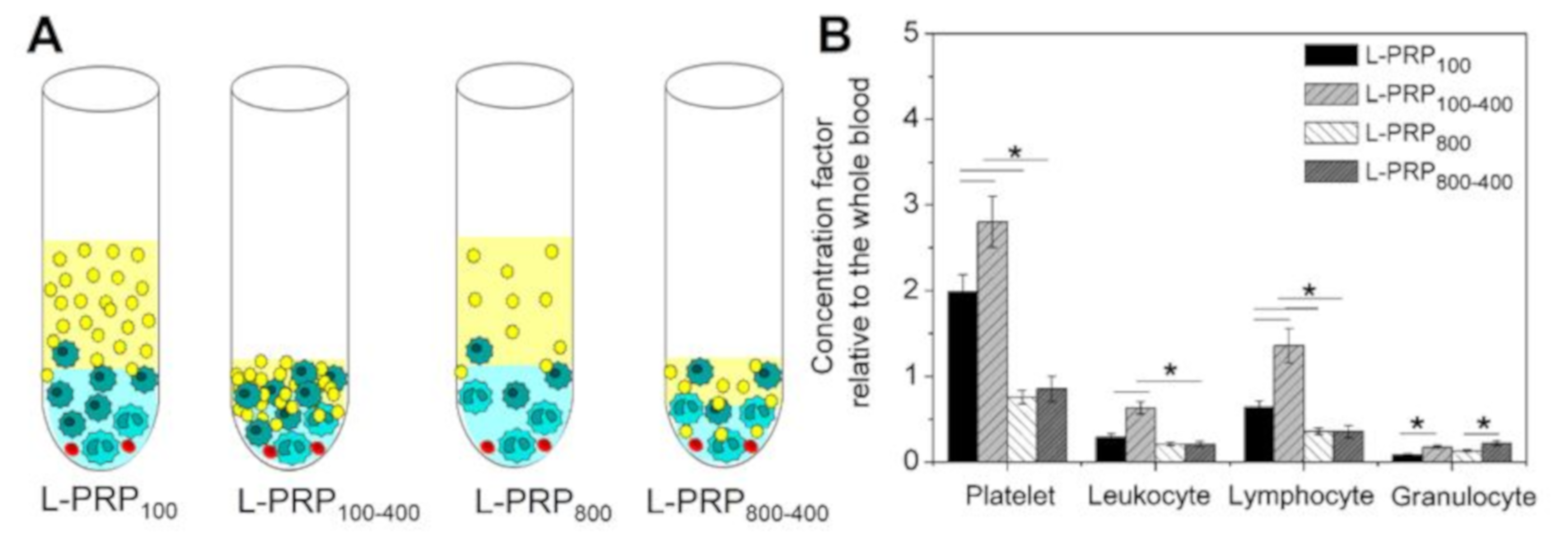
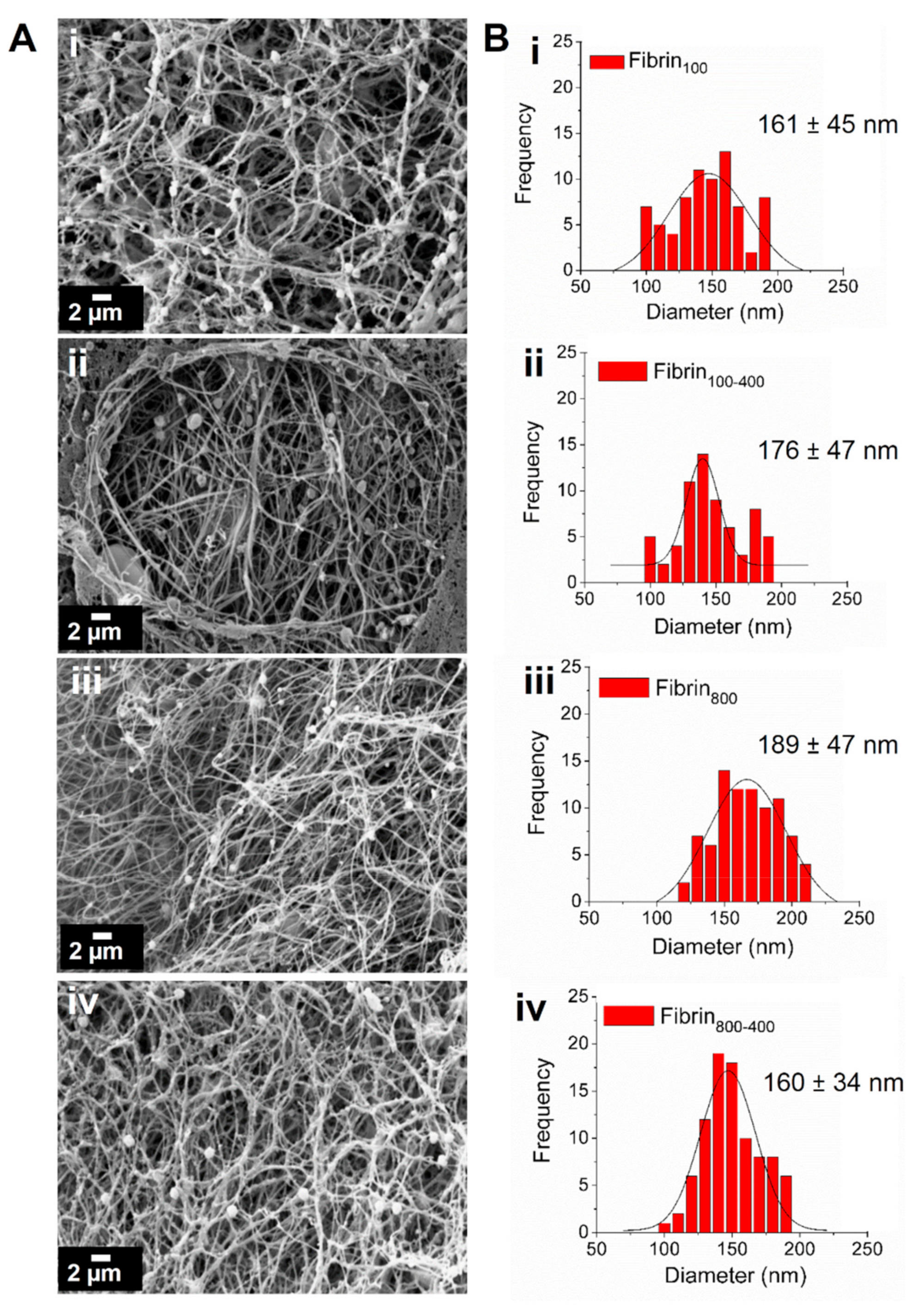
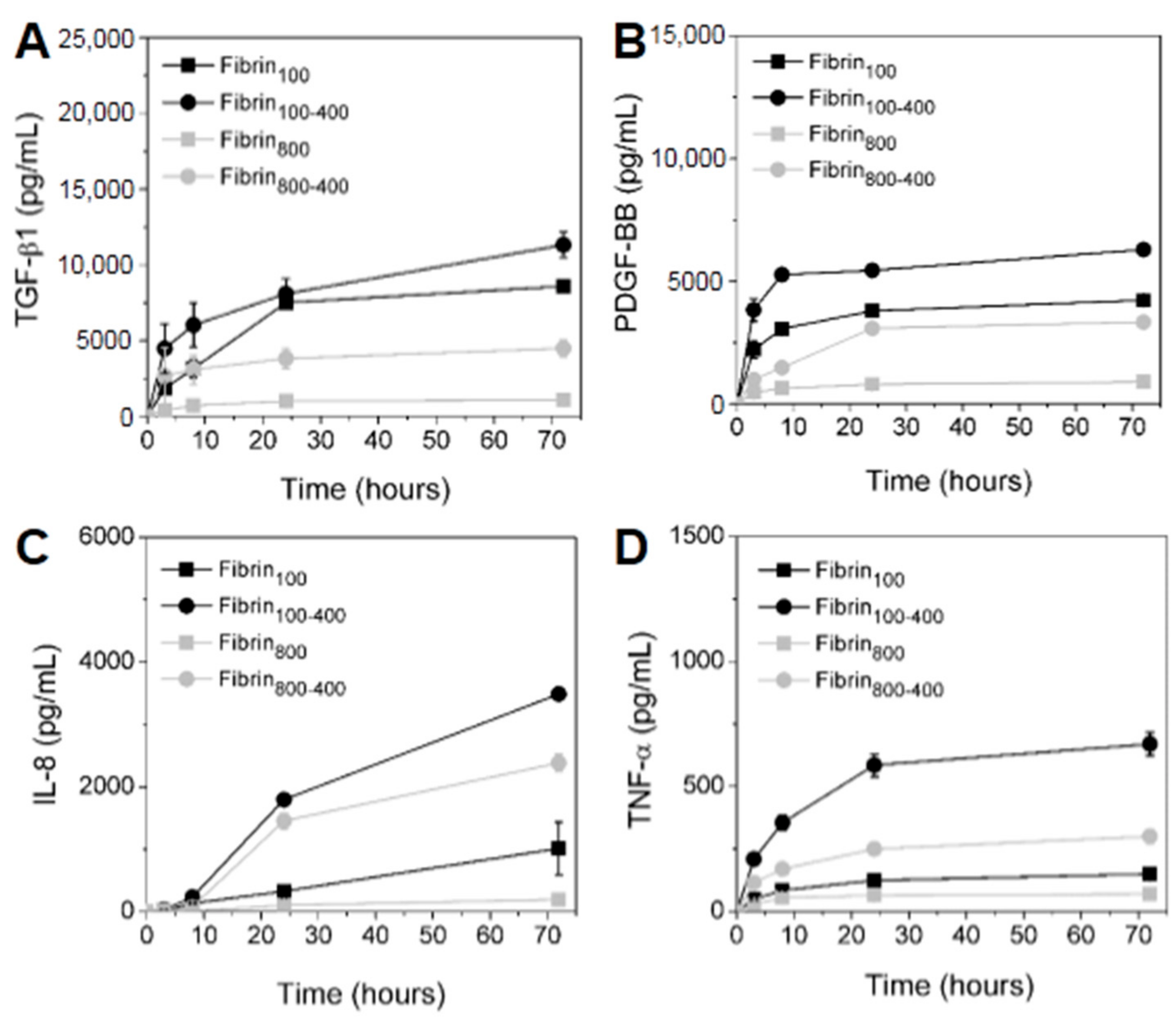
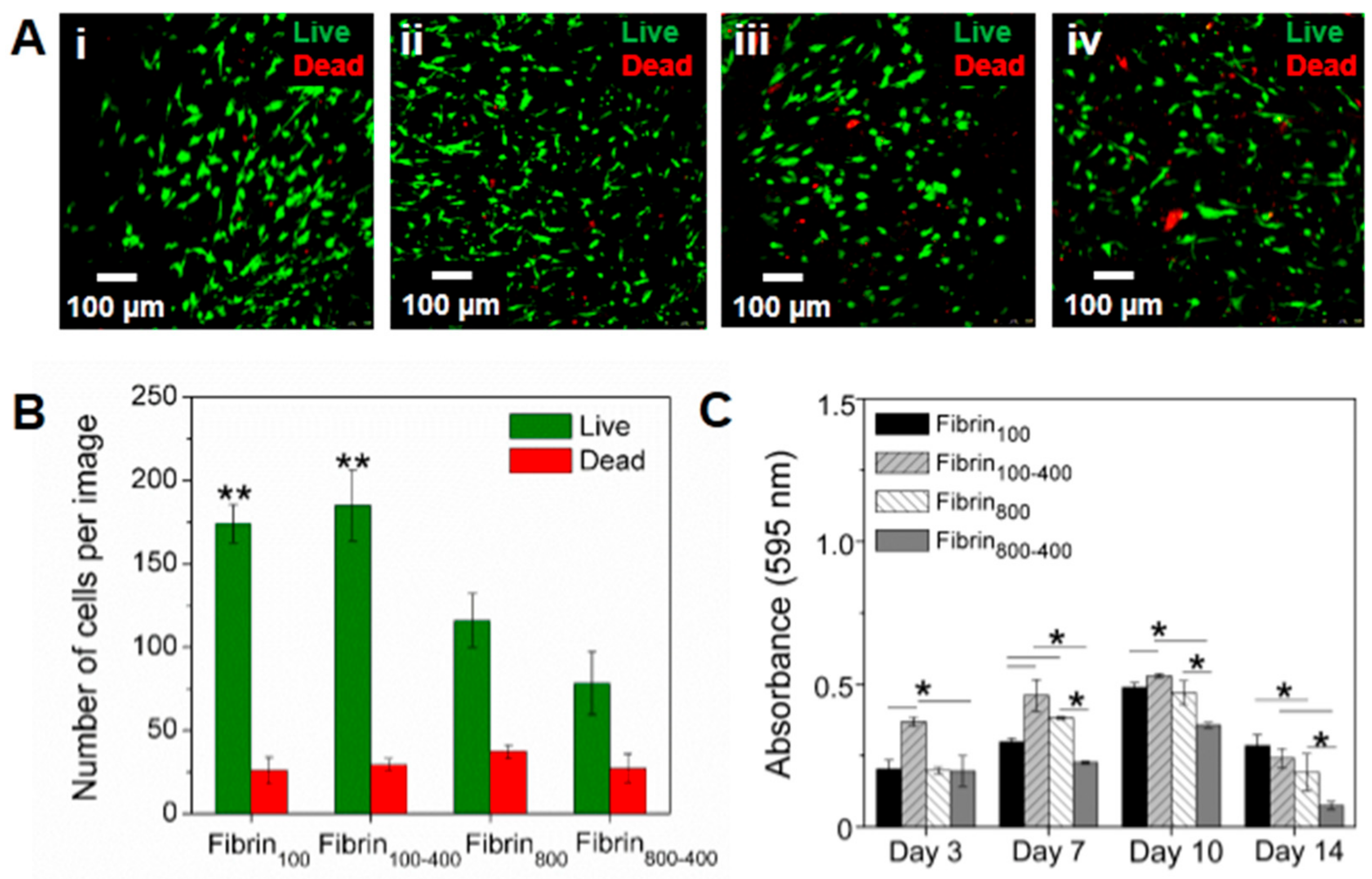
2. Results
3. Discussion
4. Material and Methods
4.1. Whole Blood Collection
4.2. L-PRP Preparation
4.3. Concentration Factor Calculation
4.4. Fibrin Nanofiber Formation
4.5. Scanning Electron Microscopy (SEM) Analysis
4.6. Kinetics of GFs and Cytokines Release
4.7. Isolation and Culture of h-AdMSCs in Fibrin Nanofibers
4.8. Assessment of h-AdMSCs Survival and Growth
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cieslik-Bielecka, A.; Skowroński, R.; Jędrusik-Pawłowska, M.; Pierchała, M. The application of L-PRP in AIDS patients with crural chronic ulcers: A pilot study. Adv. Med. Sci. 2018, 63, 140–146. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Teresa, M.; Ruiz, P.; Cenacchi, A.; Maria, P.; Maurilio, F. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sport. Traumatol Arthrosc 2012, 20, 2082–2091. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Weglein, A.; Sampson, S.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016, 12, 1–10. [Google Scholar]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Fortier, L.; Cole, B.J. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2015, 44, 792–800. [Google Scholar] [CrossRef]
- Zotti, F.; Albanese, M.; Rodella, L.F.; Nocini, P.F. Platelet-rich plasma in treatment of temporomandibular joint dysfunctions: Narrative review. Int. J. Mol. Sci. 2019, 20, 277. [Google Scholar] [CrossRef]
- Pravin, A.J.S.; Sridhar, V.; Srinivasan, B.N. Autologous Platelet Rich Plasma (Prp) Versus Leucocyte-Platelet Rich Fibrin (L-Prf) in Chronic Non-Healing Leg Ulcers-a Randomised, Open Labelled, Comparative Study. J. Evol. Med. Dent. Sci. 2016, 5, 7460–7462. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; De la Fuente, M.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surgery, Sport. Traumatol. Arthrosc. 2012, 20, 1657–1665. [Google Scholar] [CrossRef]
- Bielecki, T.; Ehrenfest, D.M.D.; Everts, P.A.; Wiczkowski, A. The Role of Leukocytes from L-PRP/L-PRF in Wound Healing and Immune Defense: New Perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Rohman, G.; Langueh, C.; Ramtani, S.; Lataillade, J.; Lutomski, D.; Senni, K.; Changotade, S. The Use of Platelet-Rich Plasma to Promote Cell Recruitment into Low-Molecular-Weight. Polymers. 2019, 1–22. [Google Scholar]
- Jubert, N.J.; Rodríguez, L.; Reverté-Vinaixa, M.M.; Navarro, A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis. A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop. J. Sport. Med. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Middleton, K.K.; Barro, V.; Muller, B.; Terada, S.; Fu, F.H. Evaluation of the Effects of Platelet-Rich Plasma (PRP) Therapy Involved in the Healing of Sports-Related Soft Tissue Injuries. Iowa Orthop. J. 2012, 32, 150–163. [Google Scholar]
- Zhou, Y.; Zhang, J.; Wu, H.; Hogan, M.V.; Wang, J.H. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells—implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res. Ther. 2015, 1–13. [Google Scholar] [CrossRef]
- Delong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles. Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L.; Malheiros Luzo, Â.C.; Belangero, W.D.; et al. Contributions for classification of platelet rich plasma - Proposal of a new classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Shimojo, A.A.M.; Perez, A.G.M.; Lana, J.F.S.D.; Santana, M.H.A. Distribution, recovery and concentration of platelets and leukocytes in L-PRP prepared by centrifugation. Colloid Surf. B Biointerfaces 2018, 161, 288–295. [Google Scholar] [CrossRef]
- Perez, A.G.M.; Lana, J.F.S.D.; Rodrigues, A.A.; Luzo, A.C.M.; Belangero, W.D.; Santana, M.H.A. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014, 2014, 176060. [Google Scholar] [CrossRef]
- Söderström, A.C.; Nybo, M.; Nielsen, C.; Vinholt, P.J. The effect of centrifugation speed and time on pre-analytical platelet activation. Clin. Chem. Lab. Med. 2016, 54, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Brandl, F.; Appel, B.; Wiese, H.; Maier, G.; Wenzel, M.; Staudenmaier, R.; Goepferich, A.; Blunk, T. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007, 28, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, K.; Dietl, S.; Ludwig, N.; Berberich, O.; Hoefner, C.; Storck, K.; Blunk, K.; Bauer-Kreisel, P. Engineering vascularized adipose tissue using the stromal-vascular fraction and fibrin hydrogels. Tissue Eng. Part A. 2015, 21, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D.; Lawrence, R.; Felson, D.; Helmick, C.; Arnold, L.; Choi, H.; et al. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.; Troya, M.; Padilla, S.; Orive, G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, A.A.M.; Perez, M.M.G.; Galdames, S.E.M.; Brissac, I.C.S.; Santana, M.H.A. Performance of PRP Associated with Porous Chitosan as a Composite Scaffold for Regenerative Medicine. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Zhao, C.; Guo, S.; Liu, S.; Jia, W.; Tuan, R.S.; Zhang, C. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials 2012, 33, 7008–7018. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Bielecki, T.; Jimbo, R.; Barbe, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Wasterlain, A.S.; Braun, H.J.; Dragoo, J.L. Contents and Formulations of Platelet-Rich Plasma. Oper. Tech. Orthop. 2012, 22, 33–42. [Google Scholar] [CrossRef]
- Perez, A.G.M.; Rodrigues, A.A.; Luzo, A.C.M.; Lana, J.F.S.D.; Belangero, W.D.; Santana, M.H.A. Fibrin network architectures in pure platelet-rich plasma as characterized by fiber radius and correlated with clotting time. J. Mater. Sci. Mater. Med. 2014, 25, 1967–1977. [Google Scholar] [CrossRef]
- Crane, D.; Everts, P.A.M. Platelet Rich Plasma (PRP) Matrix Grafts. Pract. PAIN Manag. 2008, 1–10. [Google Scholar]
- Caplan, A.I.; Correa, D. PDGF in Bone Formation and Regeneration: New Insights into a Novel Mechanism Involving MSCs. J. Orthop. Res. 2011, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Chaly, Y.V.; Selvan, R.S.; Fegeding, K.V.; Kolesnikova, T.S.; Voitenok, N.N. Expression of Il-8 Gene in Human Monocytes and Lymphocytes: Differential Regulation by tnf and Il-1. Cytokine 2000, 12, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Bellail, A.C.; Van Meir, E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro. Oncol. 2005, 7, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Stokes, D.G.; Jimenez, S.A. Modulation of TGF-β signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthr. Cartil. 2007, 15, 1367–1377. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Parrish, W.R.; Roides, B.; Hwang, J.; Mafilios, M.; Story, B.; Bhattacharyya, S. Normal platelet function in platelet concentrates requires non-platelet cells: a comparative in vitro evaluation of leucocyte-rich (type 1a) and leucocyte-poor (type 3b) platelet concentrates. BMJ Open Sport Exerc. Med. 2016, 2, e000071. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth Factor and Catabolic Cytokine Concentrations Are Influenced by the Cellular Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Barnett Jr, M.D.; Pomeroy, G.C. Use of Platelet-Rich Plasma and Bone Marrow-Derived Mesenchymal Stem Cells in Foot and Ankle Surgery. Tech. Foot Ankle Surg. 2007, 6, 89–94. [Google Scholar] [CrossRef]
- Holmes, H.L.; Wilson, B.; Goerger, J.P.; Silverberg, J.L.; Cohen, I.; Zipfel, W.R.; Fortier, L.A. Facilitated recruitment of mesenchymal stromal cells by bone marrow concentrate and platelet rich plasma. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, S.; Ma, L.; Yu, J.; Guo, Y.; Wang, C. The Differential Effects of Leukocyte-Containing and Pure Platelet-Rich Plasma on Nucleus Pulposus-Derived Mesenchymal Stem Cells: Implications for the Clinical Treatment of Intervertebral Disc Degeneration. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yin, W.; Zhang, Y.; Qi, X.; Chen, Y.; Xie, X.; Zhang, C. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci. Rep. 2017, 7, 43301. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.G.M.; Lichy, R.; Lana, J.F.S.D.; Rodrigues, A.A.; Luzo, A.C.M.; Belangero, W.D.; Santana, M.H.A. Prediction and modulation of platelet recovery by discontinuous centrifugation of whole blood for the preparation of pure platelet-rich plasma. Biores. Open Access 2013, 2, 307–314. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Sanchez, M.; Azofra, J.; del Mar Zalduendo, M.; Nurden, P.; Nurden, A.T. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. 2005, 23, 281–286. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Zalduendo, M.M.; De la Fuente, M.; Azofra, J.; Andía, I. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology 2007, 46, 1769–1772. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Del Corso, M.; Kang, B.; Nally, M.; Lanata, N.; Quirynen, M.; Dohan, D.M.; et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Dohle, E.; El Bagdadi, K.; Sader, R.; Choukroun, J.; Kirkpatrick, C.J.; Ghanaati, S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 1–13. [Google Scholar] [CrossRef]
- Hutton, D.L.; Moore, E.M.; Gimble, J.M.; Grayson, W.L. Platelet-Derived Growth Factor and Spatiotemporal Cues Induce Development of Vascularized Bone Tissue by Adipose-Derived Stem Cells. TISSUE Eng. Part. A 2013, 19, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Zachariae, C.C.; Norihisa, Y.; Ortaldo, J.R.; Hishinuma, A.; Matsushimat, A.N.D.K. IL-8 gene expression and production in human peripheral blood lymphocyte subsets. J. Immunol. 1991, 146, 3815–3823. [Google Scholar] [PubMed]
- Dubravec, D.B.; Spriggst, D.R.; Mannick, J.A.; Rodrick, M.L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor a. Proc. Natl. Acad. Sci. USA 1990, 87, 6758–6761. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J. Clin. Invest. 1996, 97, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.R.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, tgf-beta and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSC): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and ost. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Manzini, B.M.; da Silva Santos Duarte, A.; Sankaramanivel, S.; Ramos, A.L.; Latuf-Filho, P.; Escanhoela, C.; Kharmandayan, P.; Olalla Saad, S.T.; Boin, I.; Malheiros Luzo, Â.C. Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis. Cytotherapy 2015, 17, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Blashki, D.; Buchanan, R.M.; Yazdi, I.K.; Ferrari, M.; Simmons, P.J.; Tasciotti, E. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 2012, 33, 5308–5316. [Google Scholar] [CrossRef]
- Higuera, G.; Schop, D.; Janssen, F.; van Dijkhuizen-Radersma, R.; van Boxtel, T.; van Blitterswijk, C.A. Quantifying In Vitro Growth and Metabolism Kinetics of Human Mesenchymal Stem Cells Using a Mathematical Model. Tissue Eng. Part. A 2009, 15, 2653–2663. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Condition | Adaptation Time (Day) | Range of Exponential Phase (Day) | µmax * (day−1) | td ** (day) |
|---|---|---|---|---|
| Fibrin100 | ~4 | 4–9 | 0.15 | 4.53 |
| Fibrin100-400 | <<3 | <3 | *** | *** |
| Fibrin800 | ~3 | 3–7 | 0.16 | 4.33 |
| Fibrin800-400 | ~6 | 6–10 | 0.14 | 4.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo, B.A.G.; Luzo, Â.C.M.; Lana, J.F.S.D.; Santana, M.H.A. Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers. Molecules 2019, 24, 2729. https://doi.org/10.3390/molecules24152729
de Melo BAG, Luzo ÂCM, Lana JFSD, Santana MHA. Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers. Molecules. 2019; 24(15):2729. https://doi.org/10.3390/molecules24152729
Chicago/Turabian Stylede Melo, Bruna Alice Gomes, Ângela Cristina Malheiros Luzo, José Fabio Santos Duarte Lana, and Maria Helena Andrade Santana. 2019. "Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers" Molecules 24, no. 15: 2729. https://doi.org/10.3390/molecules24152729
APA Stylede Melo, B. A. G., Luzo, Â. C. M., Lana, J. F. S. D., & Santana, M. H. A. (2019). Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers. Molecules, 24(15), 2729. https://doi.org/10.3390/molecules24152729