Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Nanocatalysts
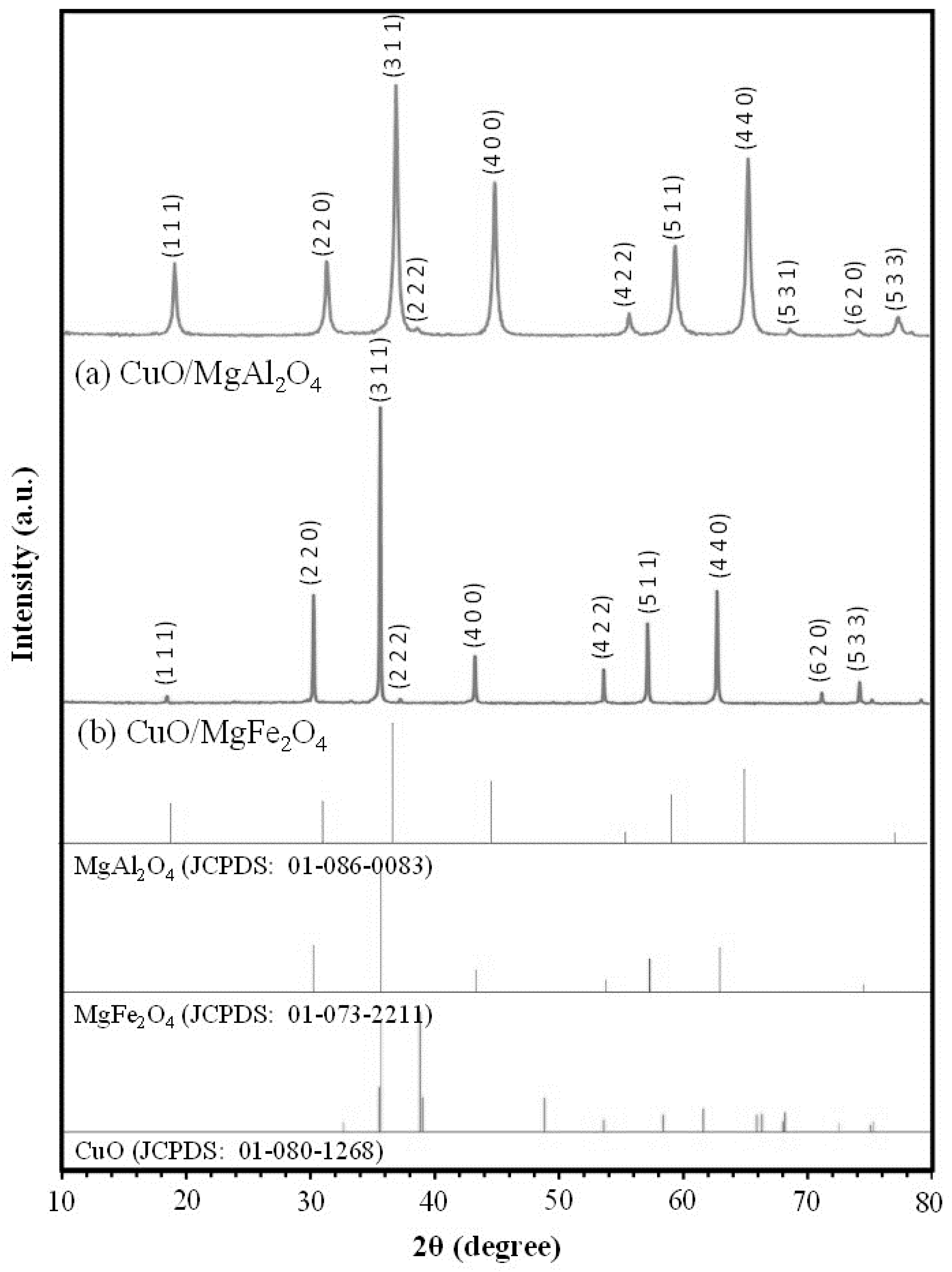
2.1.1. XRD Analysis
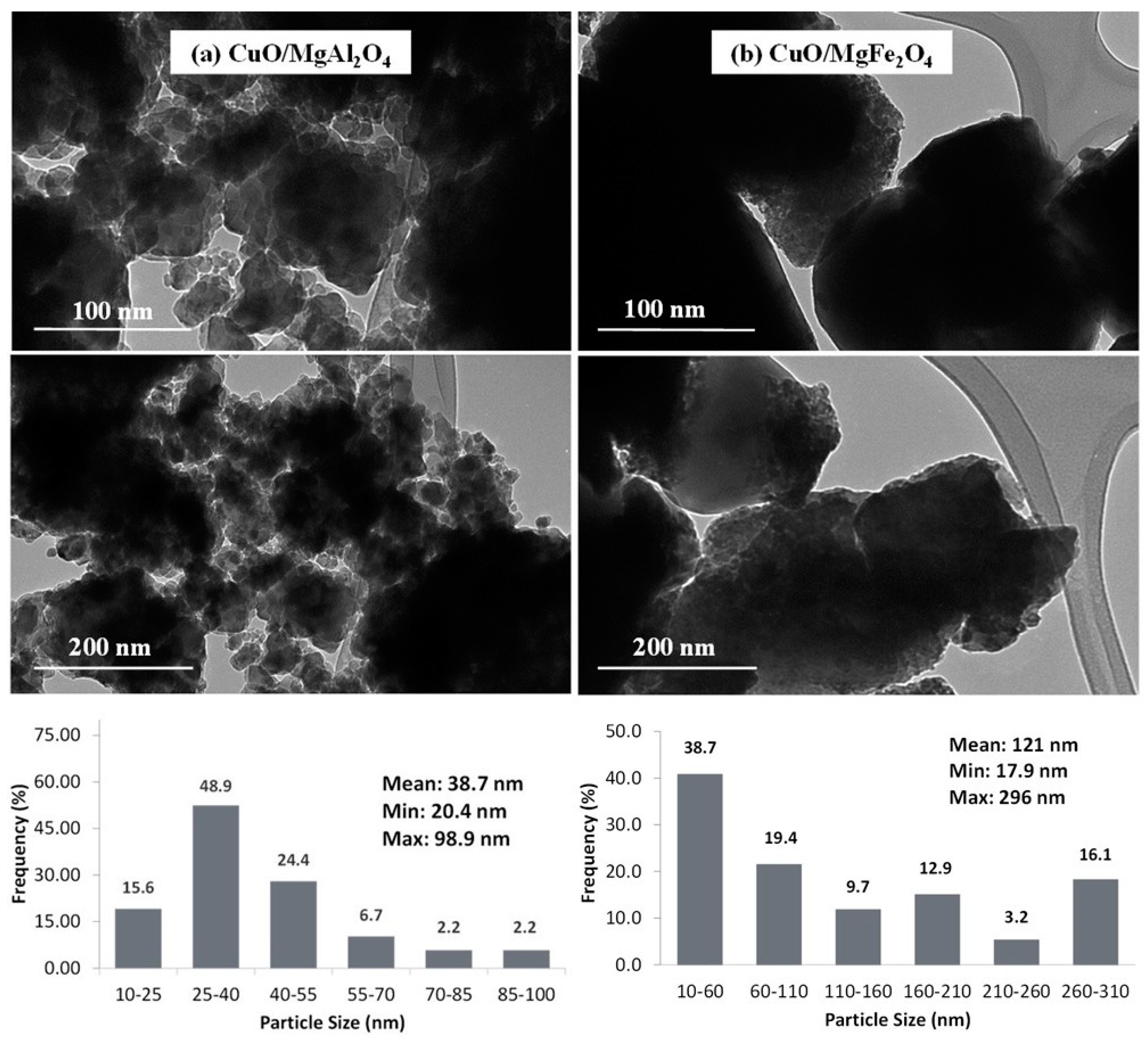
2.1.2. TEM and SEM Analysis
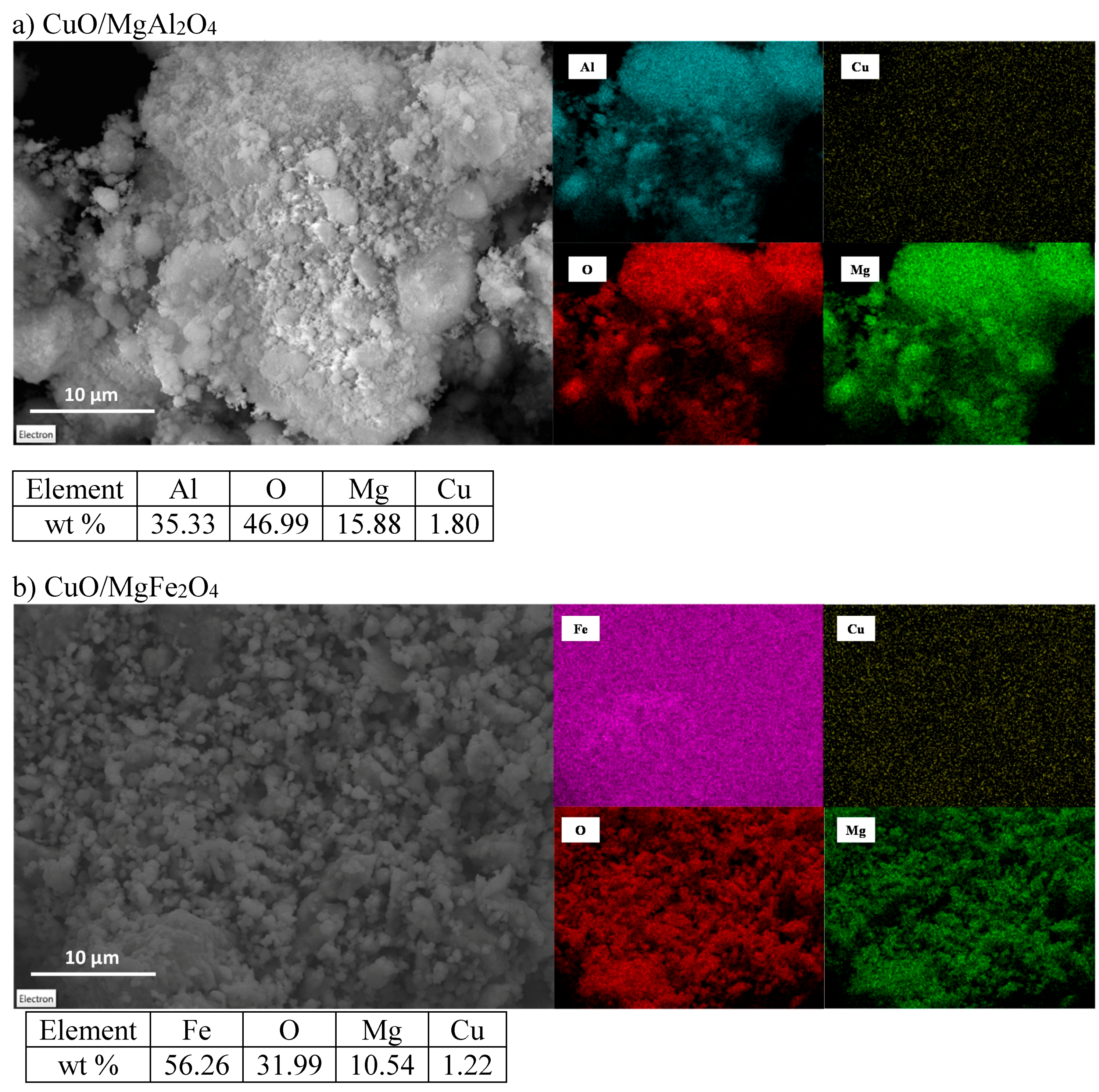
2.1.3. EDX Analysis
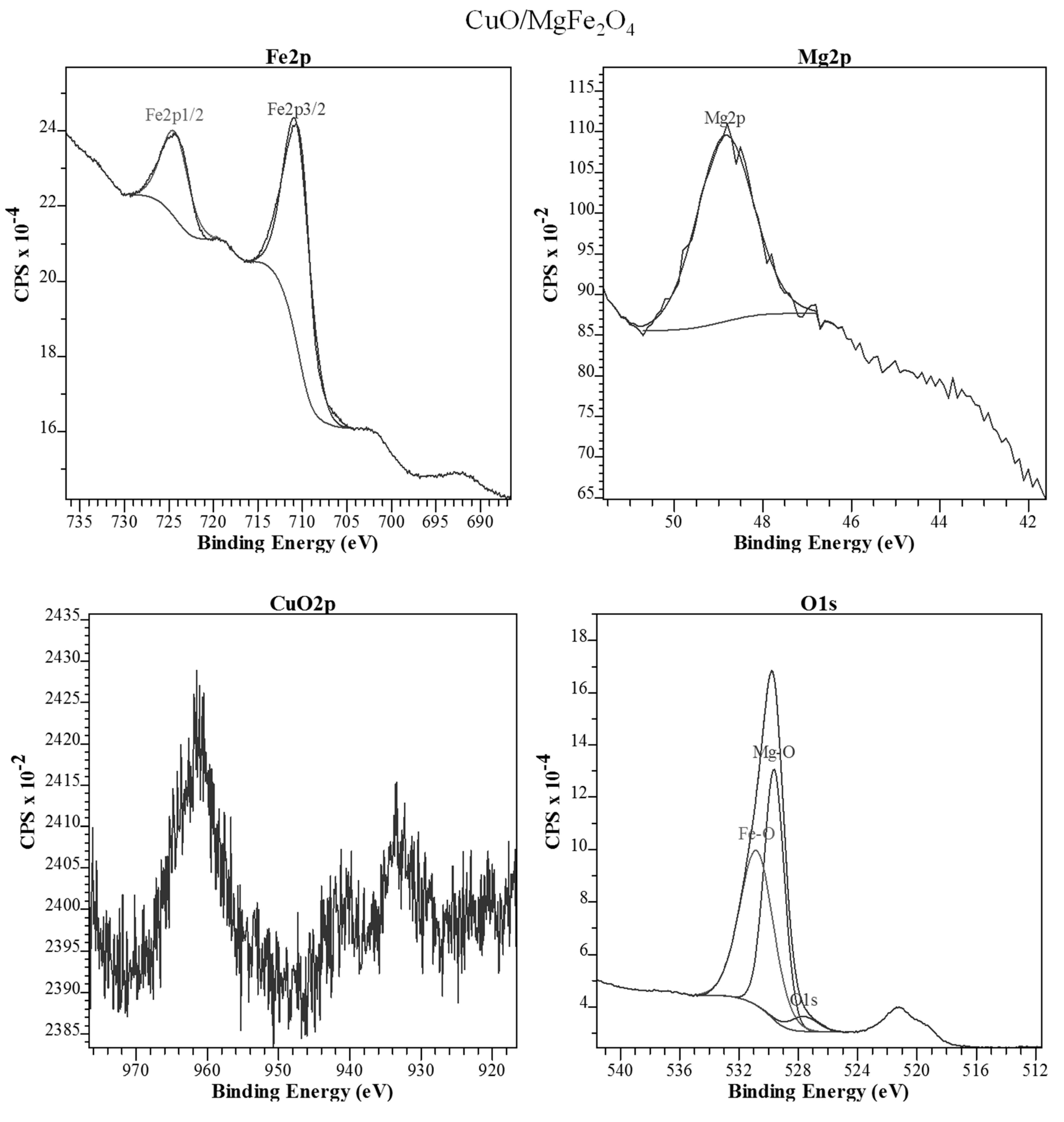
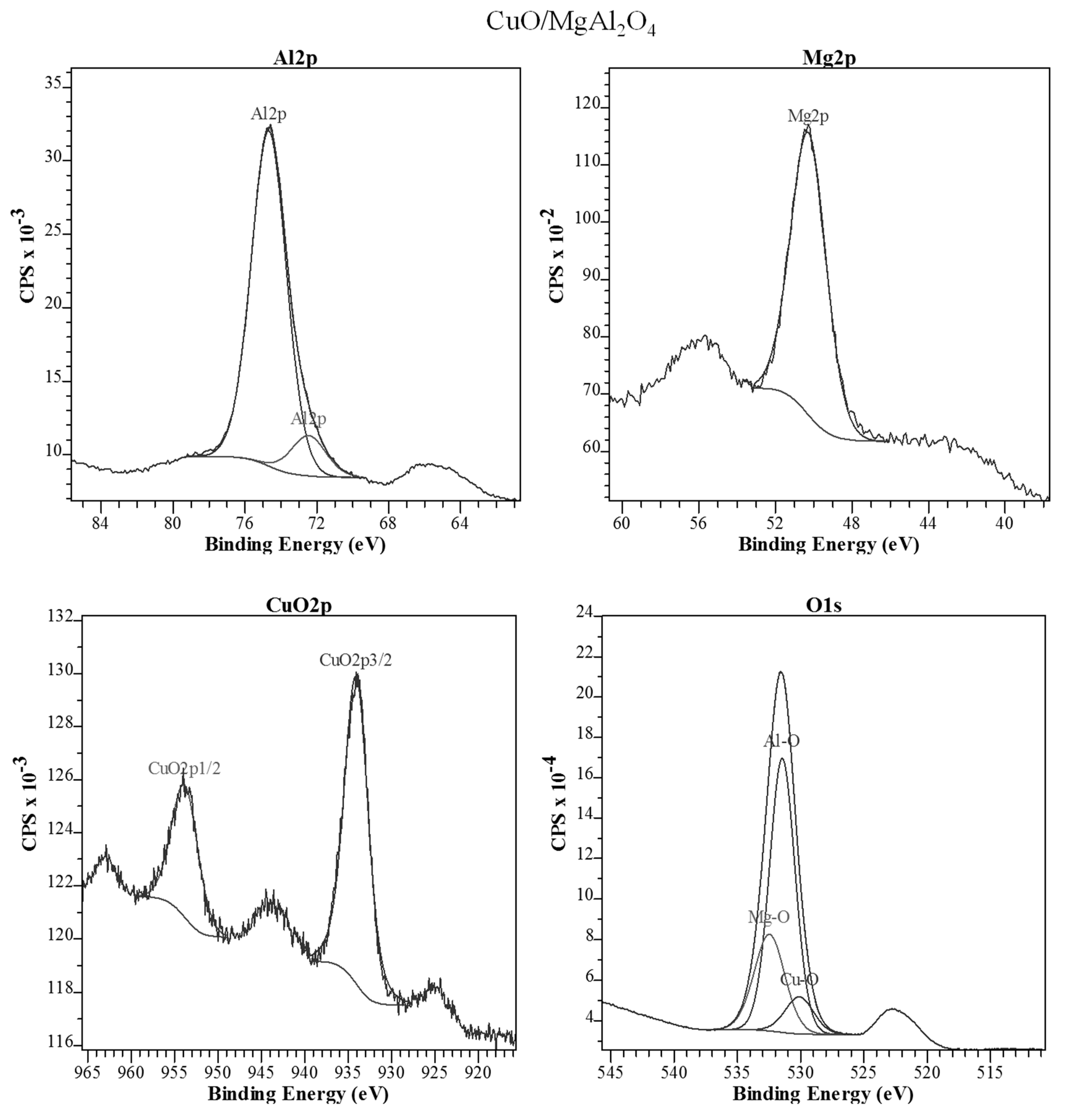
2.1.4. XPS Analysis
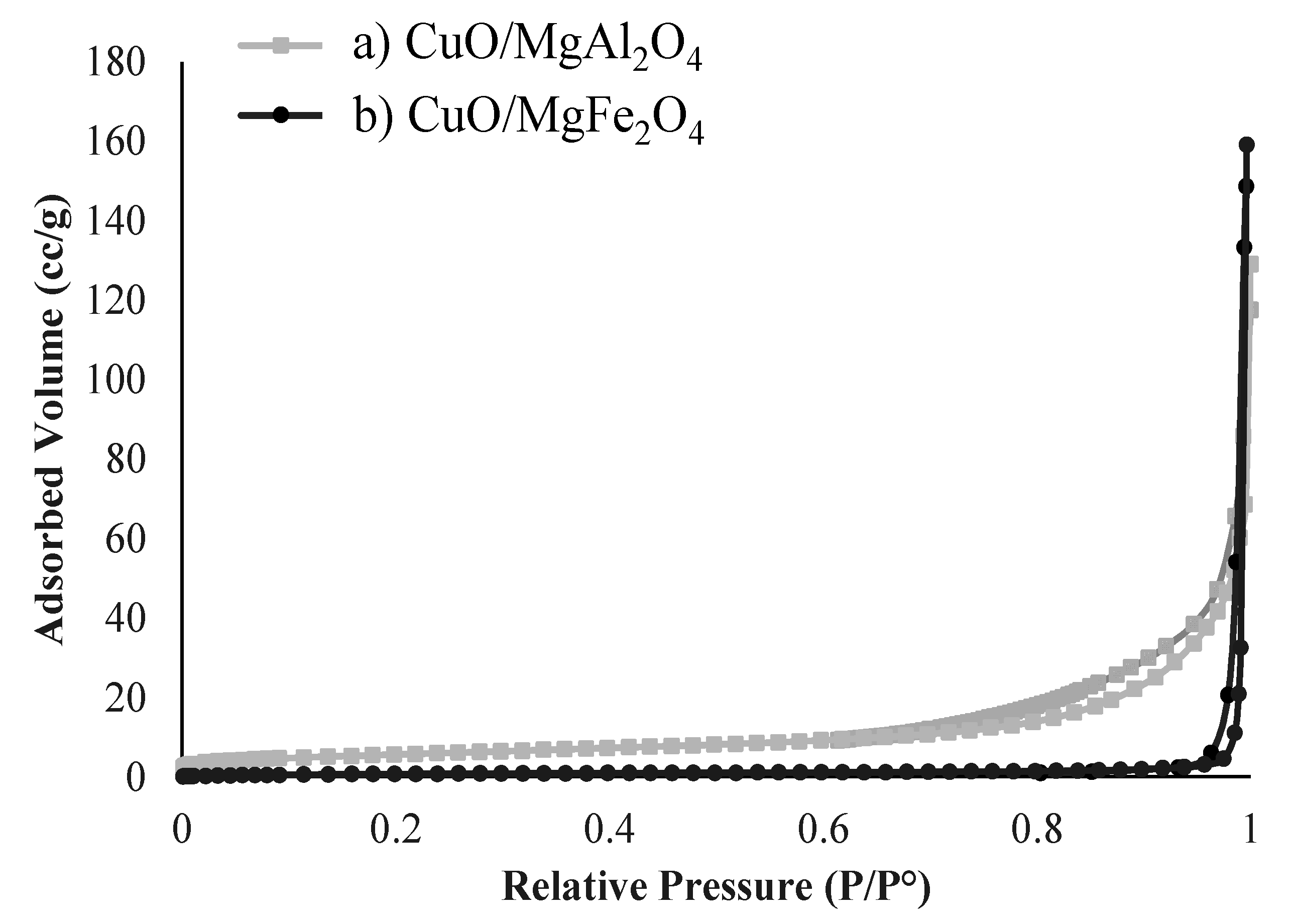
2.1.5. BET-BJH Analysis
2.1.6. Acidity
2.1.7. Magnetic Susceptibility Analysis
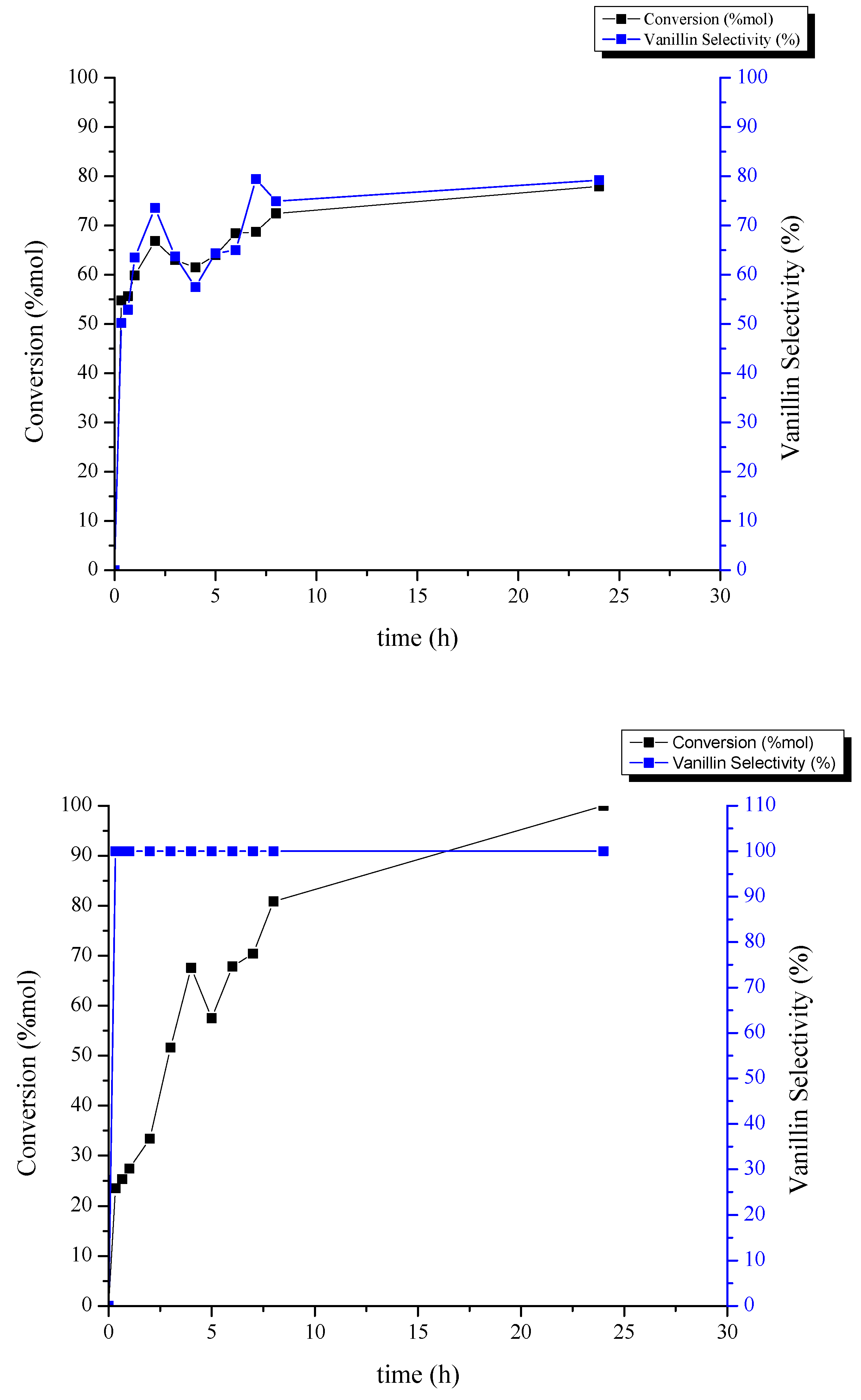
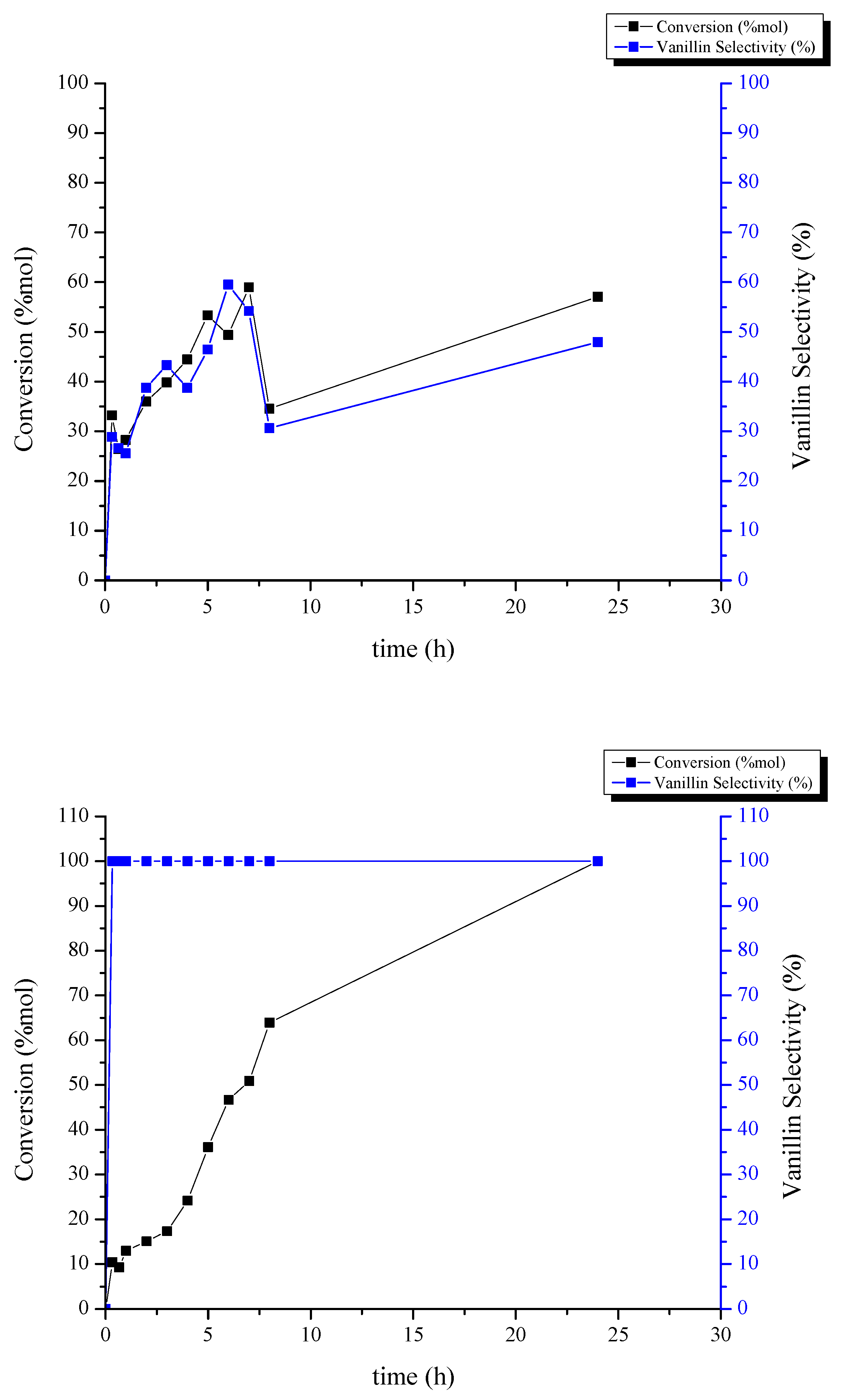
2.2. Catalytic Performance Study toward Vanillin Production
3. Conclusions
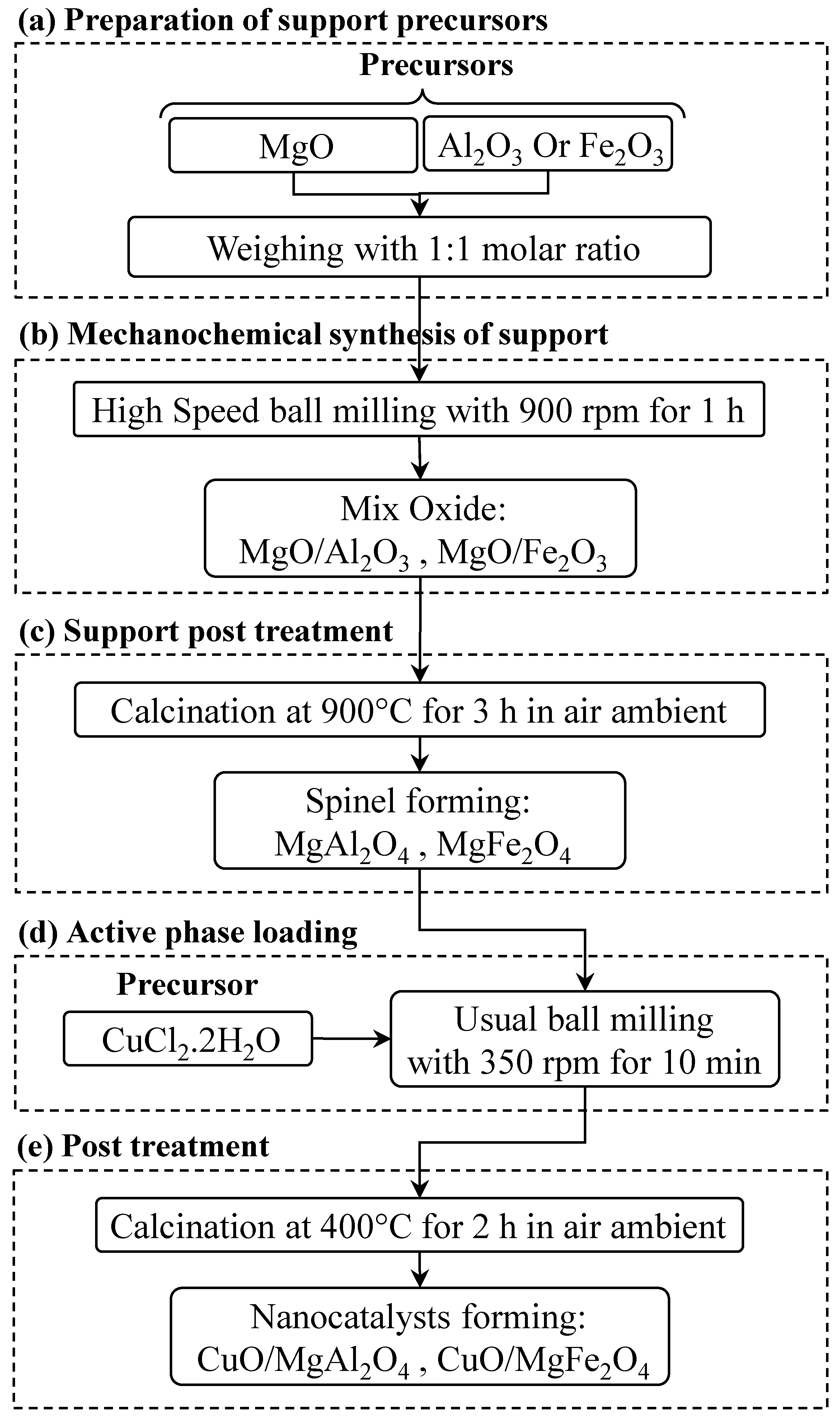
4. Materials and Methods
4.1. Materials
4.2. Preparation and Procedure of the Nanocatalysts
4.3. Characterization Techniques of the Nanocatalysts
4.4. Experimental Set-Up for the Catalytic Performance Test
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimoni, E.; Ravid, U.; Shoham, Y. Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin. J. Biotechnol. 2000, 78, 1–9. [Google Scholar] [CrossRef]
- Wu, W.; Yang, L.; Zhao, F.; Zeng, B. A vanillin electrochemical sensor based on molecularly imprinted poly(1-vinyl-3-octylimidazole hexafluoride phosphorus)−multi-walled carbon nanotubes@polydopamine–carboxyl single-walled carbon nanotubes composite. Sens. Actuators B Chem. 2017, 239, 481–487. [Google Scholar] [CrossRef]
- Mota, M.I.F.; Rodrigues Pinto, P.C.; Loureiro, J.M.; Rodrigues, A.E. Recovery of Vanillin and Syringaldehyde from Lignin Oxidation: A Review of Separation and Purification Processes. Sep. Purif. Rev. 2016, 45, 227–259. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Parrino, F.; Puma, M.A. Synthesis of vanillin in water by TiO2 photocatalysis. Appl. Catal. B Environ. 2012, 111–112, 555–561. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Menini, L.; Parreira, L.A.; Mesquita, R.A.; Kozlov, Y.N.; Shul’pin, G.B. Oxidation of isoeugenol to vanillin by the “H2O2–vanadate–pyrazine-2-carboxylic acid” reagent. J. Mol. Catal. A Chem. 2012, 363–364, 140–147. [Google Scholar] [CrossRef]
- Hua, D.; Ma, C.; Song, L.; Lin, S.; Zhang, Z.; Deng, Z.; Xu, P. Enhanced vanillin production from ferulic acid using adsorbent resin. Appl. Microbiol. Biotechnol. 2007, 74, 783–790. [Google Scholar] [CrossRef]
- Oddou, J.; Stentelaire, C.; Lesage-Meessen, L.; Asther, M.; Colonna Ceccaldi, B. Improvement of ferulic acid bioconversion into vanillin by use of high-density cultures of Pycnoporus cinnabarinus. Appl. Microbiol. Biotechnol. 1999, 53, 1–6. [Google Scholar] [CrossRef]
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef]
- Shiba, T.; Xiao, L.; Miyakoshi, T.; Chen, C.L. Oxidation of isoeugenol and coniferyl alcohol catalyzed by laccases isolated from Rhus vernicifera Stokes and Pycnoporus coccineus. J. Mol. Catal. B Enzym. 2000, 10, 605–615. [Google Scholar] [CrossRef]
- Ma, X.-K.; Daugulis, A.J. Effect of bioconversion conditions on vanillin production by Amycolatopsis sp. ATCC 39116 through an analysis of competing by-product formation. Bioprocess Biosyst. Eng. 2014, 37, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Q.; Sun, Z.-H.; Zheng, P.; He, J.-Y. Biotransformation of isoeugenol to vanillin by Bacillus fusiformis CGMCC1347 with the addition of resin HD-8. Process Biochem. 2006, 41, 1673–1676. [Google Scholar] [CrossRef]
- Hua, D.; Ma, C.; Lin, S.; Song, L.; Deng, Z.; Maomy, Z.; Zhang, Z.; Yu, B.; Xu, P. Biotransformation of isoeugenol to vanillin by a newly isolated Bacillus pumilus strain: Identification of major metabolites. J. Biotechnol. 2007, 130, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Dignum, M.J.W.; Kerler, J.; Verpoorte, R. Vanilla production: Technological, chemical, and biosynthetic aspects. Food Rev. Int. 2001, 17, 199–219. [Google Scholar] [CrossRef]
- Rodrigues Pinto, P.C.; Borges da Silva, E.A.; Rodrigues, A.E. Lignin as Source of Fine Chemicals: Vanillin and Syringaldehyde. In Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science; Baskar, C., Baskar, S., Dhillon, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 381–420. [Google Scholar]
- Ramana, S.; Rao, B.G.; Venkataswamy, P.; Rangaswamy, A.; Reddy, B.M. Nanostructured Mn-doped ceria solid solutions for efficient oxidation of vanillyl alcohol. J. Mol. Catal. A Chem. 2016, 415, 113–121. [Google Scholar] [CrossRef]
- Behling, R.; Chatel, G.; Valange, S. Sonochemical oxidation of vanillyl alcohol to vanillin in the presence of a cobalt oxide catalyst under mild conditions. Ultrason. Sonochem. 2017, 36, 27–35. [Google Scholar] [CrossRef]
- Saha, S.; Hamid, S.B.A.; Ali, T.H. Catalytic evaluation on liquid phase oxidation of vanillyl alcohol using air and H2O2 over mesoporous Cu-Ti composite oxide. Appl. Surf. Sci. 2017, 394, 205–218. [Google Scholar] [CrossRef]
- Wang, C.; Wen, C.; Lauterbach, J.; Sasmaz, E. Superior oxygen transfer ability of Pd/MnOx-CeO2 for enhanced low temperature CO oxidation activity. Appl. Catal. B Environ. 2017, 206, 1–8. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Zhang, K.; Han, Z.; Dai, H. Three-dimensionally ordered macroporous CeO2-supported Pd@Co nanoparticles: Highly active catalysts for methane oxidation. J. Catal. 2016, 342, 17–26. [Google Scholar] [CrossRef]
- Jin, M.; Kim, J.W.; Kim, J.M.; Jurng, J.; Bae, G.-N.; Jeon, J.-K.; Park, Y.-K. Effect of calcination temperature on the oxidation of benzene with ozone at low temperature over mesoporous α-Mn2O3. Powder Technol. 2011, 214, 458–462. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hou, F.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.; Cui, L. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl. Surf. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Xu, J.; Deng, Y.-Q.; Luo, Y.; Mao, W.; Yang, X.-J.; Han, Y.-F. Operando Raman spectroscopy and kinetic study of low-temperature CO oxidation on an α-Mn2O3 nanocatalyst. J. Catal. 2013, 300, 225–234. [Google Scholar] [CrossRef]
- González-Cobos, J.; Horwat, D.; Ghanbaja, J.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical activation of Au nanoparticles for the selective partial oxidation of methanol. J. Catal. 2014, 317, 293–302. [Google Scholar] [CrossRef]
- Saqlain, M.A.; Hussain, A.; Siddiq, M.; Leitão, A.A. A DFT+U study of the Mars Van Krevelen mechanism of CO oxidation on Au/TiO2 catalysts. Appl. Catal. A Gen. 2016, 519, 27–33. [Google Scholar] [CrossRef]
- Jiang, W.; Pang, Y.; Gu, L.; Yao, Y.; Su, Q.; Ji, W.; Au, C.-T. Structurally defined SnO2 substrates, nanostructured Au/SnO2 interfaces, and their distinctive behavior in benzene and methanol oxidation. J. Catal. 2017, 349, 183–196. [Google Scholar] [CrossRef]
- Deng, Q.-F.; Ren, T.-Z.; Agula, B.; Liu, Y.; Yuan, Z.-Y. Mesoporous CexZr1−xO2 solid solutions supported CuO nanocatalysts for toluene total oxidation. J. Ind. Eng. Chem. 2014, 20, 3303–3312. [Google Scholar] [CrossRef]
- Menon, U.; Galvita, V.V.; Constales, D.; Alexopoulos, K.; Yablonsky, G.; Marin, G.B. Microkinetics for toluene total oxidation over CuO–CeO2/Al2O3. Catal. Today 2015, 258, 214–224. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Wang, R.; Wang, H.; Lv, W.; Ji, S. Ultrathin willow-like CuO nanoflakes as an efficient catalyst for electro-oxidation of hydrazine. J. Power Sources 2015, 289, 22–25. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J. Enhanced CO oxidation activity of CuO/CeO2 catalyst prepared by surfactant-assisted impregnation method. J. Rare Earths 2015, 33, 1268–1274. [Google Scholar] [CrossRef]
- Rahmani Vahid, B.; Haghighi, M. Biodiesel production from sunflower oil over MgO/MgAl2O4 nanocatalyst: Effect of fuel type on catalyst nanostructure and performance. Energy Convers. Manag. 2017, 134, 290–300. [Google Scholar] [CrossRef]
- Dehghani, F.; Hashemian, S.; Shibani, A. Effect of calcination temperature for capability of MFe2O4 (M = Co, Ni and Zn) ferrite spinel for adsorption of bromophenol red. J. Ind. Eng. Chem. 2017, 48, 36–42. [Google Scholar] [CrossRef]
- Bhaduri, S.; Bhaduri, S.B. Microstructural and mechanical properties of nanocrystalline spinel and related composites. Ceram. Int. 2002, 28, 153–158. [Google Scholar] [CrossRef]
- Ganesh, I.; Bhattacharjee, S.; Saha, B.P.; Johnson, R.; Rajeshwari, K.; Sengupta, R.; Ramana Rao, M.V.; Mahajan, Y.R. An efficient MgAl2O4 spinel additive for improved slag erosion and penetration resistance of high-Al2O3 and MgO–C refractories. Ceram. Int. 2002, 28, 245–253. [Google Scholar] [CrossRef]
- Chaudhary, V.; Zhong, Y.; Parmar, H.; Sharma, V.; Tan, X.; Ramanujan, R.V. Mechanochemical Synthesis of Iron and Cobalt Magnetic Metal Nanoparticles and Iron/Calcium Oxide and Cobalt/Calcium Oxide Nanocomposites. ChemistryOpen 2018, 7, 590–598. [Google Scholar] [CrossRef]
- Bergmann, I.; Šepelák, V.; Becker, K.D. Preparation of nanoscale MgFe2O4 via non-conventional mechanochemical route. Solid State Ion. 2006, 177, 1865–1868. [Google Scholar] [CrossRef]
- Abdi, M.S.; Ebadzadeh, T.; Ghaffari, A.; Feli, M. Synthesis of nano-sized spinel (MgAl2O4) from short mechanochemically activated chloride precursors and its sintering behavior. Adv. Powder Technol. 2015, 26, 175–179. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Zu Gott. 1918, 26, 98–100. [Google Scholar]
- Rahmani Vahid, B.; Haghighi, M.; Alaei, S.; Toghiani, J. Reusability enhancement of combustion synthesized MgO/MgAl2O4 nanocatalyst in biodiesel production by glow discharge plasma treatment. Energy Convers. Manag. 2017, 143, 23–32. [Google Scholar] [CrossRef]
- Charghand, M.; Haghighi, M.; Saedy, S.; Aghamohammadi, S. Efficient hydrothermal synthesis of nanostructured SAPO-34 using ultrasound energy: Physicochemical characterization and catalytic performance toward methanol conversion to light olefins. Adv. Powder Technol. 2014, 25, 1728–1736. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Wu, G.; Gong, J. Synthesis of stable Ni-CeO2 catalysts via ball-milling for ethanol steam reforming. Catal. Today 2014, 233, 53–60. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Hu, K. Preparation of Ni/Al2O3 nanocomposite powder by high-energy ball milling and subsequent heat treatment. J. Mater. Process. Technol. 2004, 147, 236–240. [Google Scholar] [CrossRef]
- Gao, J.; Yang, S.; Li, N.; Meng, L.; Wang, F.; He, H.; Sun, C. Rapid degradation of azo dye Direct Black BN by magnetic MgFe2O4-SiC under microwave radiation. Appl. Surf. Sci. 2016, 379, 140–149. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, W.; Huo, N.; Yang, S. High rate capability and long cycle stability of Fe2O3/MgFe2O4 anode material synthesized by gel-cast processing. Chem. Eng. J. 2017, 307, 999–1007. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Li, X.; Zhao, Q.; Hou, Y. One-pot synthesis of MgFe2O4 nanospheres by solvothermal method. Mater. Lett. 2013, 96, 85–88. [Google Scholar] [CrossRef]
- Tabaza, W.A.I.; Swart, H.C.; Kroon, R.E. Luminescence of Ce doped MgAl2O4 prepared by the combustion method. Phys. B 2014, 439, 109–114. [Google Scholar] [CrossRef]
- Strohmeier, B.R. Magnesium Aluminate (MgAl2O4) by XPS. Surf. Sci. Spectra 1994, 3, 121–127. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.; Tian, X.; Yang, C.; Li, Y. Superior capability of MgAl2O4 for selenite removal from contaminated groundwater during its reconstruction of layered double hydroxides. Sep. Purif. Technol. 2017, 176, 66–72. [Google Scholar] [CrossRef]
- Fu, P.; Xu, Y.; Shi, H.; Zhang, B.; Ruan, X.; Lu, W. The effect of annealing process on the optical and microwave dielectric properties of transparent MgAl2O4 ceramics by spark plasma sintering. Opt. Mater. 2014, 36, 1232–1237. [Google Scholar] [CrossRef]
- Elmhamdi, A.; Castañeda, R.; Kubacka, A.; Pascual, L.; Nahdi, K.; Martínez-Arias, A. Characterization and catalytic properties of CuO/CeO2/MgAl2O4 for preferential oxidation of CO in H2-rich streams. Appl. Catal. B Environ. 2016, 188, 292–304. [Google Scholar] [CrossRef]
- Fernández-Garcı́a, M.; Gómez Rebollo, E.; Guerrero Ruiz, A.; Conesa, J.C.; Soria, J. Influence of Ceria on the Dispersion and Reduction/Oxidation Behaviour of Alumina-Supported Copper Catalysts. J. Catal. 1997, 172, 146–159. [Google Scholar] [CrossRef]
- Martı́nez-Arias, A.; Fernández-Garcı́a, M.; Soria, J.; Conesa, J.C. Spectroscopic Study of a Cu/CeO2Catalyst Subjected to Redox Treatments in Carbon Monoxide and Oxygen. J. Catal. 1999, 182, 367–377. [Google Scholar] [CrossRef]
- Gamarra, D.; Munuera, G.; Hungría, A.B.; Fernández-García, M.; Conesa, J.C.; Midgley, P.A.; Wang, X.Q.; Hanson, J.C.; Rodríguez, J.A.; Martínez-Arias, A. Structure—Activity Relationship in Nanostructured Copper—Ceria-Based Preferential CO Oxidation Catalysts. J. Phys. Chem. C 2007, 111, 11026–11038. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A.; Calvino, J.J.; Rodríguez-Luque, M.P. Synthesis of acidic Al-MCM-48: Influence of the Si/Al ratio, degree of the surfactant hydroxyl exchange, and post-treatment in NH4F solution. J. Catal. 2005, 230, 327–338. [Google Scholar] [CrossRef]
- Gracia, M.J.; Losada, E.; Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Activity of Gallium and Aluminum SBA-15 materials in the Friedel–Crafts alkylation of toluene with benzyl chloride and benzyl alcohol. Appl. Catal. A Gen. 2008, 349, 148–155. [Google Scholar] [CrossRef]
- Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. NH4F effect in post-synthesis treatment of Al-MCM-41 mesoporous materials. Microporous Mesoporous Mater. 2005, 84, 11–20. [Google Scholar] [CrossRef]
- Pineda, A.; Balu, A.M.; Campelo, J.M.; Luque, R.; Romero, A.A.; Serrano-Ruiz, J.C. High alkylation activities of ball-milled synthesized low-load supported iron oxide nanoparticles on mesoporous aluminosilicates. Catal. Today 2012, 187, 65–69. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds CuO/MgAl2O4 and CuO/MgFe2O4 are available from the authors. |

| Spinel | Surface Area (m2/g) | Pore Volume (cm3/g) | Mean Pore Size (nm) | Crystallite Size (nm) |
|---|---|---|---|---|
| CuO/MgAl2O4 | 20 | 0.09 | 18.2 | 25.9 a |
| CuO/MgFe2O4 | <5 | 0.03 | 51.4 | 78.0 b |
| Nanocatalyst | Magnetic Susceptibility (10−6 m3/Kg) | Total Acidity (µmol PY/g) | Brönsted Acidity (µmol DMPY/g) 1 | Lewis Acidity (µmol PY/g) 2 |
|---|---|---|---|---|
| CuO/MgAl2O4 | - | 69 | 43 | 26 |
| CuO/MgFe2O4 | 416 | 38 | 12 | 26 |
 | |||||
|---|---|---|---|---|---|
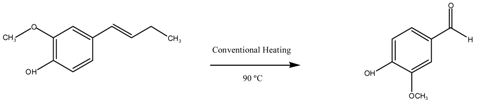
| Entry | Catalyst | Time (h) | Conversion (%mol) | Selectivity | |
| Vanillin | Others | ||||
| 1 | Blank | 2 | 18 | 7 | 93 |
| 2 | CuO/MgAl2O4 | 2 | 67 | 74 | 26 |
| 3 | 1 Reuse CuO/MgAl2O4 | 2 | 70 | 76 | 24 |
| 4 | 2 Reuse CuO/MgAl2O4 | 2 | 63 | 64 | 36 |
| 5 | 3 Reuse CuO/MgAl2O4 | 2 | 40 | 42 | 58 |
| 6 | 4 Reuse CuO/MgAl2O4 | 2 | 29 | 28 | 72 |
| 7 | Blank | 5 | 20 | 11 | 89 |
| 8 | CuO/MgFe2O4 | 5 | 53 | 46 | 54 |
| 9 | 1 Reuse CuO/MgFe2O4 | 5 | 50 | 45 | 55 |
| 10 | 2 Reuse CuO/MgFe2O4 | 5 | 29 | 23 | 77 |
| 11 | 3 Reuse CuO/MgFe2O4 | 5 | 17 | 9 | 91 |
| 12 | 4 Reuse CuO/MgFe2O4 | 5 | 17 | 8 | 92 |
| Reaction Conditions: 8 mL acetonitrile, 5 mmol isoeugenol, 20 mmol H2O2, 100 mg catalyst, 90 °C. | |||||
 | |||||
|---|---|---|---|---|---|
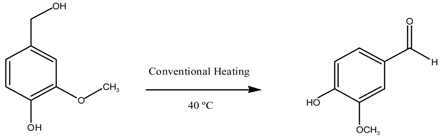
| Entry | Catalyst | Time (h) | Conversion (%mol) | Selectivity | |
| Vanillin | Others | ||||
| 1 | Blank | 8 | 21 | 0 | 100 |
| 2 | CuO/MgAl2O4 | 8 | 81 | 100 | - |
| 3 | 1 Reuse CuO/MgAl2O4 | 8 | 84 | 100 | - |
| 4 | 2 Reuse CuO/MgAl2O4 | 8 | 79 | 100 | - |
| 5 | 3 Reuse CuO/MgAl2O4 | 8 | 56 | 100 | - |
| 6 | 4 Reuse CuO/MgAl2O4 | 8 | 29 | 100 | - |
| 7 | Blank | 8 | 21 | 0 | 100 |
| 8 | CuO/MgFe2O4 | 8 | 64 | 100 | - |
| 9 | 1 Reuse CuO/MgFe2O4 | 8 | 65 | 100 | - |
| 10 | 2 Reuse CuO/MgFe2O4 | 8 | 42 | 100 | - |
| 11 | 3 Reuse CuO/MgFe2O4 | 8 | 26 | 100 | - |
| 12 | 4 Reuse CuO/MgFe2O4 | 8 | 25 | 100 | - |
| Reaction Conditions: 8 mL acetonitrile, 5 mmol vanillin alcohol, 20 mmol H2O2, 100 mg catalyst, 40 °C | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmanivahid, B.; Pinilla-de Dios, M.; Haghighi, M.; Luque, R. Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol. Molecules 2019, 24, 2597. https://doi.org/10.3390/molecules24142597
Rahmanivahid B, Pinilla-de Dios M, Haghighi M, Luque R. Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol. Molecules. 2019; 24(14):2597. https://doi.org/10.3390/molecules24142597
Chicago/Turabian StyleRahmanivahid, Behgam, Maria Pinilla-de Dios, Mohammad Haghighi, and Rafael Luque. 2019. "Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol" Molecules 24, no. 14: 2597. https://doi.org/10.3390/molecules24142597
APA StyleRahmanivahid, B., Pinilla-de Dios, M., Haghighi, M., & Luque, R. (2019). Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol. Molecules, 24(14), 2597. https://doi.org/10.3390/molecules24142597







