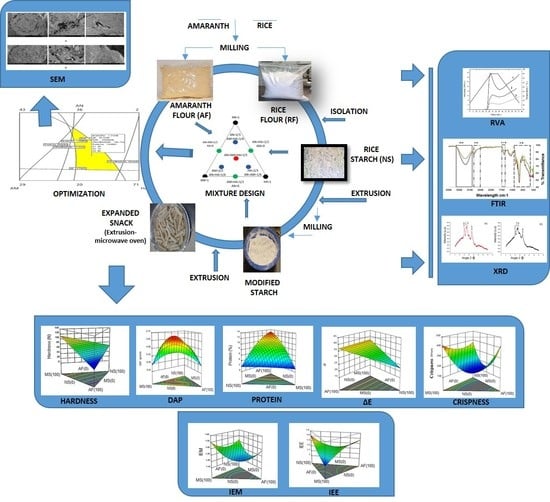
Development of an Expanded Snack of Rice Starch Enriched with Amaranth by Extrusion Process
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Obtention of Rice Flour and Amaranth
2.3. Isolation of Rice Starch
2.4. Acetylation of Rice Starch
Degree of Substitution (DS)
2.5. Differential Scanning Calorimetry (DSC)
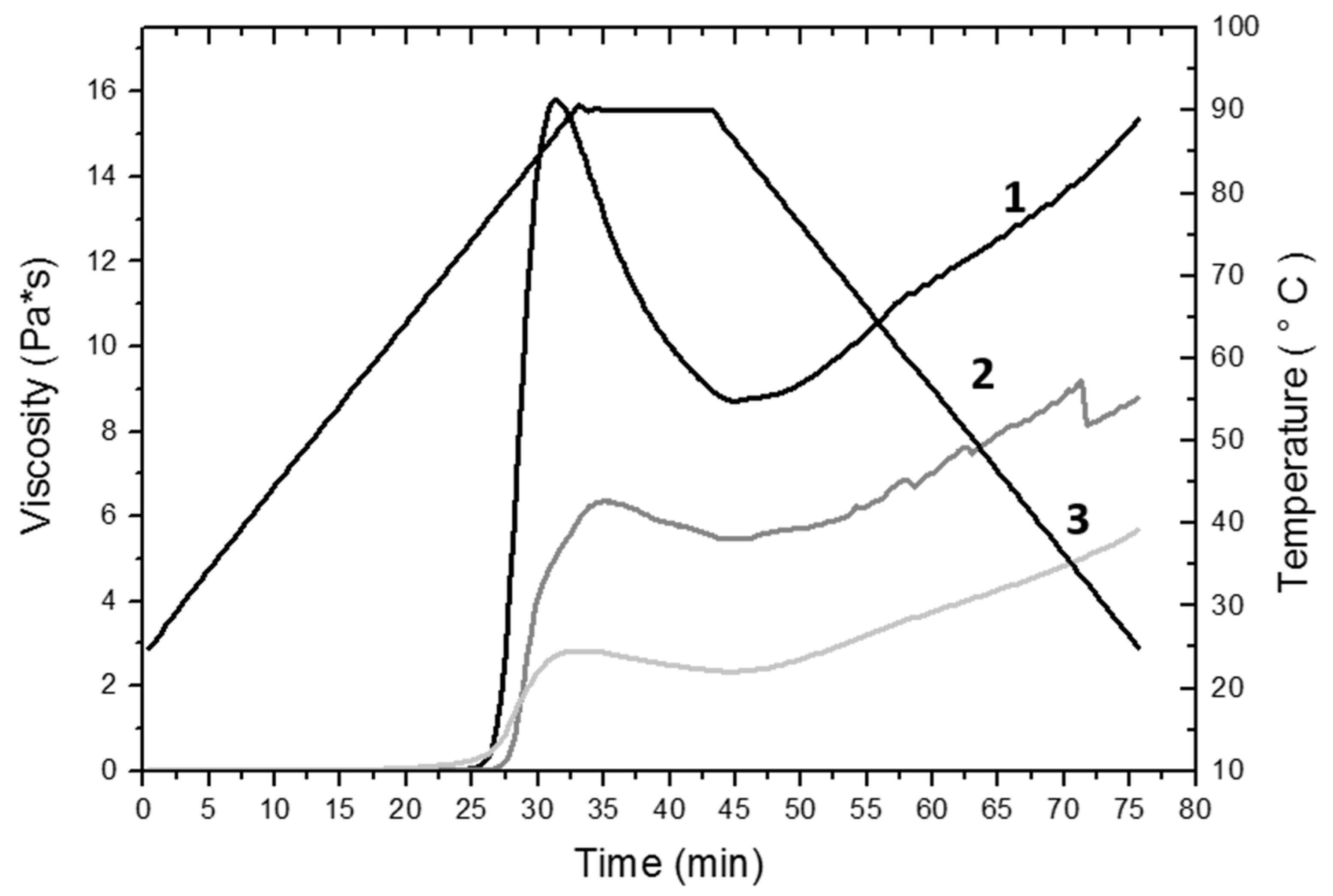
2.6. Pasting Properties (RVA)
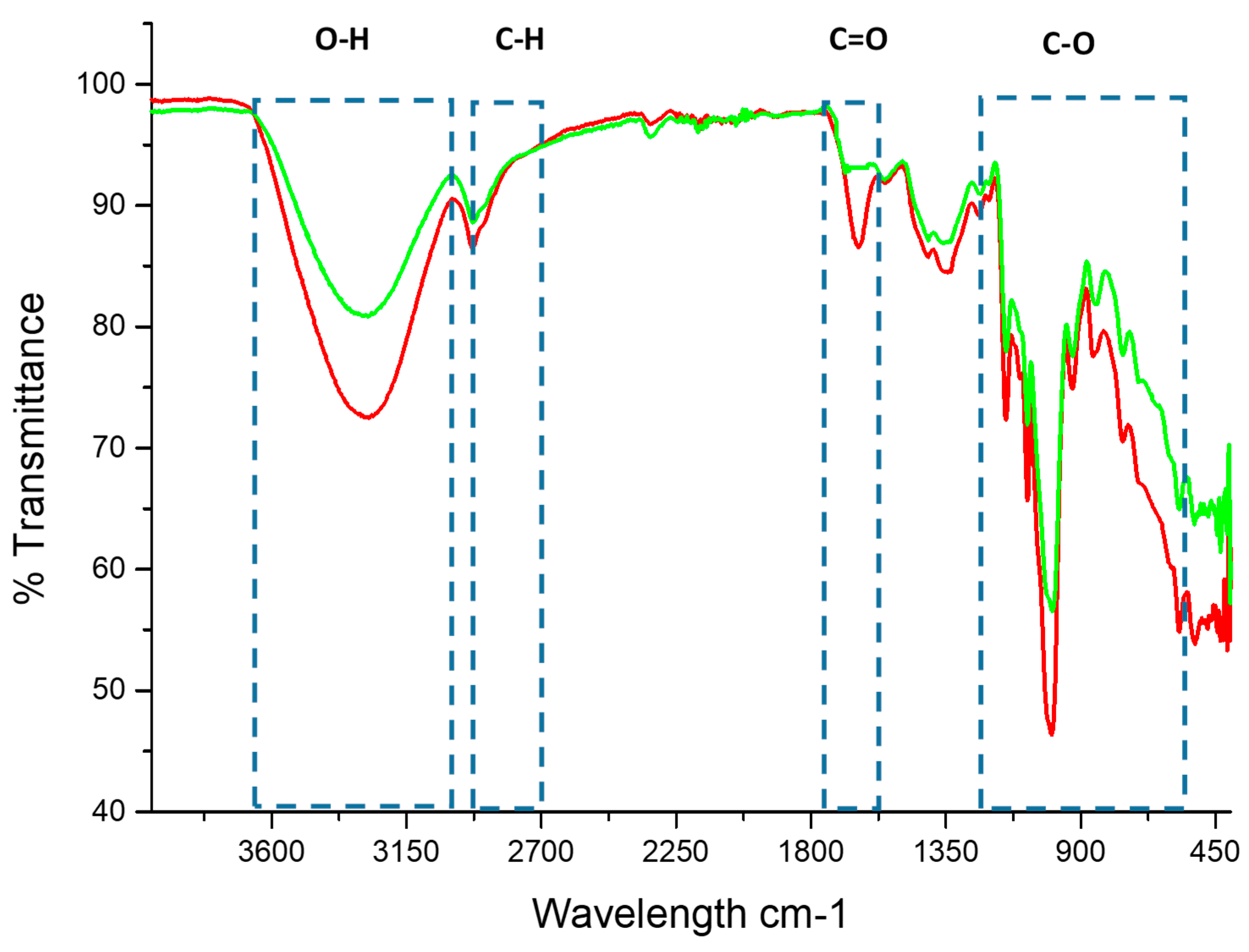
2.7. Infrared Transmission Spectroscopy with Fourier Transform (FT-IR)
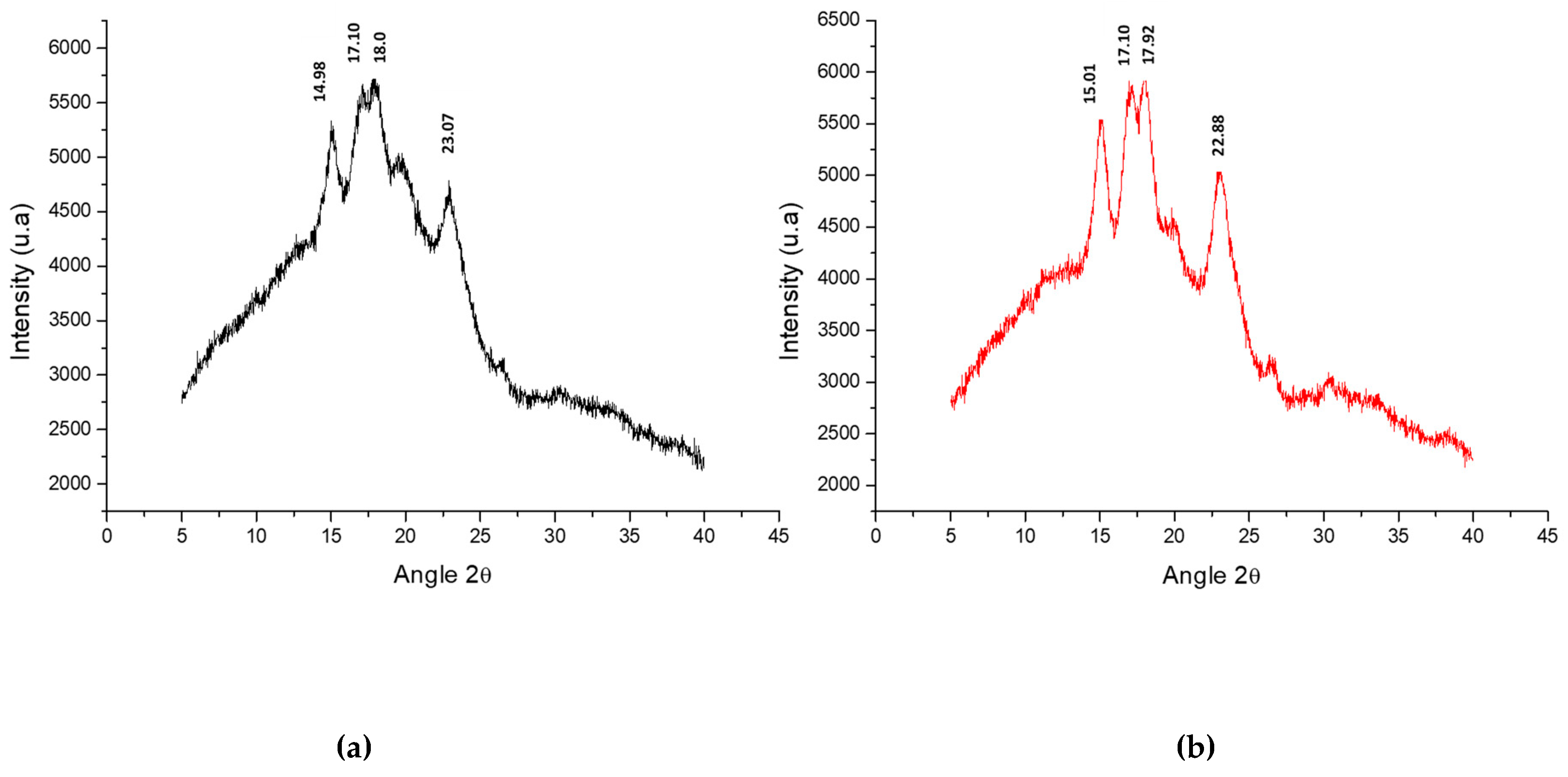
2.8. X-Ray Diffraction Analysis (XRD) and Crystallinity Percentage (CP)
2.9. Formulation, Extrusion and Microwave Expansion
2.10. Hardness of the Snack
2.11. Expansion Index of the Snack (EI)
2.12. Apparent Density of the Snacks (DAP)
2.13. Determination of Proteins
2.14. Color Change (ΔE)
2.15. Scanning Electron Microscopy (SEM)
2.16. Determination of Resistant Starch (RS)
2.17. Experimental Design and Results Analysis
3. Results and Discussion
3.1. Degree of Acetylation of Modified Starch
3.2. Thermal Characterization
3.3. Pasting Properties (RVA)
3.4. Infrared Transmission Spectroscopy with Fourier Transform (FT-IR)
3.5. X-ray Diffraction Analysis (XRD) and Crystallinity Percentage (CP)
3.6. Characterization of the Snack
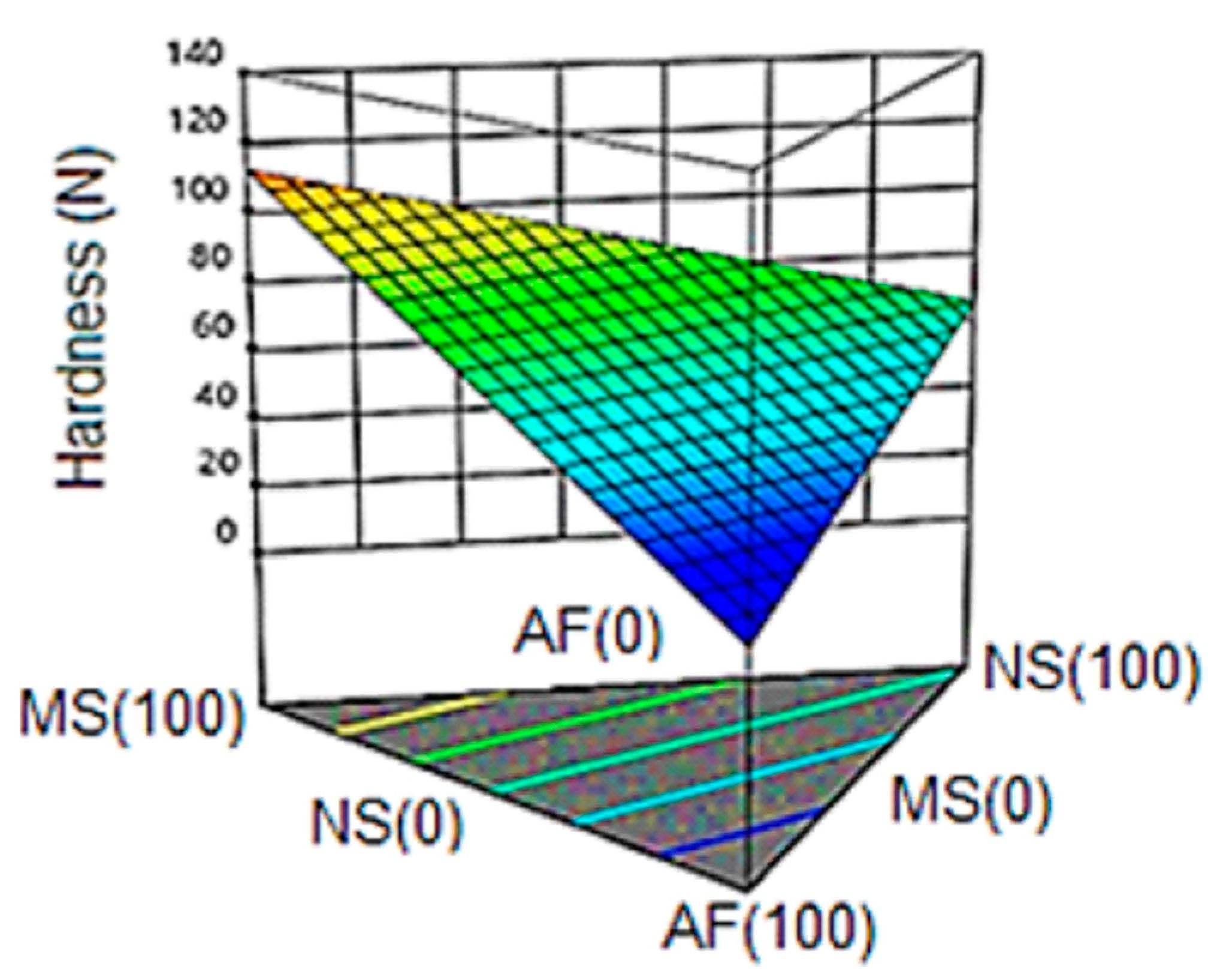
3.6.1. Hardness
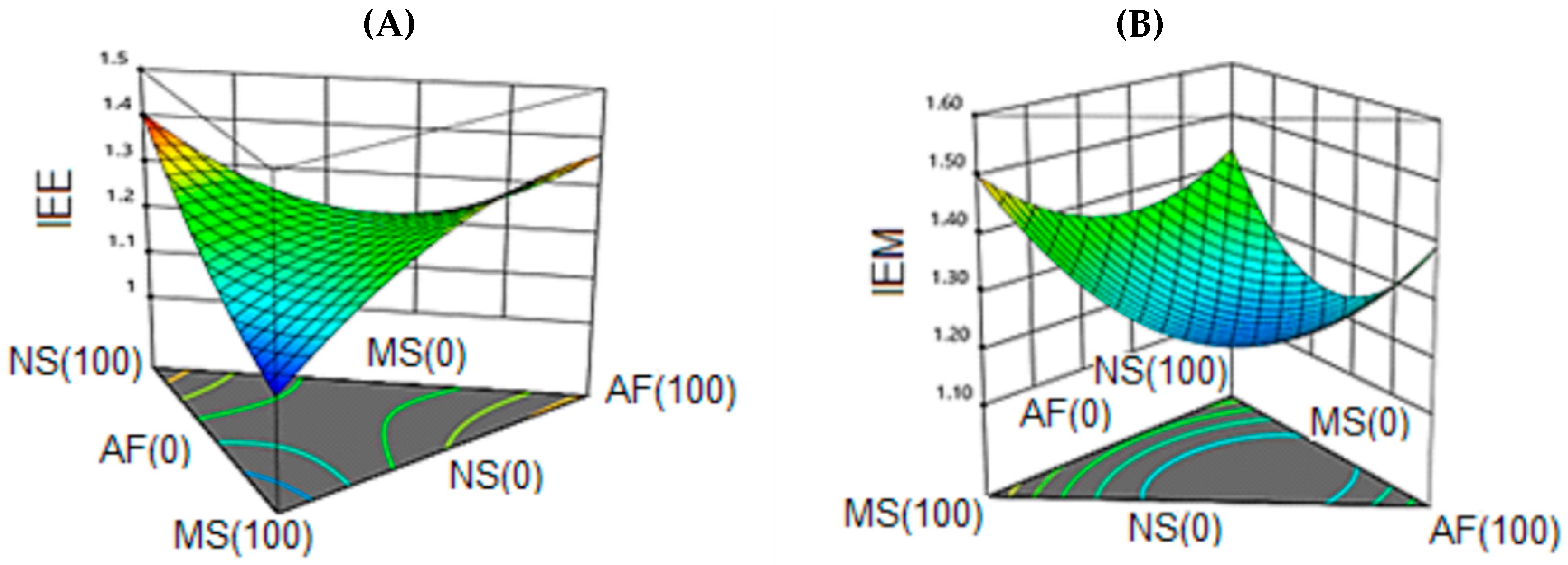
3.6.2. Expansion Index (EI).
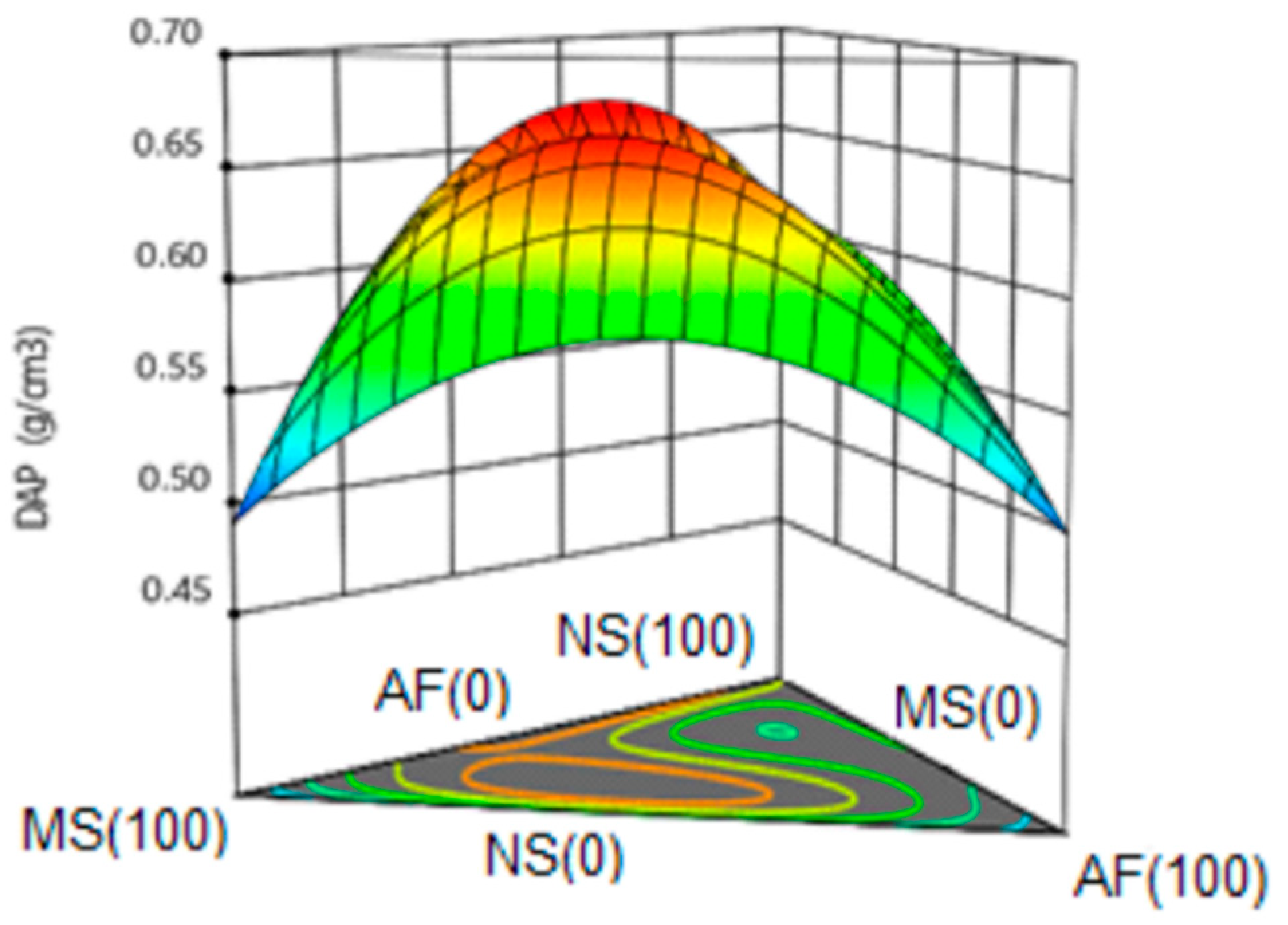
3.6.3. Apparent Density (DAP).
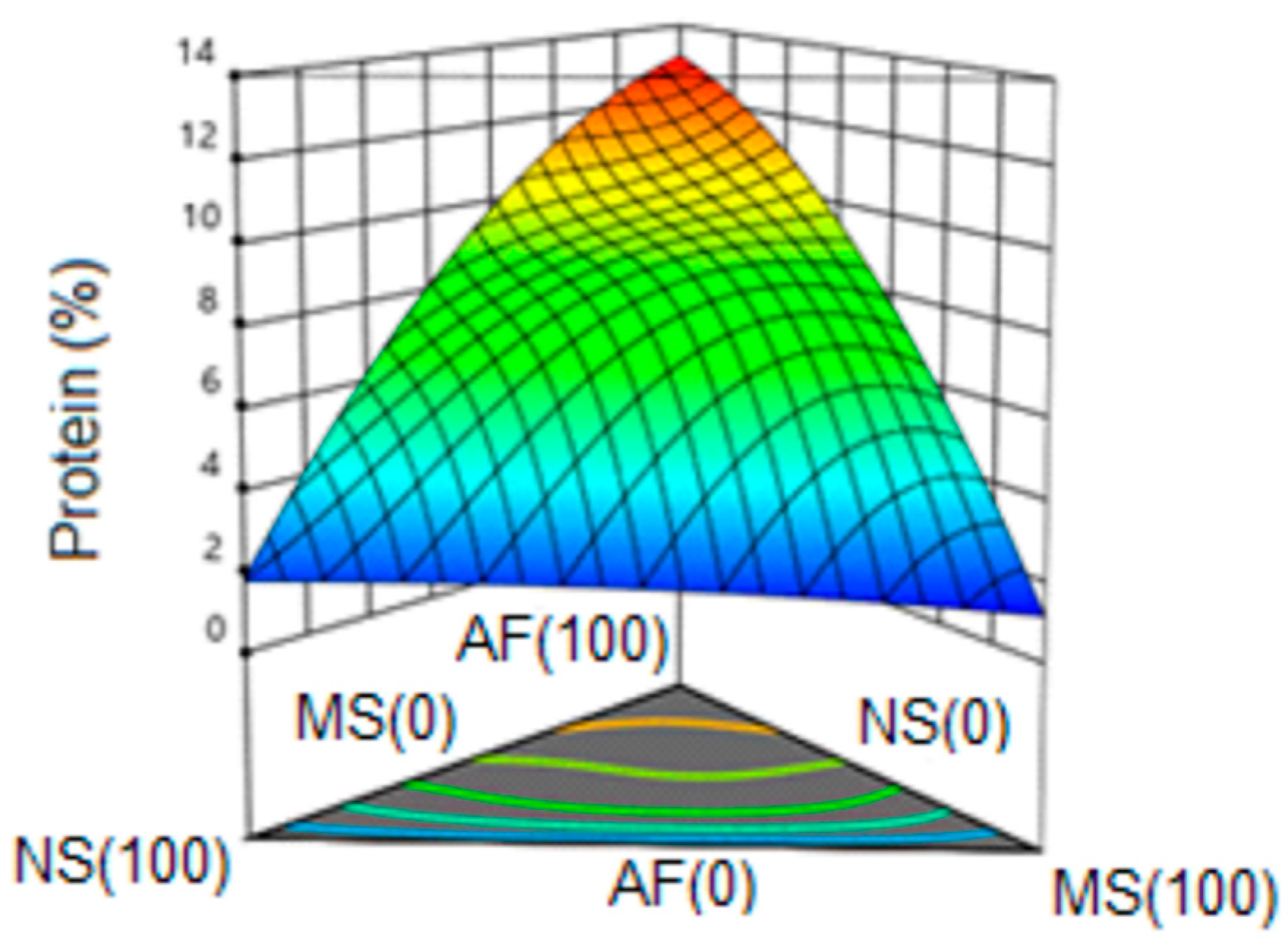
3.6.4. Protein
3.6.5. Color Change (ΔE)
3.6.6. Crispness
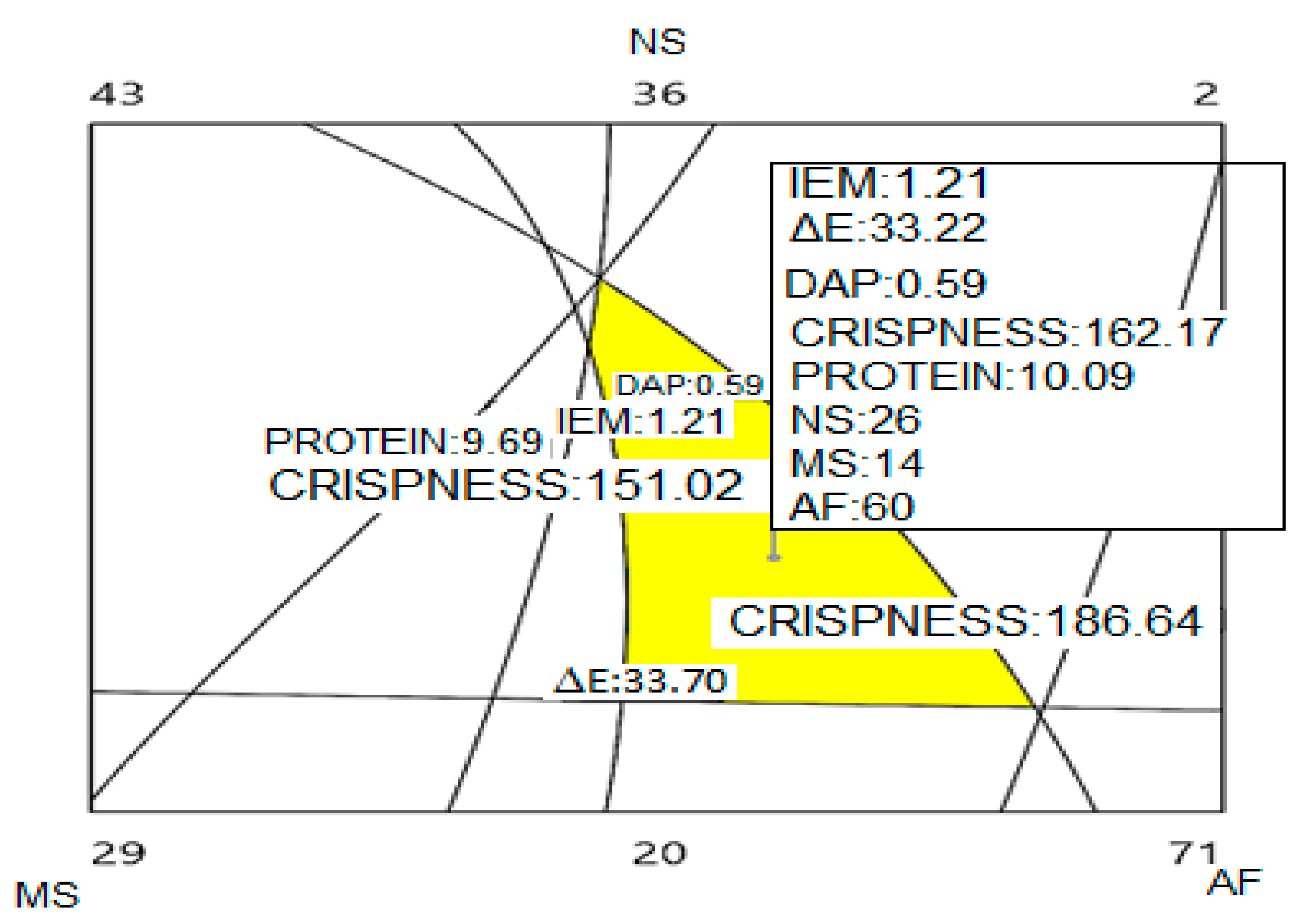
3.7. Optimization of the Final Product
3.7.1. Resistant Starch (RS)
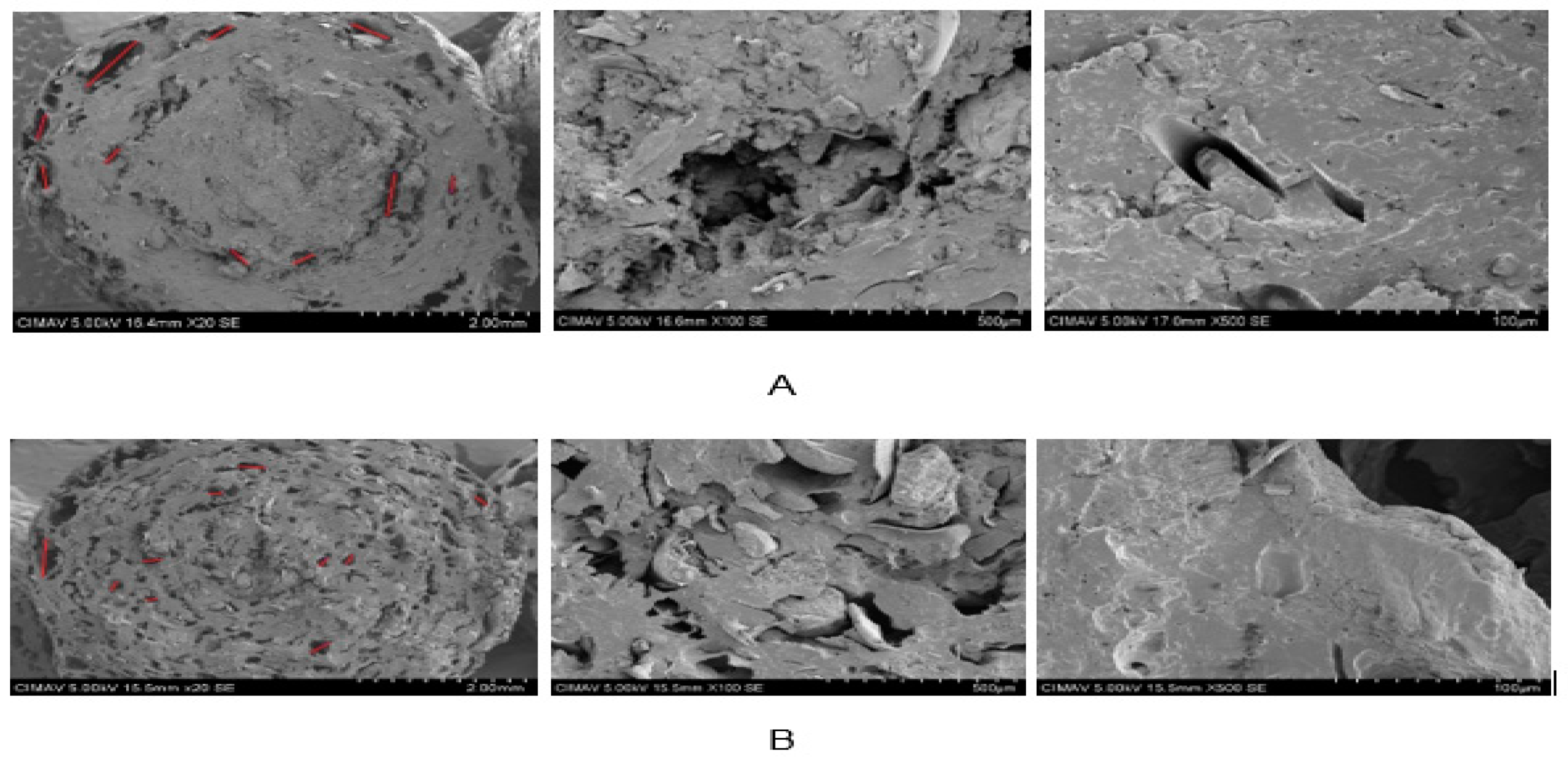
3.7.2. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rtveladze, K.; Marsh, T.; Barquera, S.; Romero, L.M.S.; Levy, D.; Melendez, G.; Webber, L.; Kilpi, F.; McPherson, K.; Brown, M. Obesity prevalence in Mexico: Impact on health and economic burden. Public Health Nutr. 2013, 17, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Dibonaventura, M.D.; Meincke, H.; Lay, A.L.; Fournier, J.; Bakker, E.; Ehrenreich, A. Obesity in Mexico: Prevalence, comorbidities, associations with patient outcomes, and treatment experiences. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.; Caterson, I.; Seidell, J.; James, W. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [PubMed]
- Singh, N.; Nakaura, Y.; Inouchi, N.; Nishinaria, K. Fine Structure, Thermal and Viscoelastic Properties of Starches Separated from Indica Rice Cultivars. Starch 2007, 59, 10–20. [Google Scholar] [CrossRef]
- Ding, Q.-B.; Ainsworth, P.; Tucker, G.; Marson, H. The effect of extrusion conditions on the physicochemical properties and sensory characteristics of rice-based expanded snacks. J. Food Eng. 2005, 66, 283–289. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Singh, N. Characteristics of acetylated starches prepared using starches separated from different rice cultivars. J. Food Eng. 2005, 70, 117–127. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Zieba, T.; Szummy, A.; Kapelko, M. Properties of retrograded and acetylated starch preparation. Part 1. Structure, susceptibilityto amylase and qualityof gelatinization. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar]
- Santos, B.D.; Fávaro-Trindade, A.; Grosso, C.S.F.; Raimundo, C. Preparo e caracterização de microcápsulas de oleoresina de páprica obtidas por atomização. Ciênc. Tecnol. Aliment. 2005, 25, 322–326. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M. Preparation of Acetylated Distarch Adipates by Extrusion. LWT Food Sci. Technol. 2001, 34, 384–389. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Contreras-Ramos, S.M.; Romero-Manilla, R.; Soloza-Feria, J.; Jiménez-Aparicio, A. Chemical and functional properties of modified starch from banana Musa paradisiaca L. (Var. Macho). Agrociencia 2002, 36, 169–180. [Google Scholar]
- Paredes-López, O.; Bello-Pérez, L.A.; López, M.G. Amylopectin: Structural, gelatinisation and retrogradation studies. Food Chem. 1994, 50, 411–417. [Google Scholar] [CrossRef]
- American-Association-of-Cereal-Chemists-(AACC). Approved Methods of the American Association of Cereal Chemists, 10th ed.; The association: St. Paul, MN, USA, 2000. [Google Scholar]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martínez-Bustos, F.; Grosso, C.R.F. Effect of surfactants on the functional properties of gelatin-based edible films. J. Food Eng. 2011, 103, 129–136. [Google Scholar] [CrossRef]
- Remya, R.; Jyothia, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Limón-Valenzuela, V.; Martínez-Bustos, F.; Aguilar-Palazuelos, E.; Caro-Corrales, J.J.; Zazueta-Morales, J.J. Physicochemical Evaluation and Optimization of Enriched Expanded Pellets with Milk Protein Concentrate. Cereal Chem. 2010, 87, 612–618. [Google Scholar] [CrossRef]
- Gujska, E.; Khan, K. Effect of Temperature on Properties of Extrudates from High Starch Fractions of Navy, Pinto and Garbanzo Beans. J. Food Sci. 1990, 55, 466–499. [Google Scholar] [CrossRef]
- Pérez-Navarrete, C.; Cruz-Estrada, R.H.; Chel-Guerrero, L.; Betancur-Ancona, D. Physical characterization of extruders prepared with corn flour mixtures QPM (Zea mays L.) and lima beans (Phaseolus lunatus L.). Mex. J. Chem. Eng. 2016, 5, 145–155. [Google Scholar]
- Association-of-Official-Analytical-Chemists-(AOAC). Official Methods of Analysis, 15th ed.; AOAC: Washintong, DC, USA, 2005. [Google Scholar]
- American Society for Testing and Materials (ASTM). Standard Test Methods for Color Determination of Plastic Pellets; ASTM D6290; ASTM International: West Conshohocken, PA, USA, 2018; Available online: www.astm.org (accessed on 3 March 2018).
- Hu, X.; Xu, X.; Jin, Z.; Tian, Y.; Bai, Y.; Xie, Z. Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP). J. Food Eng. 2011, 106, 262–266. [Google Scholar] [CrossRef]
- Goñi, I.; García-Diz, L.; Mañas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef]
- Stat-Ease. Design-Expert Version 9.0.0.; Suite 1991; Stat-Ease, Inc.: Minneapolis, MN, USA, 2014. [Google Scholar]
- Colussi, R.; Halal, S.L.M.E.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; Zavareze, E.D.R.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- Salcedo-Mendoza, J.G.; Rodríguez-Lora, M.C.; Figueroa-Florez, J.A. Effect of acetylation on structural and functional properties of starches from cassava (Manihot esculenta Crantz) and yam (Dioscorea alata cv. Diamante 22). Revista Mexicana de Ingenieria Química 2016, 15, 787–796. [Google Scholar]
- Shon, K.-J.; Yoo, B. Effect of Acetylation on Rheological Properties of Rice Starch. Starch 2006, 58, 177–185. [Google Scholar] [CrossRef]
- Colussi, R.; Pintoa, V.Z.; Mello, S.L.; Vaniera, N.L.; Villanova, F.A.; Silvad, R.M.E.; Zavarezea, E.D.R.; Dias, A.R.G. Structural, morphological, and physicochemical properties ofacetylated high-, medium-, and low-amylose rice starches. Carbohydr. Polym. 2014, 103, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Pineda–Gómez, P.; Coral, D.F.; Larciniegas, M.; Rorales–Rivera, A.; Rodríguez-García, M.E. Papel del agua en la gelatinización del almidón de maíz: Estudio por calorimetría diferencial de barrido. Ingeniería y Ciencia 2010, 6, 129–141. [Google Scholar]
- Sing, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Fukai, S.; Prakash, S.; Bhandari, B. Effect of starch modification in the whole white rice grains on physicochemical properties of two contrasting rice varieties. J. Cereal Sci. 2018, 80, 143–149. [Google Scholar] [CrossRef]
- Liu, H.; Ramsden, L.; Corke, H.; Kong, H. Physical Properties of Cross-linked and Acetylated Normal and Waxy Rice Starch. Starch 1999, 51, 249–252. [Google Scholar] [CrossRef]
- Liang, X.; King, J.M. Rice Starch with Added Amino Acids. Pasting and Crystalline Property Differences of Commercial and Isolated. J. Food Sci. 2003, 68, 832–838. [Google Scholar] [CrossRef]
- Fitgerald, M.A.; Martin, M.; Ward, R.M.; Park, W.D.; Shead, H.J. Viscosity of Rice Flour: A Rheological and Biological Study. J. Agric. Food Chem. 2003, 51, 2295–2299. [Google Scholar] [CrossRef]
- Souza, V.F.D.; Ortiz, J.A.R.; Nascimiento, E.M.D.G.C.D. Pasting properties of expanded extrudate and pellets from corn flour and rice flour. Braz. J. Food Technol. 2011, 14, 106–114. [Google Scholar] [CrossRef][Green Version]
- Hagenimana, A.; Ding, X.; Fang, T. Evaluation of rice flour modified by extrusion cooking. J. Cereal Sci. 2006, 43, 38–46. [Google Scholar] [CrossRef]
- Martínez, M.M.; Calviño, A.; Rosell, C.M.; Gómez, M. Effect of Different Extrusion Treatments and Particle Size Distribution on the Physicochemical Properties of Rice Flour. Food Bioprocess Technol. Int. J. 2014, 7, 2657–2665. [Google Scholar] [CrossRef]
- Puncha-Arnon, S.; Uttapap, D. Rice starch vs. rice flour: Differences in their properties when modified by heat–moisture treatment. Carbohydr. Polym. 2013, 91, 85–91. [Google Scholar] [CrossRef] [PubMed]
- González, Z.; Pérez, E. Effect of Acetylation on Some Properties of Rice Starch. Starch 2002, 54, 148–154. [Google Scholar] [CrossRef]
- Sujka, K.; Koczo, P.; Ceglinska, A.; Reder, M.; Ciemniewska, H. The Application of FT-IR Spectroscopy for Quality Control of Flours Obtained from Polish Producers. J. Anal. Methods Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Kaushik, N.; Mahanta, C.L. Physicochemical, morphological, thermal and IR spectral changes in the properties of waxy rice starch modified with vinyl acetate. J. Food Sci. Technol. 2014, 51, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch 2012, 64, 598–606. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M. Synthesis and Characterization of Starch Acetates with High Substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef]
- Tang, H.; Liu, X.; Li, Y.; Dong, S. Acetylated debranched rice starch: Structure, characterization, and functional properties. Int. J. Food Prop. 2017, 20, 2118–2126. [Google Scholar] [CrossRef]
- Cai, J.; Mana, J.; Huang, J.; Liu, Q.; Wei, W.; Wei, C. Relationship between structure and functional properties of normalrice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef]
- Iturriaga, L.; López, B.; Añon, M. Thermal and physicochemical characterization of seven argentine rice flours and starches. Food Res. Int. 2004, 37, 439–447. [Google Scholar] [CrossRef]
- Mohan, B.H.; Gopala, A.; Malleshia, N.G.; Tharanathan, R.N. Characteristics of native and enzymatically hydrolyzed ragi (Eleusine coracana) and rice (Oryza sativa) starches. Carbohydr. Polym. 2005, 59, 43–50. [Google Scholar] [CrossRef]
- Shih, F.; King, J.; Daigle, K.; An, H.-J.; Ali, R. Physicochemical Properties of Rice Starch Modified by Hydrothermal Treatments. Cereal Chem. 2007, 84, 527–531. [Google Scholar] [CrossRef]
- Sha, X.S.; Xiang, Z.J.; Bin, L.; Jing, L.; Bin, Z.; Jiao, Y.J.; Kun, S.R. Preparation and physical characteristics of resistant starch (type 4) in acetylated indica rice. Food Chem. 2012, 134, 149–154. [Google Scholar] [CrossRef]
- Meza, Y.M.; Herández, J.J.I.; Meraz, F.G.; Díaz, P.O. Formulation of a snack by extrusion with a mixture of banana flour and amaranth. Res. Dev. Food Sci. Technol. 2016, 1, 728–733. [Google Scholar]
- Dokić, L.P.; Bodroža-Solarov, M.I.; Hadnadev, M.S.; NikolićI, R. Properties of extruded snacks supplemented with amaranth grain grits. Acta Period. Technol. 2009, 40, 17–24. [Google Scholar] [CrossRef]
- Diaz, J.M.R.; Kirjoranta, S.; Tenitz, S.; Penttilä, P.A.; Serimaa, R.; Lampi, A.-M.; Jouppila, K. Use of amaranth, quinoa and kañiwa in extruded corn-based snacks. J. Cereal Sci. 2013, 58, 59–67. [Google Scholar] [CrossRef]
- Taverna, L.G.; Leonel, M.; Mischan, M.M. Changes in physical properties of extruded sour cassava starch and quinoa flour blend snacks. Food Sci. Technol. 2012, 32, 826–834. [Google Scholar] [CrossRef]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Ready-to-eat snack products: The role of extrusion technology in developing consumer acceptable and nutritious snacks. Int. J. Food Sci. Technol. 2013, 48, 893–902. [Google Scholar] [CrossRef]
- Pilli, T.D.; Derossi, A.; Talja, R.A.; Jouppila, K.; Severini, C. Study of starch-lipid complexes in model system and real food produced using extrusion-cooking technology. Innov. Food Sci. Emerg. Technol. 2011, 12, 610–616. [Google Scholar] [CrossRef]
- Ilo, S.; Liu, Y.; Berghofer, E. Extrusion Cooking of Rice Flour and Amaranth Blends. LWT Food Sci. Technol. 1999, 32, 79–88. [Google Scholar] [CrossRef]
- Aguilar-Palazuelos, E.; Zazueta-Morales, J.D.J.; Harumi, E.N.; Martínez-Bustos, F. Optimization of extrusion process for production of nutritious pellets. Food Sci. Technol. 2012, 32, 34–42. [Google Scholar] [CrossRef]
- Chávez-Jáuregui, R.N.; Silva, M.E.M.P.; Areas, J.A.G. Extrusion Cooking Process for Amaranth (Amaran. Caudatus L.). J. Food Sci. 2000, 65, 1009–1015. [Google Scholar]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Effect of Brewer’s spent grain and temperature on physical properties of expanded extrudates from rice. LWT Food Sci. Technol. 2017, 79, 145–151. [Google Scholar] [CrossRef]
- Seth, D.; Rajamanickam, G. Development of extruded snacks using soy, sorghum, millet and rice blend–A response surface methodology approach. Int. J. Food Sci. Technol. 2012, 47, 1526–1531. [Google Scholar] [CrossRef]
- González-Soto, R.A.; Sánchez-Hernández, L.; Solorza-Feria, J.; Núñez-Santiago, C.; Flores-Huicochea, E.; Bello-Pérez, L.A. Resistant Starch Production from Non-conventional Starch Sources by Extrusion. Food Sci. Tech. Int. 2006, 12, 5–11. [Google Scholar] [CrossRef]
- Nissar, J.; Ahad, T.; Naik, H.; Hussain, S. Resistant Starch-Chemistry and Nutritional properties. Int. J. Food Sci. Nutr. 2017, 2, 95–108. [Google Scholar]
- Arendt, E.K.; Zannini, E. (Eds.) Amaranth. In Cereal Grains for the Food and Beverage Industries; Arendt 904 & Zannini, Editor; Woodhead Publishing: Cambridge, Sawston, UK, 2013; Volume 1, pp. 439–473. [Google Scholar]
- Parchure, A.A.; Kulkarni, P.R. Effect of food processing treatments on generation of resistant starch. Int. J. Food Sci. Nutr. 1997, 48, 257–260. [Google Scholar] [CrossRef]
- Eroglu, E.I.; Buyuktuncer, Z. The effect of varıous cookıng methods on resıstant starch content of foods. Nutr. Food Sci. 2017, 47, 522–533. [Google Scholar]
Sample Availability: Samples of the raw and modified flours are available from the authors. |


| Formulations | NS | MS | AF |
|---|---|---|---|
| 1 | 66.67 | 16.67 | 16.67 |
| 2 | 50 | 0 | 50 |
| 3 | 0 | 100 | 0 |
| 4 | 100 | 0 | 0 |
| 5 | 33.33 | 33.33 | 33.33 |
| 6 | 16.67 | 66.67 | 16.67 |
| 7 | 100 | 0 | 0 |
| 8 | 0 | 0 | 100 |
| 9 | 50 | 50 | 0 |
| 10 | 0 | 50 | 50 |
| 11 | 50 | 50 | 0.00 |
| 12 | 0 | 100 | 0 |
| 13 | 0 | 0 | 100 |
| 14 | 50 | 0 | 50 |
| 15 | 16.67 | 16.67 | 66.67 |
| RF | NS | MS | |
|---|---|---|---|
| T0 (°C) | 81.63 ± 0.2a | 73.15 ± 0.5b | 61.15 ± 1.3c |
| Tg (°C) | 82.63 ± 0.2a | 78.35 ± 0.2b | 63.90 ± 1.1c |
| Tf (°C) | 85.25 ± 0.5a | 83.50 ± 0.1a | 66.95 ± 1.6b |
| ΔHg (J/g) | 8.64 ± 0.0a | 6.25 ± 0.60b | 1.35 ± 0.02c |
| Rice Flour | Native Starch | Modified Starch | |
|---|---|---|---|
| µmax, Pa*s, (cP) Heating (25–90 °C, 2 °C/min) | 15.8 ± 0.14a (15 800 ± 0.14a) | 6.35 ± 0.16b (6 350 ± 0.16b) | 2.88 ± 1.15c (2 880 ± 1.15c) |
| µmin, Pa*s (cP) Stability (90 °C, 10 min) | 8.78 ± 0.19a (8 780 ± 0.19a) | 5.50 ± 0.26b (5 500 ± 0.26b) | 2.34 ± 0.74c (2 340 ± 0.74c) |
| µf, Pa*s (cP) Cooling (90–25 °C, 2 °C/min) | 15.35 ± 0.21a (15 350 ± 0.21a) | 8.77 ± 0.27b (8 770 ± 0.27b) | 6.45 ± 1.11b (6 450 ± 1.11b) |
| µr, Pa*s (cP) | 6.1 ± 0.07a (6 100 ± 0.07a) | 3.6 ± 0.05b (3 600 ± 0.05b) | 3.6 ± 0.04b (3 600 ± 0.04b) |
| Formulation | NS | MS | AF | Hardness (N) | IEE | IEM | DAP (g/cm3) | Protein (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.667 | 0.167 | 0.167 | 32 | 1.32 | 1.32 | 0.57 | 6.07 |
| 2 | 0.500 | 0.000 | 0.500 | 35 | 1.13 | 1.15 | 0.58 | 9.30 |
| 3 | 0.000 | 1.000 | 0.000 | 121 | 1.08 | 1.57 | 0.48 | 1.05 |
| 4 | 1.000 | 0.000 | 0.000 | 76 | 1.39 | 1.40 | 0.60 | 1.72 |
| 5 | 0.333 | 0.333 | 0.333 | 58 | 1.24 | 1.24 | 0.61 | 8.58 |
| 6 | 0.167 | 0.667 | 0.167 | 54 | 1.13 | 1.14 | 0.65 | 6.29 |
| 7 | 1.000 | 0.000 | 0.000 | 74 | 1.41 | 1.45 | 0.61 | 1.83 |
| 8 | 0.000 | 0.000 | 1.000 | 33 | 1.36 | 1.37 | 0.50 | 13.34 |
| 9 | 0.500 | 0.500 | 0.000 | 112 | 1.17 | 1.39 | 0.67 | 1.60 |
| 10 | 0.000 | 0.500 | 0.500 | 52 | 1.29 | 1.23 | 0.58 | 9.11 |
| 11 | 0.500 | 0.500 | 0.000 | 113 | 1.21 | 1.38 | 0.66 | 1.79 |
| 12 | 0.000 | 1.000 | 0.000 | 120 | 1.09 | 1.48 | 0.50 | 1.24 |
| 13 | 0.000 | 0.000 | 1.000 | 36 | 1.37 | 1.37 | 0.49 | 12.99 |
| 14 | 0.500 | 0.000 | 0.500 | 36 | 1.27 | 1.28 | 0.57 | 9.42 |
| 15 | 0.167 | 0.167 | 0.667 | 37 | 1.29 | 1.31 | 0.61 | 10.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-Gallo, L.; Galicia-García, T.; Estrada-Moreno, I.; Mendoza-Duarte, M.; Márquez-Meléndez, R.; Portillo-Arroyo, B.; Soto-Figueroa, C.; Leal-Ramos, Y.; Sanchez-Aldana, D. Development of an Expanded Snack of Rice Starch Enriched with Amaranth by Extrusion Process. Molecules 2019, 24, 2430. https://doi.org/10.3390/molecules24132430
Castellanos-Gallo L, Galicia-García T, Estrada-Moreno I, Mendoza-Duarte M, Márquez-Meléndez R, Portillo-Arroyo B, Soto-Figueroa C, Leal-Ramos Y, Sanchez-Aldana D. Development of an Expanded Snack of Rice Starch Enriched with Amaranth by Extrusion Process. Molecules. 2019; 24(13):2430. https://doi.org/10.3390/molecules24132430
Chicago/Turabian StyleCastellanos-Gallo, Lilisbet, Tomás Galicia-García, Iván Estrada-Moreno, Mónica Mendoza-Duarte, Rubén Márquez-Meléndez, Beatriz Portillo-Arroyo, Cesar Soto-Figueroa, Yarely Leal-Ramos, and Daniela Sanchez-Aldana. 2019. "Development of an Expanded Snack of Rice Starch Enriched with Amaranth by Extrusion Process" Molecules 24, no. 13: 2430. https://doi.org/10.3390/molecules24132430
APA StyleCastellanos-Gallo, L., Galicia-García, T., Estrada-Moreno, I., Mendoza-Duarte, M., Márquez-Meléndez, R., Portillo-Arroyo, B., Soto-Figueroa, C., Leal-Ramos, Y., & Sanchez-Aldana, D. (2019). Development of an Expanded Snack of Rice Starch Enriched with Amaranth by Extrusion Process. Molecules, 24(13), 2430. https://doi.org/10.3390/molecules24132430