Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen
Abstract
1. Introduction
2. Results and Discussion
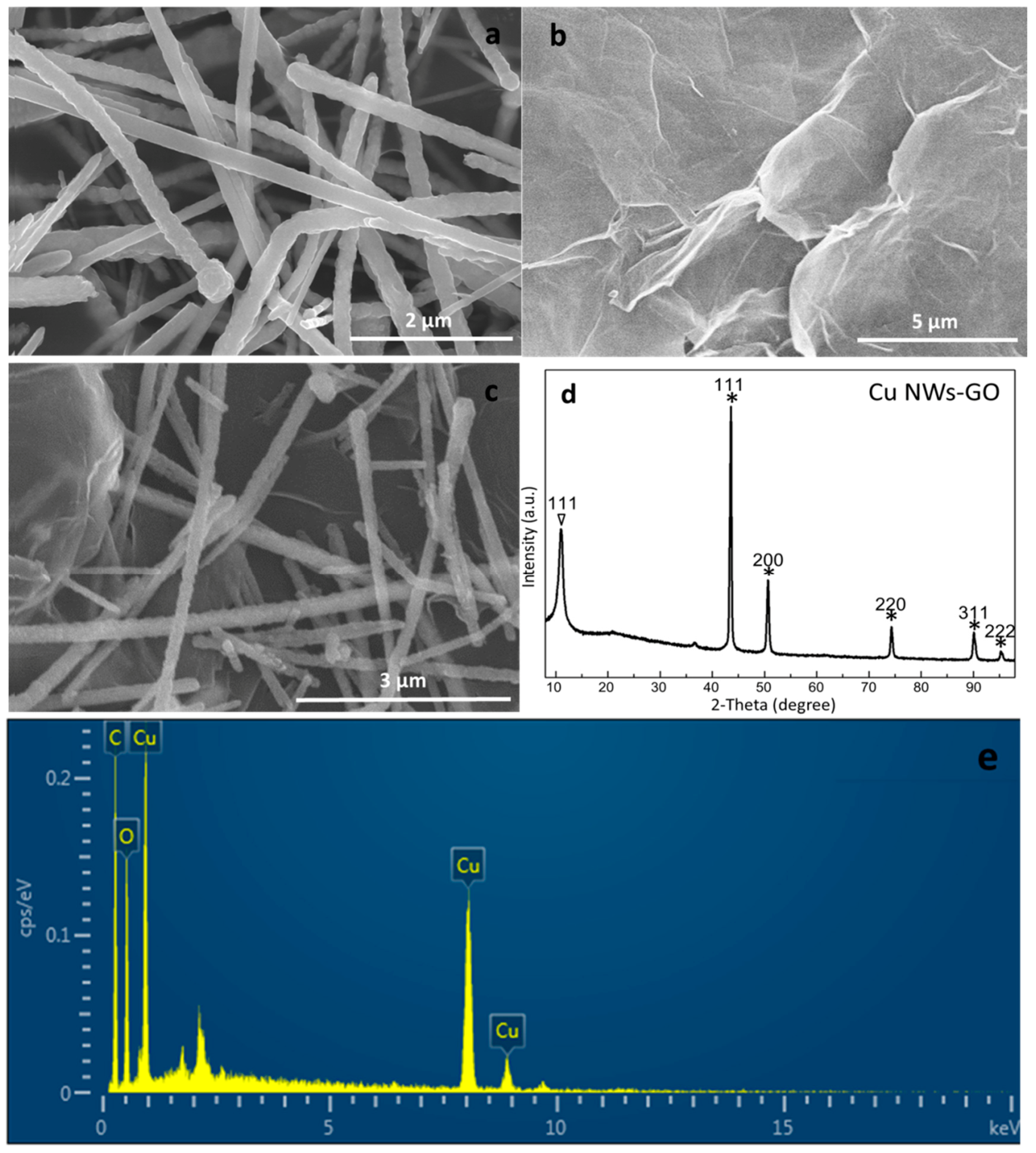
2.1. Characterization of the Copper Nanowires–Graphene Oxide (Cu NWs–GO) Nanocomposite
2.2. Voltammetric Behaviors of the Modified Electrode
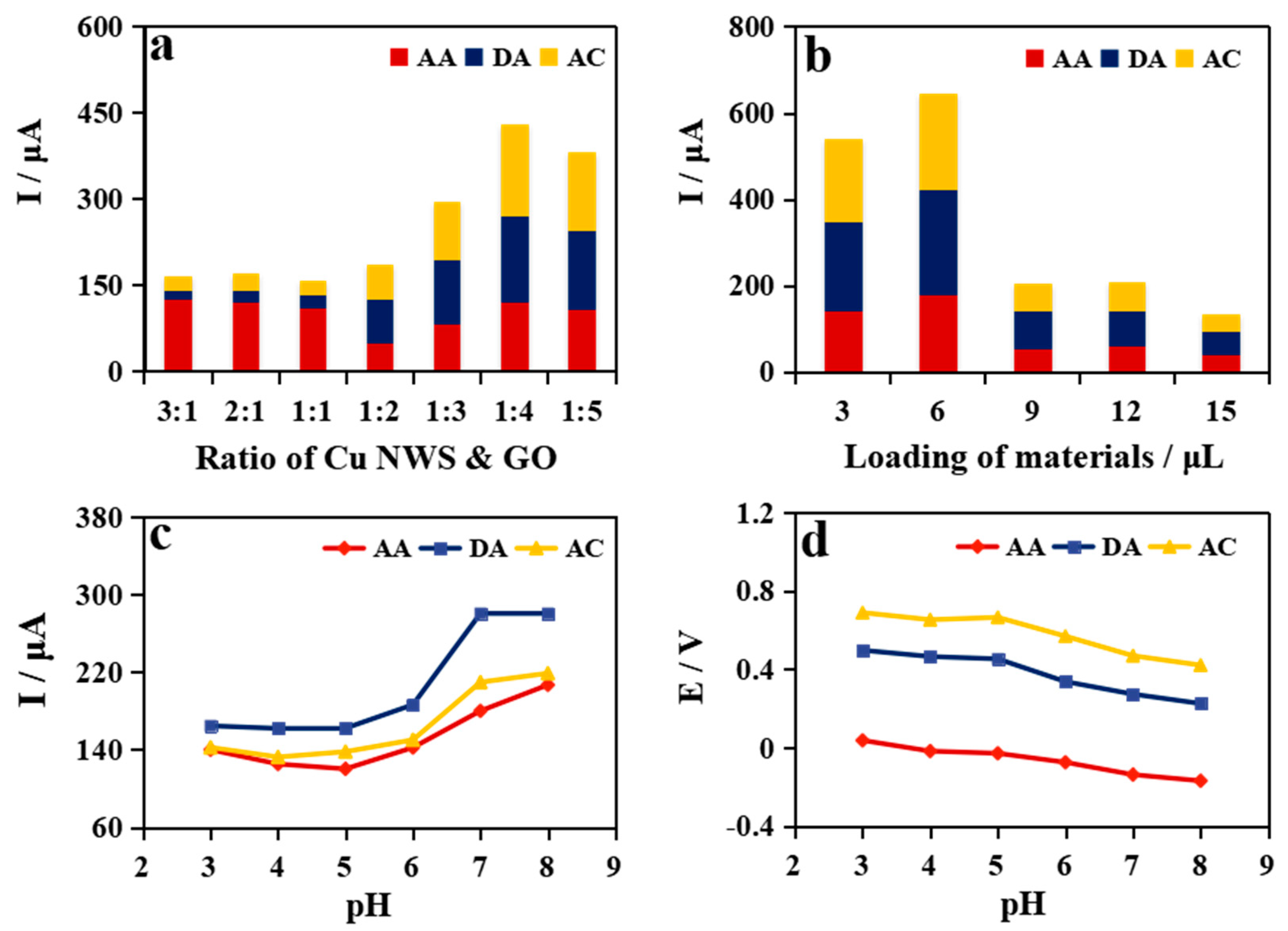
2.3. Optimization of Detection Conditions
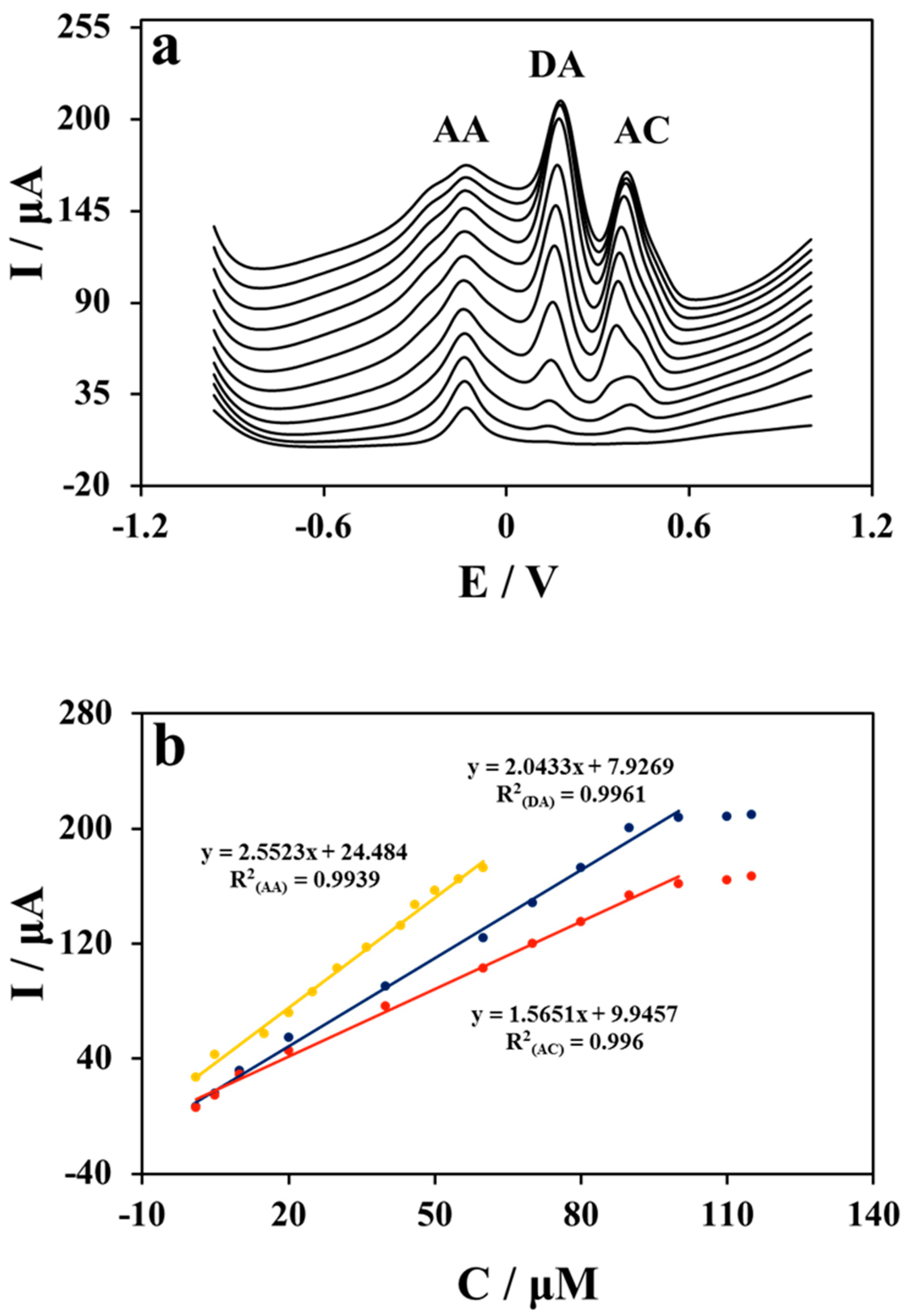
2.4. Simultaneous Detection of Ascorbic Acid (AA), Dopamine (DA), and Acetaminophen (AC) in Nafion/Cu NWs–GO/GCE
2.5. Interference, Reproducibility and Stability
2.6. Detection Application in Human Serum Sample
3. Experiment
3.1. Reagents and Materials
3.2. Characterization and Electrochemical Measurements
3.3. Preparation of Nanocomposite Modified Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Zhang, G.; Shi, L.; Pan, S.; Liu, W.; Pan, H. Au/ZnO hybrid nanocatalysts impregnated in N-doped graphene for simultaneous determination of ascorbic acid, acetaminophen and dopamine. Mater. Sci. Eng. C 2016, 65, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Madrakian, T.; Haghshenas, E.; Afkhami, A. Simultaneous determination of tyrosine, acetaminophen and ascorbic acid using gold nanoparticles/multiwalled carbon nanotube/glassy carbon electrode by differential pulse voltammetric method. Sens. Actuators B Chem. 2014, 193, 451–460. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Fu, L.; Cao, Z.; Ye, W.; Li, H.; Guo, P.; Zhao, X.S. Electrospun gamma-Fe2O3 nanofibers as bioelectrochemical sensors for simultaneous determination of small biomolecules. Anal. Chim. Acta 2018, 1026, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Habibi, B.; Pournaghi-Azar, M.H. Simultaneous determination of ascorbic acid, dopamine and uric acid by use of a MWCNT modified carbon-ceramic electrode and differential pulse voltammetry. Electrochim. Acta 2010, 55, 5492–5498. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, Y.K.; Lee, C.; Kim, M.H.; Lee, Y. Real-time direct electrochemical sensing of ascorbic acid over rat liver tissues using RuO2 nanowires on electrospun TiO2 nanofibers. Biosens. Bioelectron. 2016, 77, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, S.; He, W.; Uyama, H.; Xie, Q.; Zhang, L.; Yang, F. Electrochemical sensor based on carbon-supported NiCoO2 nanoparticles for selective detection of ascorbic acid. Biosens. Bioelectron. 2014, 55, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Wei, S.; Chen, S.; Ou, X.; Lu, Q. Overoxidized polyimidazole/graphene oxide copolymer modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid, guanine and adenine. Biosens. Bioelectron. 2014, 57, 232–238. [Google Scholar] [CrossRef]
- Noroozifar, M. Solid-phase iodine as an oxidant in flow injection analysis: Determination of ascorbic acid in pharmaceuticals and foods by background correction. Talanta 2003, 61, 173–179. [Google Scholar] [CrossRef]
- Alarcón-Ángeles, G.; Perez-Lopez, B.; Palomar-Pardavé, M.; Ramírez-Silva, M.; Alegret, S.; Merkoçi, A. Enhanced host–guest electrochemical recognition of dopamine using cyclodextrin in the presence of carbon nanotubes. Carbon 2008, 46, 898–906. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Lee, J.-H.; Oh, B.-K.; Choi, J.-W. 3-D nanoporous gold thin film for the simultaneous electrochemical determination of dopamine and ascorbic acid. Electrochem. Commun. 2010, 12, 1756–1759. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M.; Pournaghi-Azar, M.H. Simultaneous determination of acetaminophen and dopamine using SWCNT modified carbon–ceramic electrode by differential pulse voltammetry. Electrochim. Acta 2011, 56, 2888–2894. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lai, C.; Zhang, Y.; Deng, J.; Li, H.; Yao, S. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 2016, 903, 69–80. [Google Scholar] [CrossRef]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.-P.; Zhou, H. Long-Persistent Enzyme-MOF Nanoreactor Activates Non-toxic Paracetamol for Cancer Therapy. Angew. Chem. Int. Edit. 2018, 57, 5725–5730. [Google Scholar] [CrossRef]
- Bui, M.-P.N.; Li, C.A.; Han, K.N.; Pham, X.-H.; Seong, G.H. Determination of acetaminophen by electrochemical co-deposition of glutamic acid and gold nanoparticles. Sens. Actuators B Chem. 2012, 174, 318–324. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Wang, L.; Lin, S.; Wang, C.; Chen, X. Sensitive detection of acetaminophen based on Fe3O4 nanoparticles-coated poly(diallyldimethylammonium chloride)-functionalized graphene nanocomposite film. Talanta 2012, 88, 181–186. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Tavakkoli, N.; Ahmadi, N.; Davar, F. Simultaneous determination of acetaminophen, dopamine and ascorbic acid using a PbS nanoparticles Schiff base-modified carbon paste electrode. Comptes Rendus Chimie 2015, 18, 438–448. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Jia, Z.; Huo, D.; Liu, Q.; Zhong, D.; Hu, Y.; Yang, M.; Bian, M.; Hou, C. In-situ growth of gold nanoparticles on a 3D-network consisting of a MoS2/rGO nanocomposite for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Mikrochim. Acta 2019, 186, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, J.; Xu, H.; Gao, S.; Jiang, T.; Zhang, S.; Jin, J. Gold nanorods decorated with graphene oxide and multi-walled carbon nanotubes for trace level voltammetric determination of ascorbic acid. Mikrochim. Acta 2018, 186, 17. [Google Scholar] [CrossRef] [PubMed]
- Zang, G.; Hao, W.; Li, X.; Huang, S.; Gan, J.; Luo, Z.; Zhang, Y. Copper nanowires-MOFs-graphene oxide hybrid nanocomposite targeting glucose electro-oxidation in neutral medium. Electrochim. Acta 2018, 277, 176–184. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and Electronic Structure of Graphene-Oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef]
- Tang, J.; Jiang, S.; Liu, Y.; Zheng, S.; Bai, L.; Guo, J.; Wang, J. Electrochemical determination of dopamine and uric acid using a glassy carbon electrode modified with a composite consisting of a Co(II)-based metalorganic framework (ZIF-67) and graphene oxide. Mikrochim. Acta 2018, 185, 486. [Google Scholar] [CrossRef]
- Ejaz, A.; Jeon, S. A highly stable and sensitive GO-XDA-Mn2O3 electrochemical sensor for simultaneous electrooxidation of paracetamol and ascorbic acid. Electrochim. Acta 2017, 245, 742–751. [Google Scholar] [CrossRef]
- Tığ, G.A. Development of electrochemical sensor for detection of ascorbic acid, dopamine, uric acid and l-tryptophan based on Ag nanoparticles and poly(l-arginine)-graphene oxide composite. J. Electroanal. Chem. 2017, 807, 19–28. [Google Scholar]
- He, S.; He, P.; Zhang, X.; Zhang, X.; Liu, K.; Jia, L.; Dong, F. Poly(glycine)/graphene oxide modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of dopamine, uric acid, guanine and adenine. Anal. Chim. Acta 2018, 1031, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.-Y.; Li, H.; Piner, R.; Zarbin, A.J.G.; Ruoff, R.S. Reduced Graphene Oxide/Copper Nanowire Hybrid Films as High-Performance Transparent Electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, L.; Manuzzi, D.; Monteros, H.V.E.D.L.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens. Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J.; Veerapandian, D.M. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Wang, F.; Hu, Y.; Wang, E.; Rao, Z.; Zhang, X. In situ reduction of graphene oxide to improve the thermal and wettability properties of urea-melamine-modified phenol formaldehyde resin composites. Mater. Res. Express 2018, 6, 025302. [Google Scholar] [CrossRef]
- Oko, D.N.; Garbarino, S.; Zhang, J.; Xu, Z.; Chaker, M.; Ma, D.; Guay, D.; Tavares, A.C. Dopamine and ascorbic acid electro-oxidation on Au, AuPt and Pt nanoparticles prepared by pulse laser ablation in water. Electrochim. Acta 2015, 159, 174–183. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, Y.L.; Yu, A.; Lee, J.; Lee, S.C.; Lee, C.; Kim, M.H.; Lee, Y. Electrospun iridium oxide nanofibers for direct selective electrochemical detection of ascorbic acid. Sens. Actuators B Chem. 2014, 196, 480–488. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Y.; Wu, W.; Liu, M.; Mei, S.; Zhou, Y.; Jing, T. Highly selective and sensitive determination of dopamine by the novel molecularly imprinted poly(nicotinamide)/CuO nanoparticles modified electrode. Biosens. Bioelectron. 2015, 67, 121–128. [Google Scholar] [CrossRef]
- Bessems, J.G.M.; Vermeulen, N.P.E. Paracetamol (Acetaminophen)-Induced Toxicity: Molecular and Biochemical Mechanisms, Analogues and Protective Approaches. Crit. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, B.; Ding, Y.; Li, L.; Ouyang, X. Fabrication of cuprous oxide nanoparticles-graphene nanocomposite for determination of acetaminophen. J. Electroanal. Chem. 2015, 757, 88–93. [Google Scholar] [CrossRef]
- He, P.; Wang, W.; Du, L.; Dong, F.; Deng, Y.; Zhang, T. Zeolite A functionalized with copper nanoparticles and graphene oxide for simultaneous electrochemical determination of dopamine and ascorbic acid. Anal. Chim. Acta 2012, 739, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Aziz, A.; Wang, H.; Wang, Z.; Wang, W.; Ajmal, M.; Xiao, F.; Chen, X.; Liu, H. Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Microchim. Acta 2019, 186, 61. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, E.; Tashkhourian, J. Sonication-assisted preparation of a nanocomposite consisting of reduced graphene oxide and CdSe quantum dots, and its application to simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2018, 185, 456. [Google Scholar] [CrossRef] [PubMed]
- Shahbakhsh, M.; Noroozifar, M. Copper polydopamine complex/multiwalled carbon nanotubes as novel modifier for simultaneous electrochemical determination of ascorbic acid, dopamine, acetaminophen, nitrite and xanthine. J. Solid State Electrochem. 2018, 22, 3049–3057. [Google Scholar] [CrossRef]
- Liu, X.; Shangguan, E.; Li, J.; Ning, S.; Guo, L.; Li, Q. A novel electrochemical sensor based on FeS anchored reduced graphene oxide nanosheets for simultaneous determination of dopamine and acetaminophen. Mater. Sci. Eng. C 2017, 70, 628–636. [Google Scholar] [CrossRef]
- Felix, S.; Grace, A.N.; Jayavel, R. Sensitive electrochemical detection of glucose based on Au-CuO nanocomposites. J. Phys. Chem. Solids 2018, 122, 255–260. [Google Scholar] [CrossRef]
- Branagan, D.; Breslin, C.B. Electrochemical detection of glucose at physiological pH using gold nanoparticles deposited on carbon nanotubes. Sens. Actuators B Chem. 2019, 282, 490–499. [Google Scholar] [CrossRef]
- Salamon, J.; Sathishkumar, Y.; Ramachandran, K.; Lee, Y.S.; Yoo, D.J.; Kim, A.R.; Kumar, G.G. One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens. Bioelectron. 2015, 64, 269–276. [Google Scholar] [CrossRef]
- Kumar, B.; Murali, A.; Giri, S. Upconversion Nanoplatform for FRET-Based Sensing of Dopamine and pH. ChemistrySelect 2019, 4, 5407–5414. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Electrodes | Methods | Linear Ranges (μM) | Detection Limit (μM) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| AA | DA | AC | AA | DA | AC | |||
| GO-XDA-Mn2O3/GCE | Chronoamperometric | 10–8000 | - | 1–1000 | 0.6 | - | 0.056 | [29] |
| MWCNT/GO/AuNR/GCE | DPV | 1–8000 | - | - | 0.087 | - | - | [24] |
| GO-ZIF67/GCE | DPV | - | 0.2–80 | - | - | 0.05 | - | [28] |
| p-GLY/GO/GCE | DPV | - | 0.2–62 | - | - | 0.011 | - | [31] |
| AgNPs/P(Arg)-GO/GCE | DPV | 4–2400 (only change CAA) | 0.05–50 (only change CDA) | - | 0.984 | 0.01 | - | [30] |
| Cu2O/GR/GCE | DPV | - | - | 0.02-1.3 | - | - | 0.0067 | [42] |
| CuZEA/RGO/GCE | DPV | 20–200 (only change CAA) | 0.1–19 (only change CDA) | - | 11 | 0.041 | - | [43] |
| Zn-NiAl LDH/rGO/GCE | DPV | 0.5–11 | 0.001–1 | - | 0.0135 | 0.0001 | - | [44] |
| 3D-MoS2/rGO/Au/GCE | DPV | 2–5400 | 0.3–198.3 | - | 1.46 | 0.15 | - | [23] |
| RGO-CdSe QD/GCE | DPV | 390–1000 (only change CAA) | 4.9–74 (only change CDA) | - | 66 | 0.11 | - | [45] |
| Fc-S-Au/C NC/graphene/GCE | DPV | 8–400 | 0.2–2.5 | 0.5–46 | 1.0 | 0.05 | 0.1 | [17] |
| Cu2+@PDA-MWCNTs/GCE | DPV | 5–175 | 4–125 | 5–75 | 0.82 | 0.45 | 0.87 | [46] |
| Fe3O4@Au-S-Fc/GS-chitosan/GCE | DPV | 6–350 | 0.5–50 | 0.4–32 | 1.0 | 0.1 | 0.05 | [16] |
| PSNSB/CPE | DPV | 2.5–1050 | 0.05–120 | 0.033–158 | 0.02 | 0.002 | 0.005 | [22] |
| Au/ZnO/N-doped graphene/GCE | DPV | 30–13,000 (only change CAA) | 2–180 (only change CDA) | 5–3100 (only change CAC) | 5.0 | 0.4 | 0.8 | [1] |
| Cu NWs/GO/GCE | DPV | 1–60 | 1–100 | 1–100 | 0.05 | 0.41 | 0.04 | this work |
| Samples | Added (μM) | Found (μM) | Recoveries | Relative Standard Deviation (RSD) (%) (n = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | DA | AC | AA | DA | AC | AA | DA | AC | AA | DA | AC | |
| 1 | 50.0 | 68.0 | 70.0 | 49.1 | 66.9 | 71.2 | 98.2 | 98.4 | 101.7 | 3.3 | 1.9 | 2.1 |
| 2 | 52.0 | 72.0 | 75.0 | 50.3 | 68.0 | 71.0 | 96.8 | 94.6 | 94.7 | 1.6 | 0.8 | 1.4 |
| 3 | 54.0 | 74.0 | 78.0 | 51.8 | 70.1 | 73.8 | 96.0 | 94.8 | 94.6 | 1.0 | 0.6 | 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Zhang, Y.; Fan, J.; Liu, H.; Shi, Q.; Liu, W.; Peng, Q.; Zang, G. Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen. Molecules 2019, 24, 2320. https://doi.org/10.3390/molecules24122320
Hao W, Zhang Y, Fan J, Liu H, Shi Q, Liu W, Peng Q, Zang G. Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen. Molecules. 2019; 24(12):2320. https://doi.org/10.3390/molecules24122320
Chicago/Turabian StyleHao, Wanting, Yuchan Zhang, Jingchuan Fan, Handeng Liu, Qi Shi, Weichi Liu, Qianyu Peng, and Guangchao Zang. 2019. "Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen" Molecules 24, no. 12: 2320. https://doi.org/10.3390/molecules24122320
APA StyleHao, W., Zhang, Y., Fan, J., Liu, H., Shi, Q., Liu, W., Peng, Q., & Zang, G. (2019). Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen. Molecules, 24(12), 2320. https://doi.org/10.3390/molecules24122320






