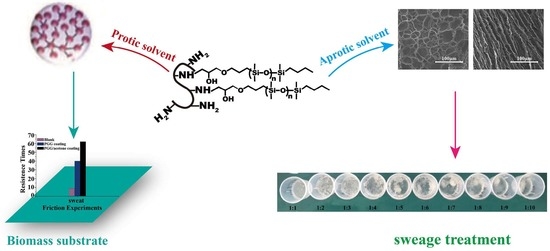
Induced Aggregation of Epoxy Polysiloxane Grafted Gelatin by Organic Solvent and Green Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Modification of Gelatin by PDMS-E
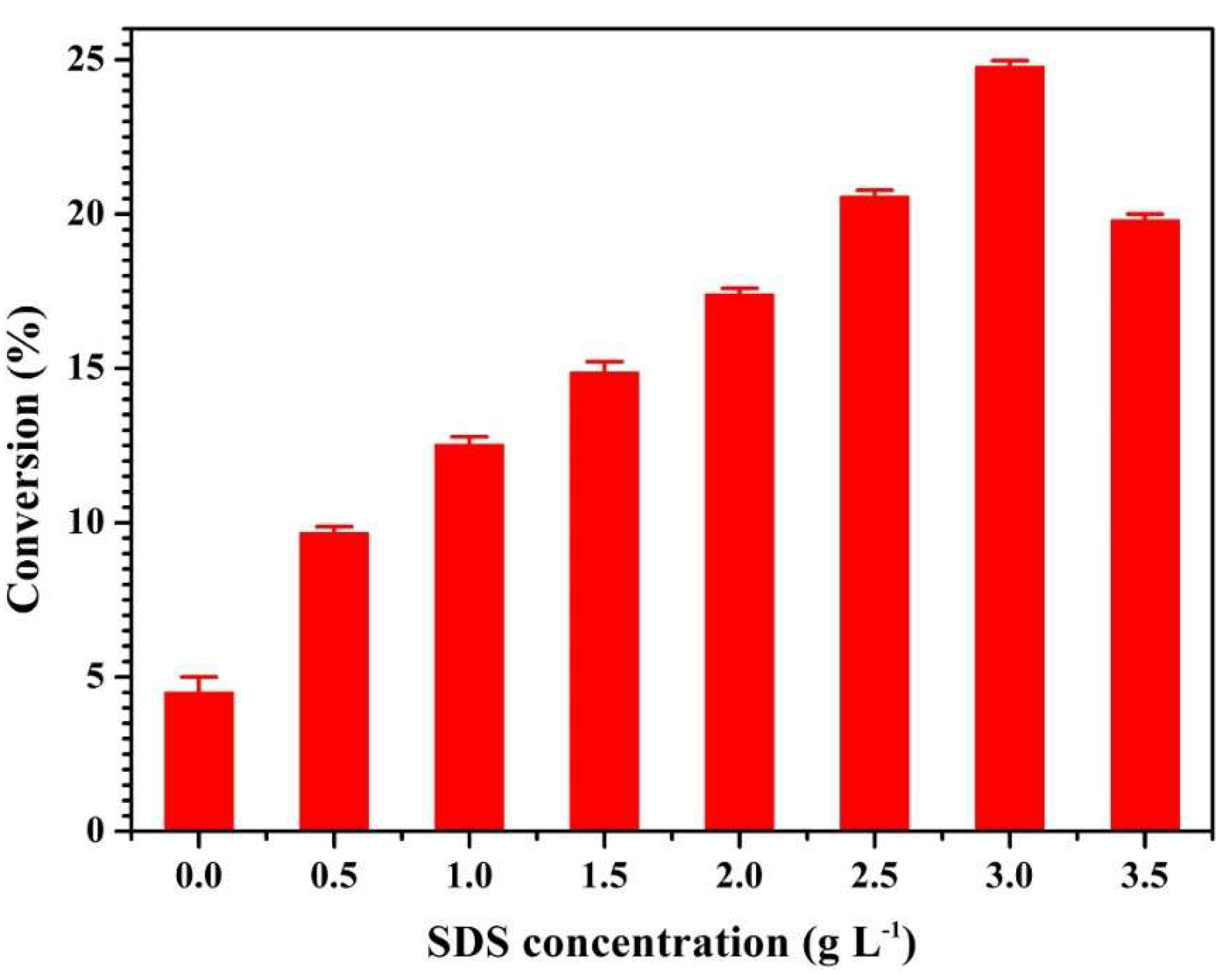
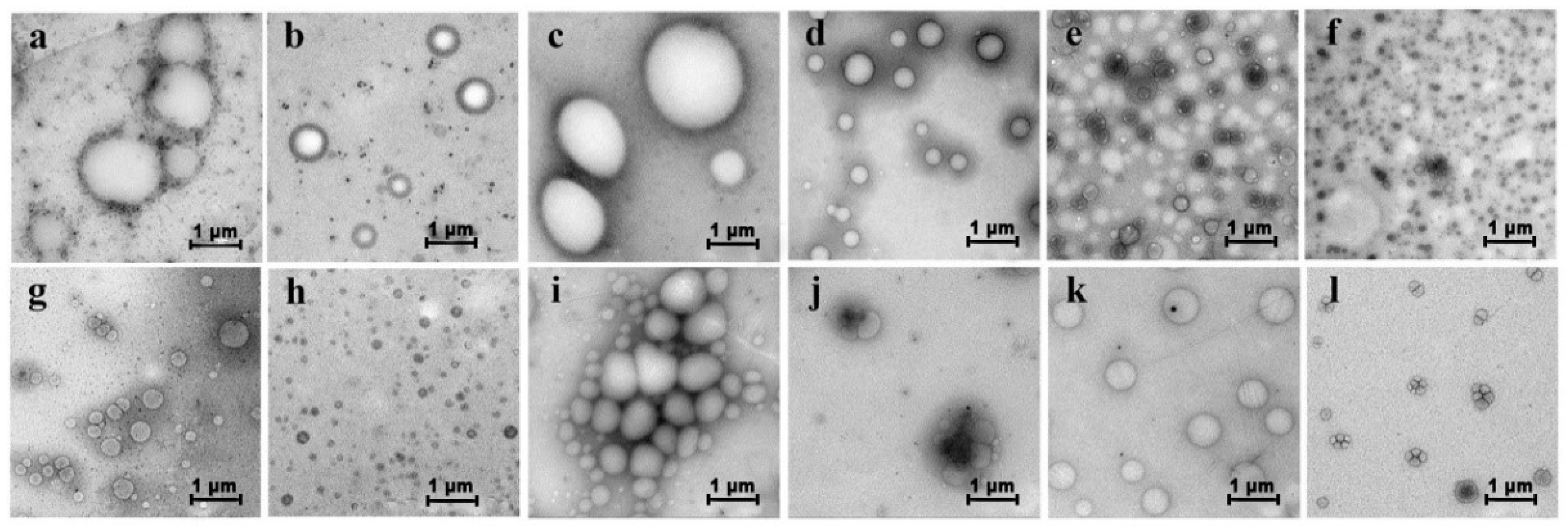
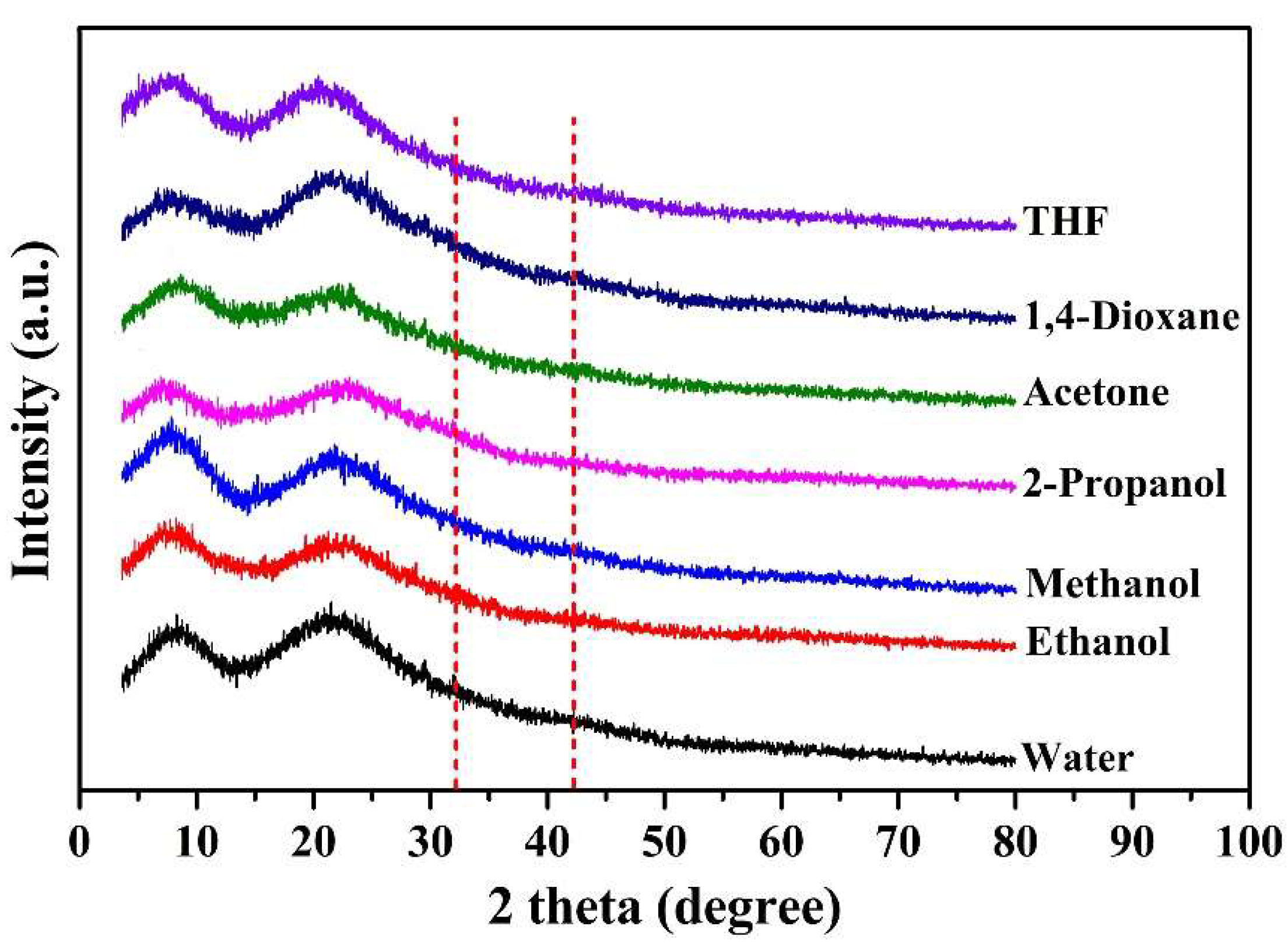
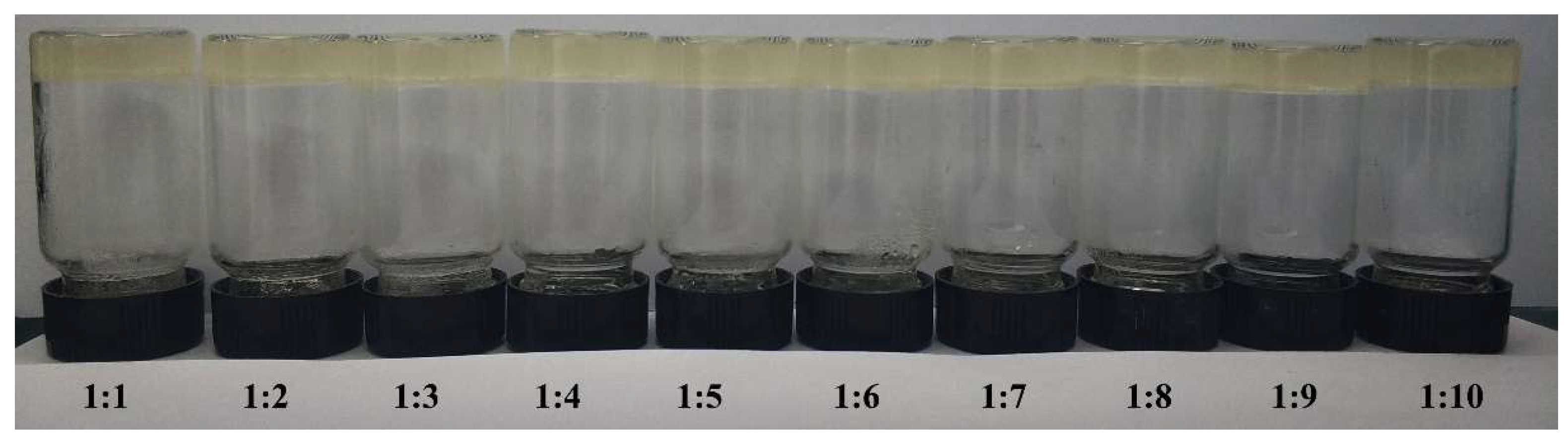
2.2. Aggregation of PGG Induced by Solvents
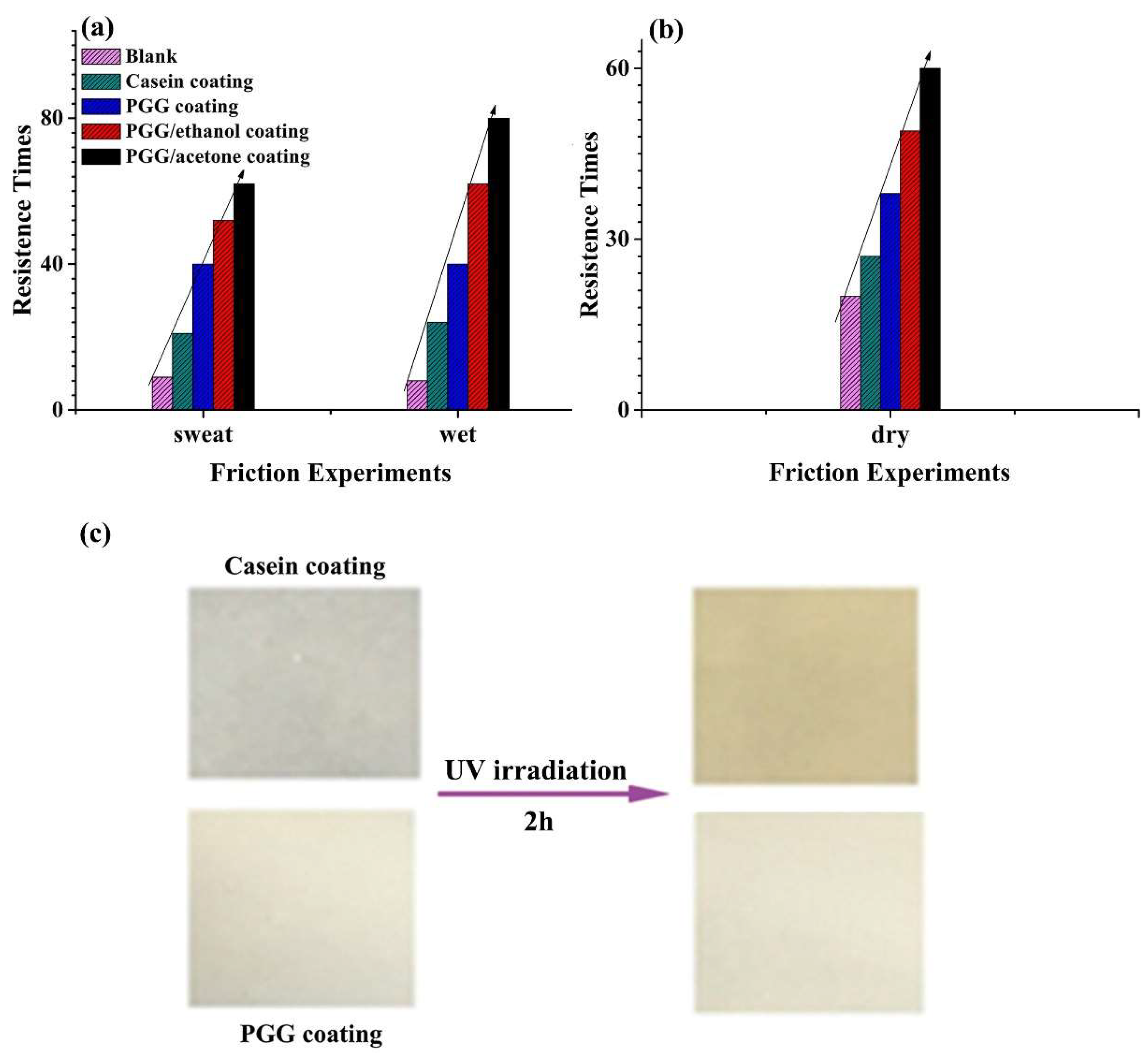
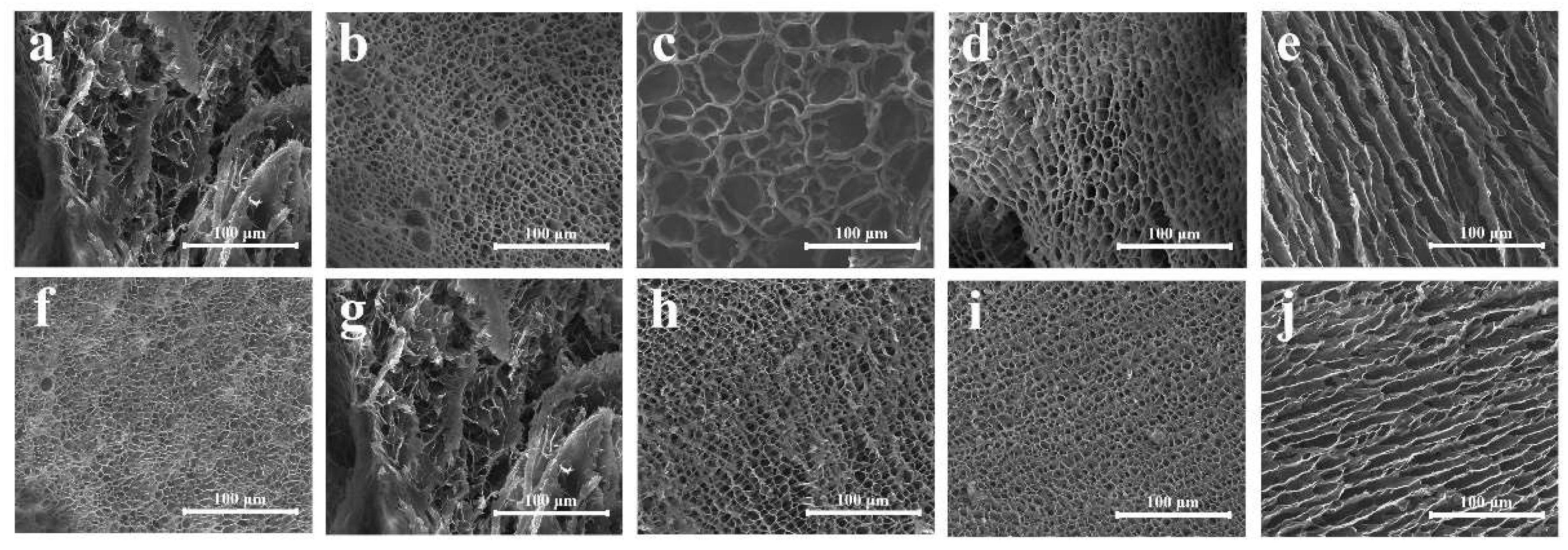
2.3. Coating Performance
2.4. Gel formation and Its Application
3. Materials and Methods
3.1. Materials
3.2. Synthesis of α–[3–(2,3–epoxy–propoxy)propyl]–ω–butyl–polydimethylsiloxanes (PDMS-E) Grafted Gelatin
3.3. Aggregation of PGG in Mixed Solvent
3.4. Characterization
3.5. Coating Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.F.; Xie, F.; Duan, P.F.; Liu, M.H. Dynamic evolution of coaxial nanotoruloid in the self-assembled naphthyl-containing l-glutamide. Langmuir 2016, 32, 12534–12541. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, D.; Zhao, K.; Du, W.; Zheng, J.; Yang, M. A water-dispersible, ferrocene-tagged peptide nanowire for amplified electrochemical immunosensing. Biosens. Bioelectron. 2013, 48, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Challa, S.R.; Medforth, C.J.; Qiu, Y.; Watt, R.K.; Peña, D.; Miller, J.E.; van Swol, F.; Shelnutt, J.A. Synthesis of peptide-nanotube platinum-nanoparticle composites. Chem. Commun. 2004, 7, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, Y.; Qi, W.; Su, Y.; Yang, Y.; He, Q.; Li, J. Self-assembly of peptide-based colloids containing lipophilic nanocrystals. Small 2008, 4, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.E.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylme-thoxycarbonyl-dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Holowka, E.P.; Pochan, D.J.; Deming, T.J. Charged polypeptide vesicles with controllable diameter. J. Am. Chem. Soc. 2005, 127, 12423–12428. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Lamprou, D.A.; Urquhart, A.J.; Yannopoulos, S.N.; Vizirianakis, I.S.; Zhang, S.; Koutsopoulos, S. Lipid-like self-assembling peptide nanovesicles for drug delivery. ACS Appl. Mater. Inter. 2014, 6, 8184–8189. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, S.K.; Verma, S. Self-assembly and potassium ion triggered disruption of peptide-based soft structures. Chem. Commun. 2007, 14, 2296–2298. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Yan, L.Y.; Wang, A.H.; Bai, S.; Yan, X.H. Trace solvent as a predominant factor to tune dipeptide self-assembly. ACS Nano 2016, 10, 2138–2143. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional supramolecular polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Izadyar, M.; Khavania, M.; Housaindokhta, M.R. A combined molecular dynamic and quantum mechanic study of the solvent and guest molecule effect on the stability and length of heterocyclic peptide nanotubes. Phys. Chem. Chem. Phys. 2015, 17, 11382–11391. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Gao, J.; Wu, Y.; Wang, X.; Zhao, R.; Mei, S.; Yang, J.; Zhai, X.; Qiu, H. Design of folic acid based supramolecular hybrid gel with improved mechanical properties in NMP/H2O for dye adsorption. React. Funct. Polym. 2018, 122, 140–147. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K. Solvent effects on supramolecular gel-phase materials: Two-component dendritic gel. Langmuir 2004, 20, 10851–10857. [Google Scholar] [CrossRef]
- Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- Gil, E.S.; Frankowski, D.J.; Bowman, M.K.; Gozen, A.O.; Hudson, S.M.; Spontak, R.J. Mixed protein blends composed of gelatin and bombyx mori silk fibroin: Effects of solvent-induced crystallization and composition. Biomacromolecules 2006, 7, 728–735. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.D.; Jiang, Q.W.; Qiao, C.D.; Cheng, J.Y. Microstructure transformation of PDMS-E grafted gelatin polymers induced by SDS and SDBS. Colloid Surf. B Biointerfaces 2013, 103, 375–380. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The β-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Lossow, C.V.; Schwarz, G. Cyclic polypeptides by solvent-induced polymerizations of α-amino acid N-carboxyanhydrides. Macromolecules 2005, 38, 5513–5518. [Google Scholar] [CrossRef]
- Luo, S.C.; Craciun, V.; Douglas, E.P. Instabilities during the formation of electroactive polymer thin films. Langmuir 2005, 21, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Choktaweesap, N.; Arayanarakul, K.; Aht-Ong, D.; Meechaisue, C.; Supaphol, P. Electrospun gelatin fibers: Effect of solvent system on morphology and fiber diameters. Polym. J. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Mason, T.O.; Chirgadze, D.Y.; Levin, A.; Adler-Abramovich, L.; Gazit, E.; Knowles, T.P.J.; Buell, A.K. Expanding the solvent chemical space for self-assembly of dipeptide nanostructures. ACS Nano 2014, 8, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, P.A.; Schaefer, C.; de Greef, T.F.A.; Meijer, E.W. Controlling chemical self-assembly by solvent-dependent dynamics. J. Am. Chem. Soc. 1348, 134, 13482–13491. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Liang, F.; Qu, X.; Wang, Q.; Zhu, J.; Yang, Z. Diblock copolymer based janus nanoparticles. Macromolecules 2015, 48, 750–755. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, properties and applications of Janus particles. Chem. Soc. Rev. 2012, 41, 4356–4378. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Zhang, C.L.; Qu, X.Z.; Liu, J.G.; Li, J.L.; Yang, Z.Z. Janus composite nanorings by combinational template synthesis and skiving micro-process. Polymer 2010, 51, 3606–3611. [Google Scholar] [CrossRef]
- Liang, F.; Liu, J.; Zhang, C.; Qu, X.Z.; Lia, J.; Yang, Z.Z. Janus hollow spheres by emulsion interfacial self-assembled sol-gel process. Chem. Commun. 2011, 47, 1231–1233. [Google Scholar] [CrossRef]
- Hanania, Z.A.N.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, L.; Zhao, S.; Ni, H.; Cao, S.; Wang, J. Cross-linked polyacrylonitrile/polyethyleneimine–polydimethylsiloxane composite membrane for solvent resistant nanofiltration. Chem. Eng. Sci. 2014, 106, 157–166. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Li, Z.; Nan, N.; Zhu, Y.; Li, Y. Forth flotation giant surfactants. Polymer 2019, 162, 58–62. [Google Scholar] [CrossRef]
- Mirsky, A.E.; Pauling, L. On the structure of native, denatured, and coagulated proteins. Proc. Natl. Acad. Sci. USA 1936, 22, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Privalov, P.L.; Kechinashvili, N.N. A thermodynamic approach to the problem of stabilization of globular protein structure: A calorimetric study. J. Mol. Biol. 1974, 86, 665–684. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Fu, F.N.; Deoliveira, D.B.; Trumble, W.R.; Sarkar, H.K.; Singh, B.R. Secondary structure estimation of proteins using the amide III region of fourier transform infrared spectroscopy: Application to analyze calcium-binding-induced structural changes in calsequestrin. Appl. Spectrosc. 1994, 48, 1432–1441. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Byler, D.M.; Brouillette, J.N.; Susi, H. Quantitative studies of protein structure by FT-IR spectral deconvolution and curve fitting. Spectroscopy 1986, 1, 29–32. [Google Scholar]
- Surewicz, W.K.; Moscarello, M.A.; Mantsch, H.H. Conformational properties of azurin in solution as determined from resolution-enhanced fourier-transform infrared spectra. Eur. J. Biochem. 1987, 167, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin filmsinfluenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.L.; Yan, X.H.; Su, Y.; Yang, Y.; Li, J.B. Solvent-induced structural transition of self-assembled dipeptide: From organogels to microcrystals. Chem. Eur. J. 2010, 16, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Chu, X.; Li, S.; Ma, M.; Hao, A. Hybrid gels assembled from fmoc–amino acid and graphene oxide with controllable properties. ChemPhysChem 2014, 15, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide hydrogels. RSC Adv. 2013, 3, 9117–9149. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Zhang, Y.; Wang, L.; Xu, B. Small molecule hydrogels based on a class of antiinflammatory agents. Chem. Commun. 2004, 2, 208–209. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Weng, W.; Li, Z.; Jamieson, A.M.; Rowan, S.J. Control of gel morphology and properties of a class of metallo-supramolecular polymers by good/poor solvent environments. Macromolecules 2009, 42, 236–246. [Google Scholar] [CrossRef]
- Ka, D.; Seo, M.; Choi, H.; Kim, E.H.; Kim, S.Y. Physical gelation of polar aprotic solvents induced by hydrogen bonding modulation of polymeric molecules. Chem. Commun. 2010, 46, 5722–5724. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Solvents | Blank | Methanol | Ethanol | 2-Propanol | Acetone | Tetrahydrofuran | 1,4-Dioxane |
|---|---|---|---|---|---|---|---|
| α-Helix | 24.45 ± 0.18 b | 27.99 ± 0.16 a | 28.07 ± 0.31 a | 14.59 ± 0.42 d | 17.73 ± 0.08 c | 24.48 ± 0.11 b | 12.55 ± 0.34 e |
| β-Sheet | 31.31 ± 0.07 b | 26.55 ± 0.20 d | 21.50 ± 0.03 f | 29.83 ± 0.17 c | 33.54 ± 0.36 a | 30.18 ± 0.23 c | 24.79 ± 0.16 e |
| β-Turn | 17.50 ± 0.12 c | 11.34 ± 0.28 d | 18.71 ± 0.15 a | 18.33 ± 0.10 ab | 18.50 ± 0.29 ab | 17.95 ± 0.13 bc | 18.65 ± 0.22 ab |
| Random Coil | 26.73 ± 0.26 f | 33.86 ± 0.40 c | 31.73 ± 0.22 d | 37.24 ± 0.06 b | 30.21 ± 0.15 e | 27.39 ± 0.17 f | 44.01 ± 0.31 a |
| α-Helix+β-Sheet | 55.76 ± 0.24 a | 54.54 ± 0.33 a | 49.57 ± 0.29 c | 44.42 ± 0.45 d | 51.27 ± 0.40 b | 54.66 ± 0.30 a | 37.34 ± 0.49 e |
| Helix/Coil | 0.91 ± 0.27 a | 0.83 ± 0.16 ab | 0.88 ± 0.06 a | 0.39 ± 0.17 ab | 0.59 ± 0.07 ab | 0.89 ± 0.18 a | 0.29 ± 0.02 b |
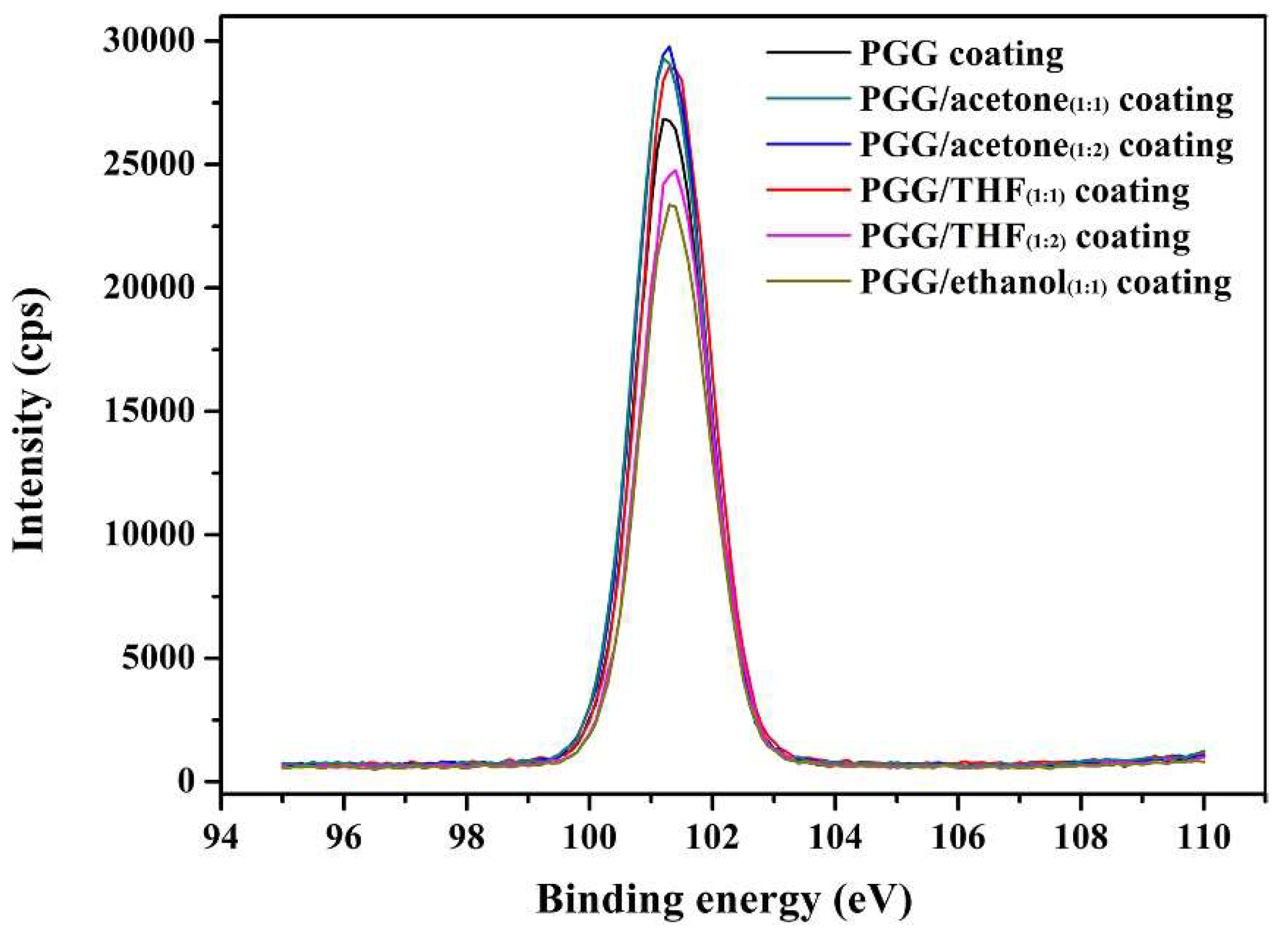
| Coating | Si 2p (%) |
|---|---|
| PGG coating | 19.52 ± 0.19 b |
| PGG/acetone(1:1) coating | 20.56 ± 0.28 a |
| PGG/acetone(1:2) coating | 20.90 ± 0.22 a |
| PGG/THF(1:1) coating | 20.34 ± 0.16 a |
| PGG/THF(1:2) coating | 18.85 ± 0.21 b |
| PGG/ethanol(1:1) coating | 18.04 ± 0.13 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, D.; Ma, H.; Xu, J.; Li, T.; Cai, Z.; Chen, H.; Zhang, J.; Dong, H. Induced Aggregation of Epoxy Polysiloxane Grafted Gelatin by Organic Solvent and Green Application. Molecules 2019, 24, 2264. https://doi.org/10.3390/molecules24122264
Zhang Z, Zhang D, Ma H, Xu J, Li T, Cai Z, Chen H, Zhang J, Dong H. Induced Aggregation of Epoxy Polysiloxane Grafted Gelatin by Organic Solvent and Green Application. Molecules. 2019; 24(12):2264. https://doi.org/10.3390/molecules24122264
Chicago/Turabian StyleZhang, Zhen, Dongmei Zhang, Huijun Ma, Jing Xu, Tianduo Li, Zhaoning Cai, Haifeng Chen, Jinghui Zhang, and Hao Dong. 2019. "Induced Aggregation of Epoxy Polysiloxane Grafted Gelatin by Organic Solvent and Green Application" Molecules 24, no. 12: 2264. https://doi.org/10.3390/molecules24122264
APA StyleZhang, Z., Zhang, D., Ma, H., Xu, J., Li, T., Cai, Z., Chen, H., Zhang, J., & Dong, H. (2019). Induced Aggregation of Epoxy Polysiloxane Grafted Gelatin by Organic Solvent and Green Application. Molecules, 24(12), 2264. https://doi.org/10.3390/molecules24122264