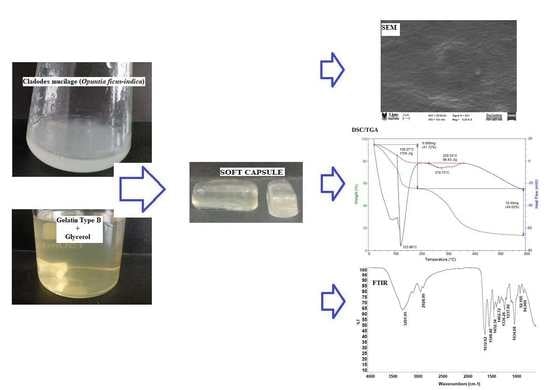
Preparation and Physicochemical Characterization of Softgels Cross-Linked with Cactus Mucilage Extracted from Cladodes of Opuntia Ficus-Indica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
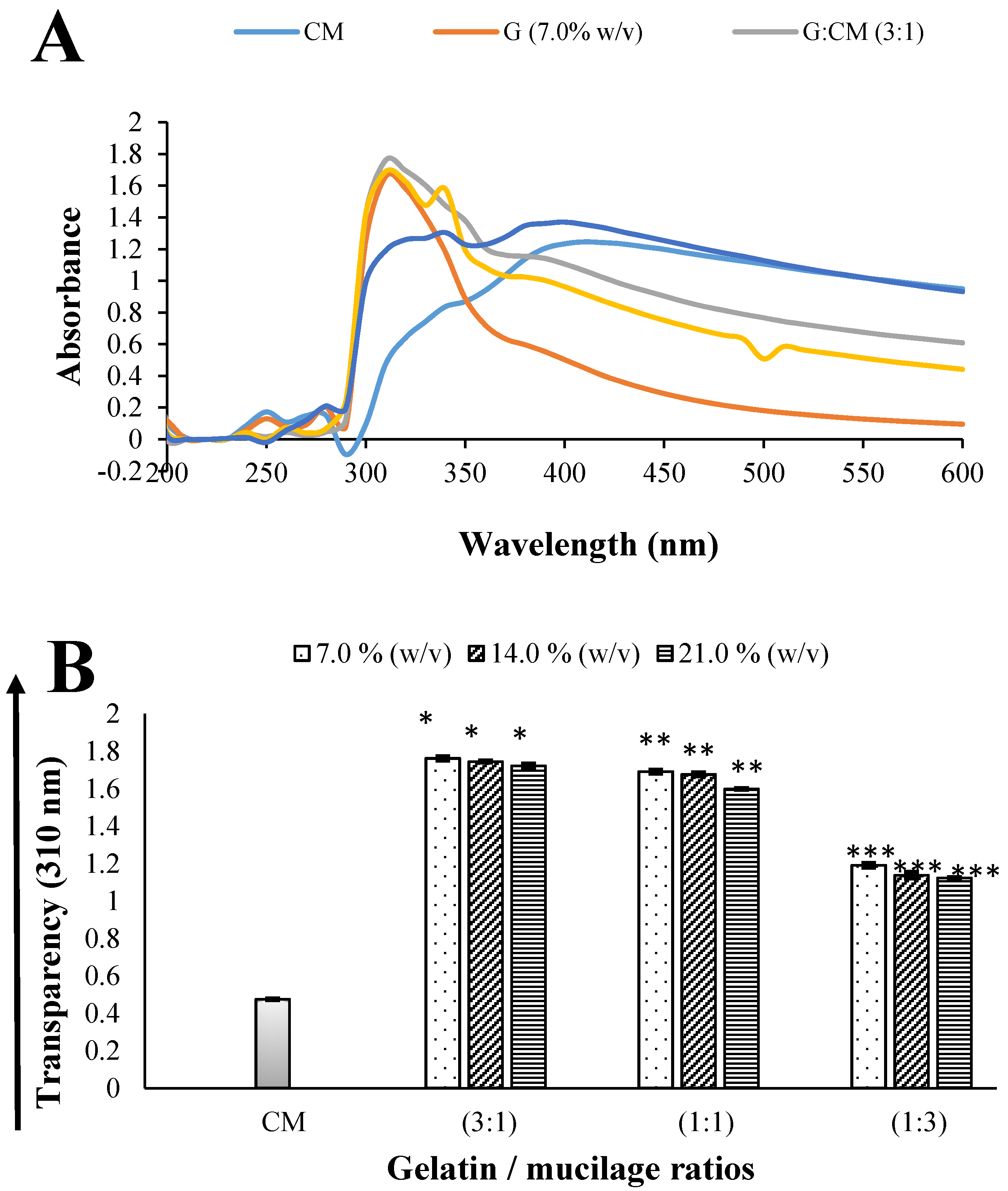
2.1.1. Transparency
2.1.2. Moisture Content
2.1.3. Solubility in Water
2.1.4. Color Parameters
2.2. Structural Properties
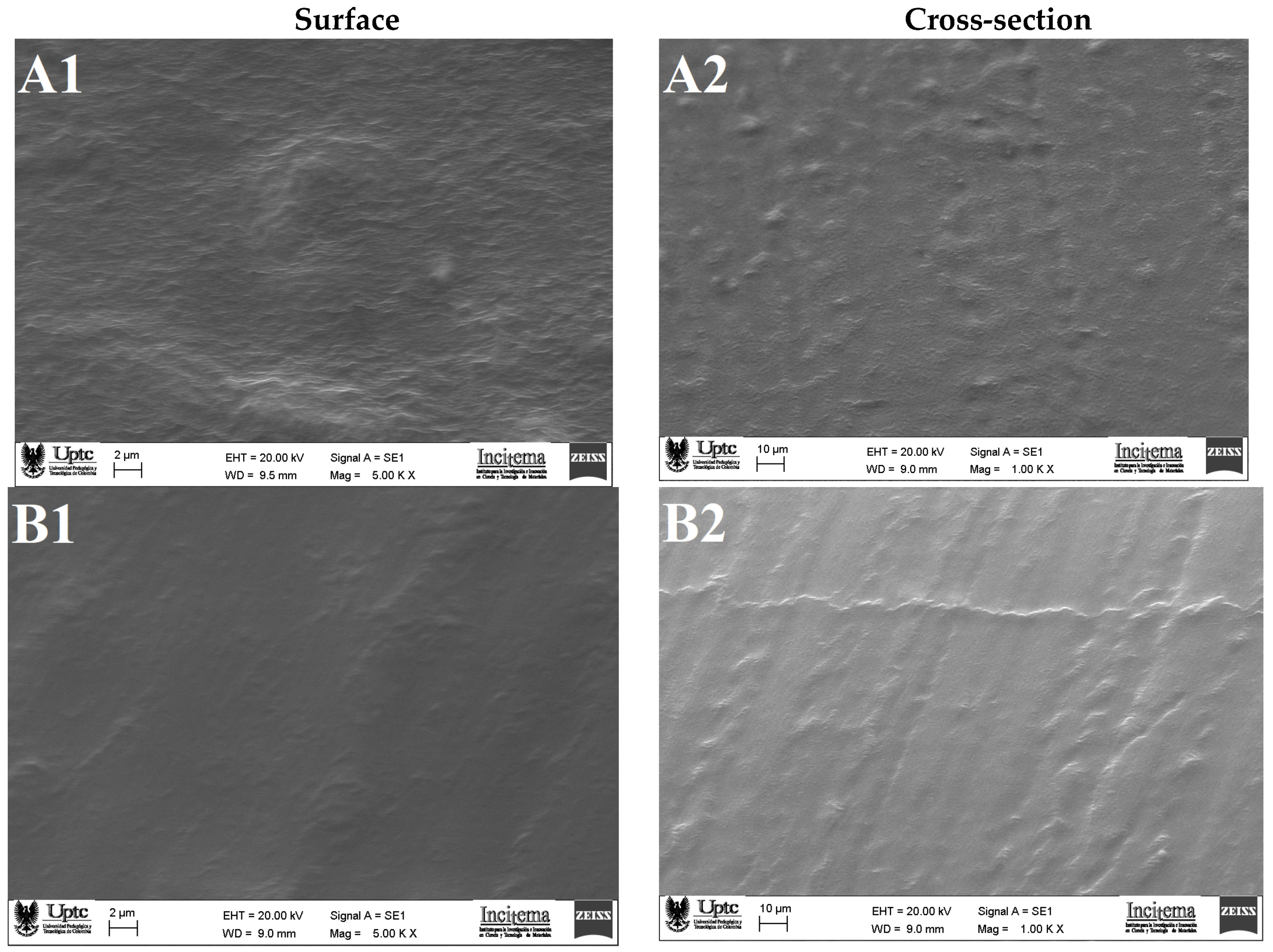
2.2.1. Scanning Electron Microscopy (SEM)
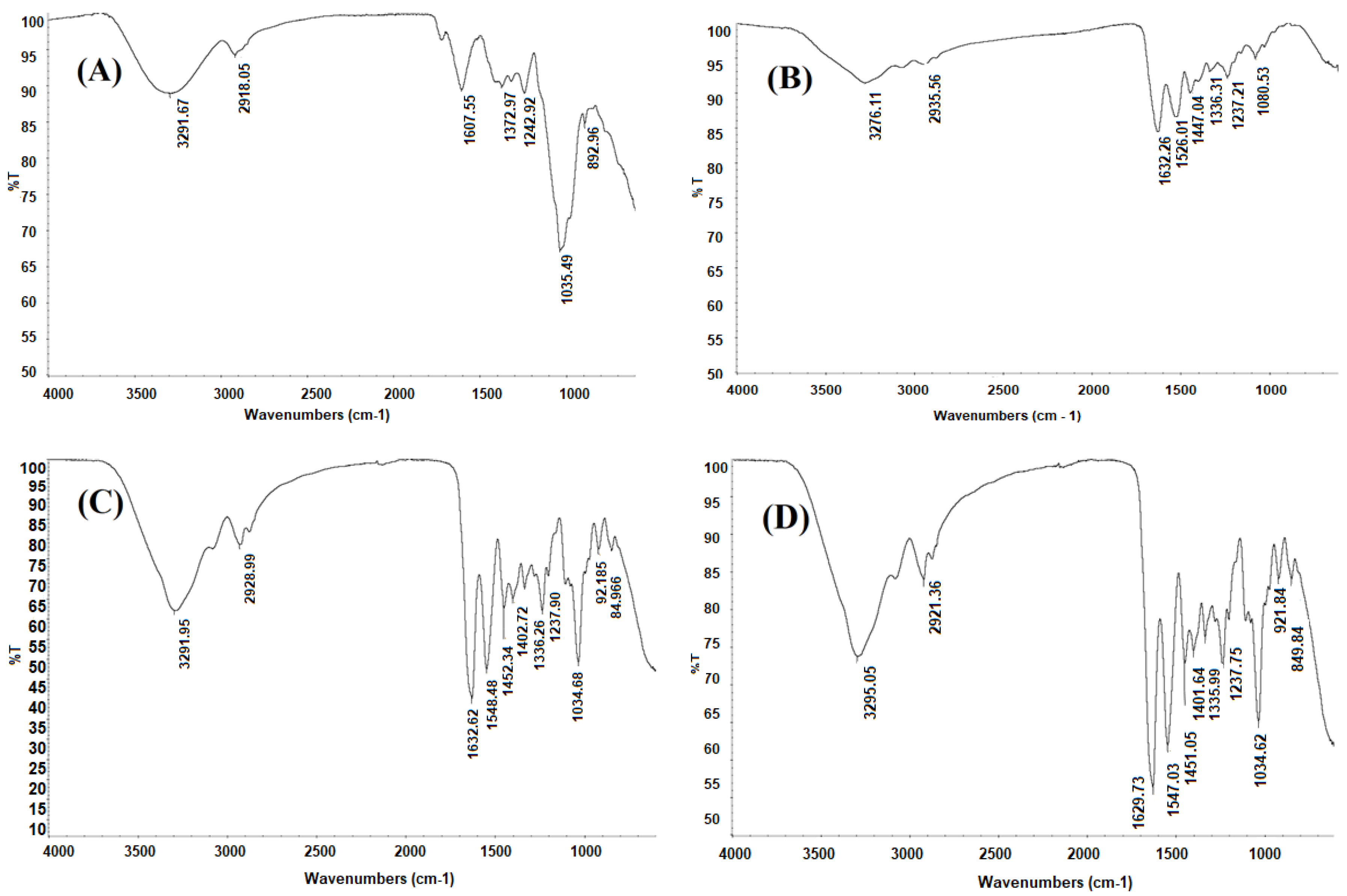
2.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
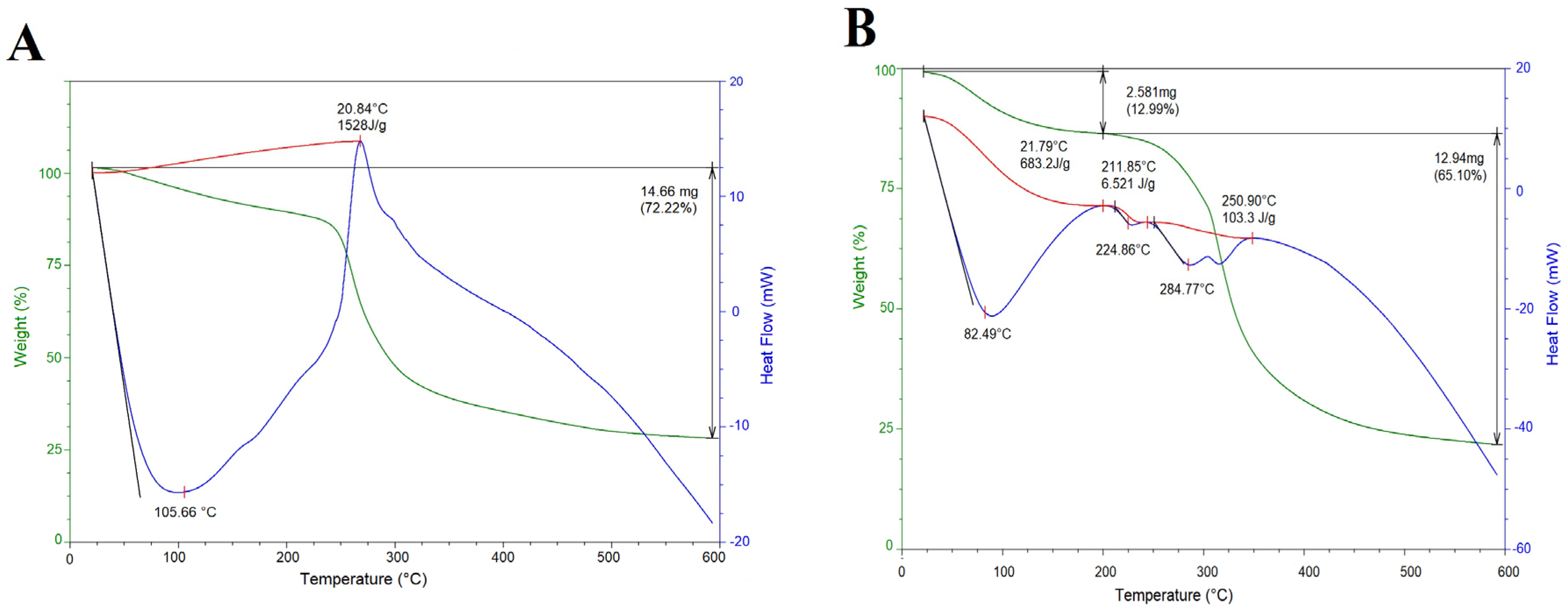
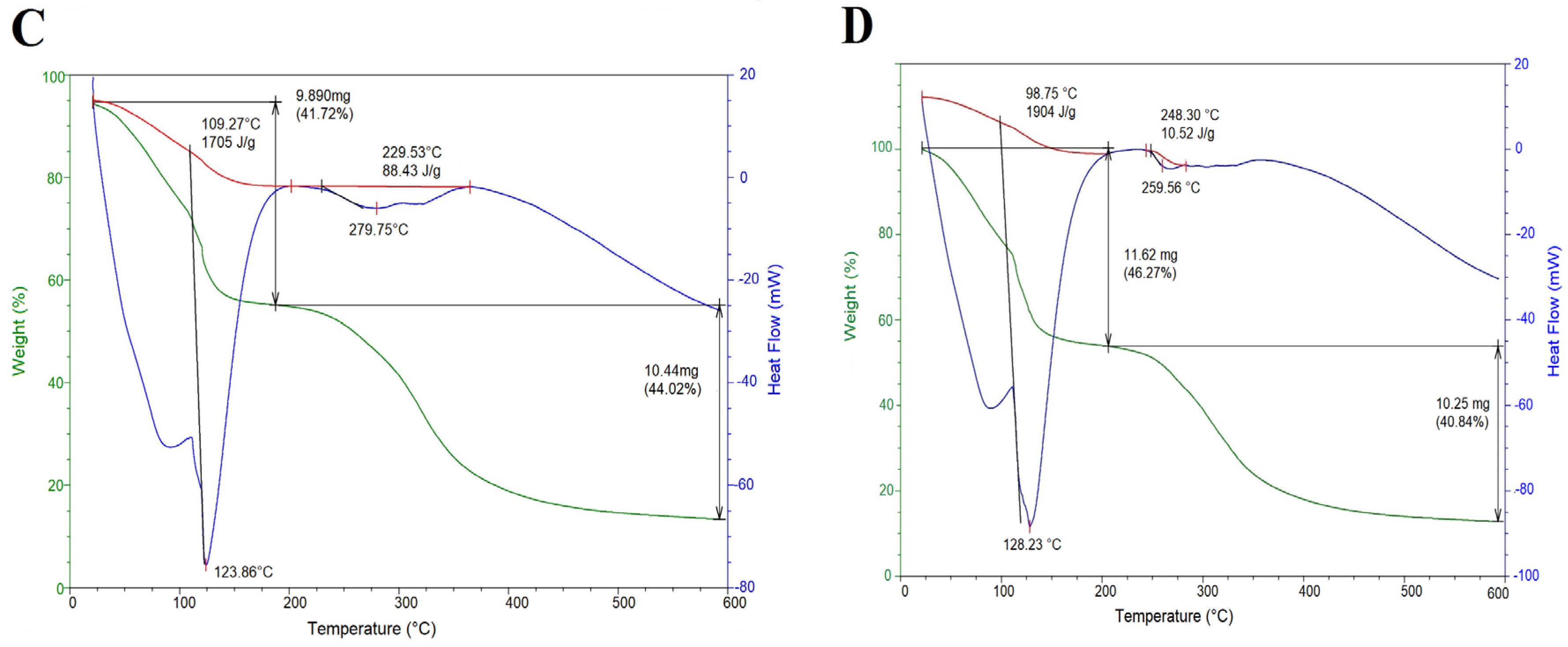
2.3. Thermal Properties
2.4. Dietary Fiber Content
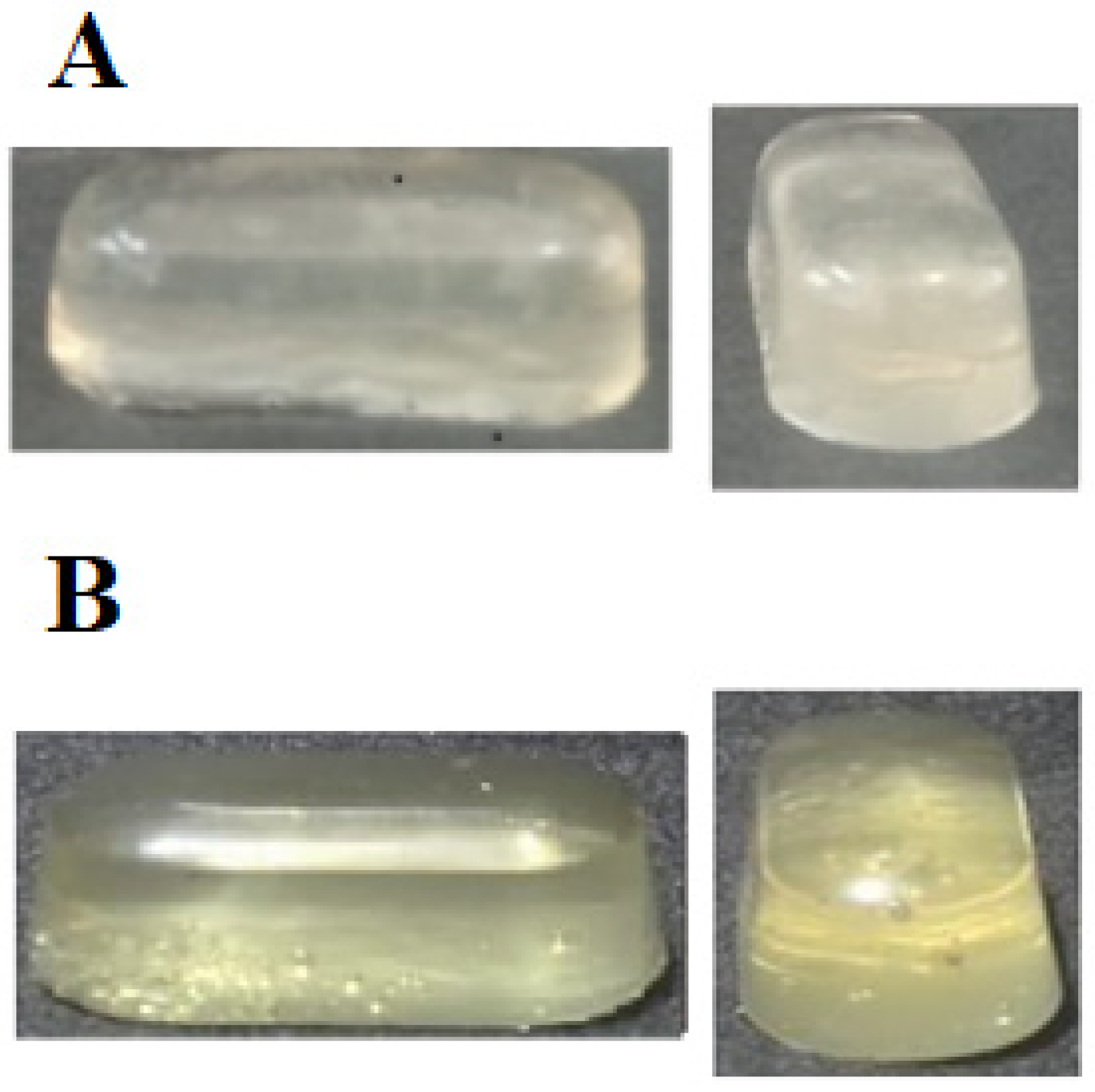
2.5. Photograph of Composite Capsules (CMC) and Control Capsules (CC)
3. Materials and Methods
3.1. Materials and Reagent
3.2. Preparation of Solutions
3.3. Formation of Soft Capsules
3.4. Physicochemical Properties
3.4.1. Transparency Measurements of Soft Capsule-Forming Solutions
3.4.2. Moisture Content
3.4.3. Solubility in Water
3.4.4. Color Parameters
3.5. Structural Properties
3.5.1. Scanning Electron Microscopy (SEM)
3.5.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Thermal Properties
3.7. Dietary Fiber Content
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gullapalli, R.P. Soft gelatin capsules (softgels). J. Pharm. Sci. 2010, 99, 4107–4148. [Google Scholar] [CrossRef] [PubMed]
- Mutalib, S.A.; Muin, N.M.; Aminah Abdullah, A.; Hassan, O.; Mustapha, W.A.W.; Sani, N.A.; Maskat, M.Y. Sensitivity of polymerase chain reaction (PCR)-southern hybridization and conventional PCR analysis for Halal authentication of gelatin capsules. LWT Food Sci. Technol. 2015, 63, 714–719. [Google Scholar] [CrossRef]
- Gullapalli, R.P.; Mazzitelli, C.L. Gelatin and Non-Gelatin Capsule Dosage Forms. J. Pharm. Sci. 2017, 106, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, I.; Jridi, M.; Nasri, R.; Dammak, A.; Hajji, M.; Nasri, M.; Barkia, A. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014, 41, 309–318. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jimenez-Munguia, M.T.J. Complex Coacervation Between Gelatin and Chia Mucilage as an Alternative of Encapsulating Agents. Food Sci. 2019, 84, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.C.; de Oliveira, M.; Ferreira, J.G.L.; Sierakowski, M.R.; Simas-Tosin, F.F.; Orth, E.S.; Riegel-Vidotti, I.C. Polyelectrolyte complexes from gum arabic and gelatin: Optimal complexation pH as a key parameter to obtain reproducible microcapsules. Food Hydrocoll. 2015, 46, 201–207. [Google Scholar] [CrossRef]
- Tanner, K.E.; Draper, P.R.; Getz, J.J.; Burnett, S.W.; Youngblood, E. Film Forming Compositions Comprising Modified Starches and Iota-Carrageenan and Methods for Manufacturing Soft Capsules Using Same. U.S. Patent US6582727, 27 June 2002. Available online: http://www.patentbuddy.com/Patent/6582727 (accessed on 3 January 2019).
- McGarvie, D.; Parolis, H. The mucilage of Opuntia ficus-indica. Carbohydr. Res. 1979, 69, 171–179. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepulveda, E.; Matsuhiro, B. Opuntia spp mucilages: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus-indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntia ficus-indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Sáenz-Hernández, C.; Corrales-García, J.; Aquino-Pérez, G. Nopalitos, Mucilage, Fiber, and Cochineal. In Cacti: Biology and Uses, 1st ed.; Nobel, P.S., Ed.; University of California Press: London, CA, USA, 2002; pp. 211–234. [Google Scholar]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, S.; Martinez-Flores, H.E.; Chavez-Moreno, C.K.; Marcias-Rodriguez, L.I.; Zavalia-Mendoza, E.; Garnica-Romo, M.G.; Chacón-García, L. Extraction and characterization of mucilage from wild species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Tripodo, M.M.; d’Aquino, A.; Mondello, M.R. Antiulcer activity of Opuntia ficus-indica (L.) Mill. (Cactaceae): Ultrastructural study. J. Ethnopharmacol. 2001, 76, 1–9. [Google Scholar] [CrossRef]
- Trombetta, D.; Puglia, C.; Perri, D.; Licata, A.; Pergolizzi, S.; Lauriano, E.R.; De Pasquale, A.; Saija, A.; Bonina, F.P. Effect of polysaccharides from Opuntia ficus-indica (L.) cladodes on the healing of dermal wounds in the rat. Phytomedicine 2006, 13, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; Miceli, N.; Mondello, M.R.; Taviano, M.F.; Galluzzo, M.; Tripodo, M.M. Opuntia ficus-indica (L.) Mill. mucilages show cytoprotective effect on gastric mucosa in rat. Phytother. Res. 2007, 25, 370–375. [Google Scholar] [CrossRef]
- Galati, E.M.; Tripodo, M.M.; Trovato, A.; d’Aquino, A.; Monforte, M.T. Biological activity of Opuntia ficus-indica cladodes II: Effect on experimental hypercholesterolemia in rats. Pharm. Biol. 2003, 41, 175–179. [Google Scholar] [CrossRef]
- De Campo, C.; Dick, M.; Pereira dos Santos, P.; Haas Costa, T.M.; Paese, K.; Stanisçuaski Guterres, S.; De Oliveira Rios, A.; Hickmann Flôres, S. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. A 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Osorio, C.; Nazareno, M.A. Encapsulation of cactus (Opuntia megacantha) betaxanthins by ionic gelation and spray drying: A comparative study. Food Res. Int. 2018, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray drying of nopal mucilage (Opuntia ficus-indica): Effects on powder properties and characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Bou, S. Novel, multifunctional mucilage composite films incorporated with cellulose nanofibers. Food Hydrocoll. 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Meznic, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Lira-Vargas, A.A.; Corrales-García, J.J.E.; Valle-Guadarrama, S.; Peña-Valdivia, C.B.; Trejo-Marquez, M.A. Biopolymeric films based on cactus (Opuntia ficus-indica) mucilage incorporated with gelatin and bees wax. J. Prof. Assoc. Cactus. 2014, 16, 51–70. [Google Scholar]
- Espino-Díaz, M.; Ornelas-Paz, J.J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and Characterization of Edible Films Based on Mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf. 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; AskariTransfer, G. Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Semenova, M.G.; Dickinson, E. Biopolymers in Food Colloids: Thermodynamics and Molecular Interactions, 1st ed.; CRC Press: London, UK, 2010; pp. 117–130. [Google Scholar]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevi, V.; Kucharek, M.; Makarewicz, M.; Adam, V. Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll. 2018, 83, 9–16. [Google Scholar] [CrossRef]
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Ma, J.; Zhong, F. Tailoring physical properties of transglutaminase-modified gelatin films by varying drying temperature. Food Hydrocoll. 2016, 58, 20–28. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Ebadi, S.; Nafchi, A.M.; Karimd, A.A.; Kiahosseini, S.R. Functional properties of dually modified sago starch/κ-carrageenan films: An alternative to gelatin in pharmaceutical capsules. Carbohydr. Polym. 2017, 160, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Esteghlal, S.; Niakousari, M.; Mohammad, S.; Hosseini, H. Physical and mechanical properties of gelatin-CMC composite films under the influence of electrostatic interactions. Int. J. Biol. Macromol. 2018, 114, 1–9. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of type and content of binary polyol mixtures on physical and mechanical properties of starch-based edible films. Carbohydr. Polym. 2008, 71, 269–276. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Ye, R.; Liu, A.; Xiao, J.; Liu, Y.; Zhao, Y. Mechanical properties and solubility in water of corn starch-collagen composite films: Effect of starch type and concentrations. Food Chem. 2017, 216, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Cao, Y.; Wang, W.; Chen, X.; Cai, J.; Wang, L.; Xiao, J. Sustained-release antimicrobial gelatin film: Effect of chia mucilage on physicochemical and antimicrobial properties. Food Hydrocoll. 2019, 87, 783–791. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on CMC, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Nharingo, T.; Moyo, M. Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. J. Environ. Manag. 2016, 166, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Duhoranimana, E.; Karangwa, E.; Lai, L.; Xu, X.; Yu, J.; Xia, S.; Zhang, X.; Muhoza, B.; Habinshuti, I. Effect of sodium carboxymethyl cellulose on complex coacervates formation with gelatin: Coacervates characterization stabilization and formation mechanism. Food Hydrocoll. 2017, 69, 111–120. [Google Scholar] [CrossRef]
- Das, M.P.; Suguna, P.R.; Prasad, K.; Vijaylakshmi, J.V.; Renuka, M. Extraction and Characterization of Gelatin: A Functional Biopolymer. Int. J. Pharm. Pharm. Sci. 2017, 9, 239–242. [Google Scholar] [CrossRef]
- Nor, M.M.; Nazmi, N.N.M.; Sarbon, N.M. Effect of different hydrophilic plasticizer concentrations on functional properties of chicken skin gelatin films. Int. Food Res. J. 2017, 24, 1910–1918. [Google Scholar]
- Manhivi, V.E.; Venter, S.; Amonsou, E.O.; Kudanga, T. Composition, thermal and rheological properties of polysaccharides from amadumbe (Colocasia esculenta) and cactus (Opuntia spp.). Carbohydr. Polym. 2018, 195, 163–169. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Furtado, R.F.; Bastos, M.S.R.; Leitão, R.C.; Benevides, S.D.; Muniz, C.R.; Cheng, H.N.; Biswas, A. Performance evaluation of cashew gum and gelatin blend for food packaging. Food Packag. Shelf 2018, 17, 57–64. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Oishi, S.; Kimura, S.; Noguchi, S.; Kondo, M.; Kondo, Y.; Shimokawa, Y.; Iwao, Y.; Itai, S. New scale-down methodology from commercial to lab scale to optimize plant-derived soft gel capsule formulations on a commercial scale. Int. J. Pharm. 2018, 535, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Quinzio, C.; Corvalán, M.; López, B.; Iturriaga, L. Studying stability against coalescence in tuna mucilage emulsions. Acta Hort. 2009, 811, 427–431. [Google Scholar] [CrossRef]
- Lafargue, D.; Lourdin, D.; Doublier, J.L. Film-forming properties of a modified starch/κ-carrageenan mixture in relation to its rheological behaviour. Carbohydr. Polym. 2007, 70, 101–111. [Google Scholar] [CrossRef]
- AOAC. Enzymatic-gravimetric method. In Official Methods of Analysis of AOAC International, 15th ed.; AOAC: Gaithersburg, MD, USA, 1995. [Google Scholar]
- InfoStat Software Statics. Available online: http://www.infostat.com.ar (accessed on 15 September 2018).
Sample Availability: Samples of the composite (CMC) and control (CC) soft capsules are available from the authors. |
| Parameter | Composite Capsule | Control Capsule |
|---|---|---|
| L* | 71.38 ± 1.93 a | 87.73 ± 0.88 b |
| a* | −1.80 ± 0.02 c | −1.97 ± 0.08 c |
| b* | 13.07 ± 0.53 d | 13.36 ± 0.43 d |
| ΔE* | 25.45 ± 1.79 e | 12.25 ± 0.87 f |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camelo Caballero, L.R.; Wilches-Torres, A.; Cárdenas-Chaparro, A.; Gómez Castaño, J.A.; Otálora, M.C. Preparation and Physicochemical Characterization of Softgels Cross-Linked with Cactus Mucilage Extracted from Cladodes of Opuntia Ficus-Indica. Molecules 2019, 24, 2531. https://doi.org/10.3390/molecules24142531
Camelo Caballero LR, Wilches-Torres A, Cárdenas-Chaparro A, Gómez Castaño JA, Otálora MC. Preparation and Physicochemical Characterization of Softgels Cross-Linked with Cactus Mucilage Extracted from Cladodes of Opuntia Ficus-Indica. Molecules. 2019; 24(14):2531. https://doi.org/10.3390/molecules24142531
Chicago/Turabian StyleCamelo Caballero, Luis R., Andrea Wilches-Torres, Agobardo Cárdenas-Chaparro, Jovanny A. Gómez Castaño, and María Carolina Otálora. 2019. "Preparation and Physicochemical Characterization of Softgels Cross-Linked with Cactus Mucilage Extracted from Cladodes of Opuntia Ficus-Indica" Molecules 24, no. 14: 2531. https://doi.org/10.3390/molecules24142531
APA StyleCamelo Caballero, L. R., Wilches-Torres, A., Cárdenas-Chaparro, A., Gómez Castaño, J. A., & Otálora, M. C. (2019). Preparation and Physicochemical Characterization of Softgels Cross-Linked with Cactus Mucilage Extracted from Cladodes of Opuntia Ficus-Indica. Molecules, 24(14), 2531. https://doi.org/10.3390/molecules24142531