Comparison of Phytochemical Differences of the Pulp of Different Peach [Prunus persica (L.) Batsch] Cultivars with Alpha-Glucosidase Inhibitory Activity Variations in China Using UPLC-Q-TOF/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Fruit Phenolic Phytochemical Contents and Alpha-glucosidase Inhibitory Activity Evaluation
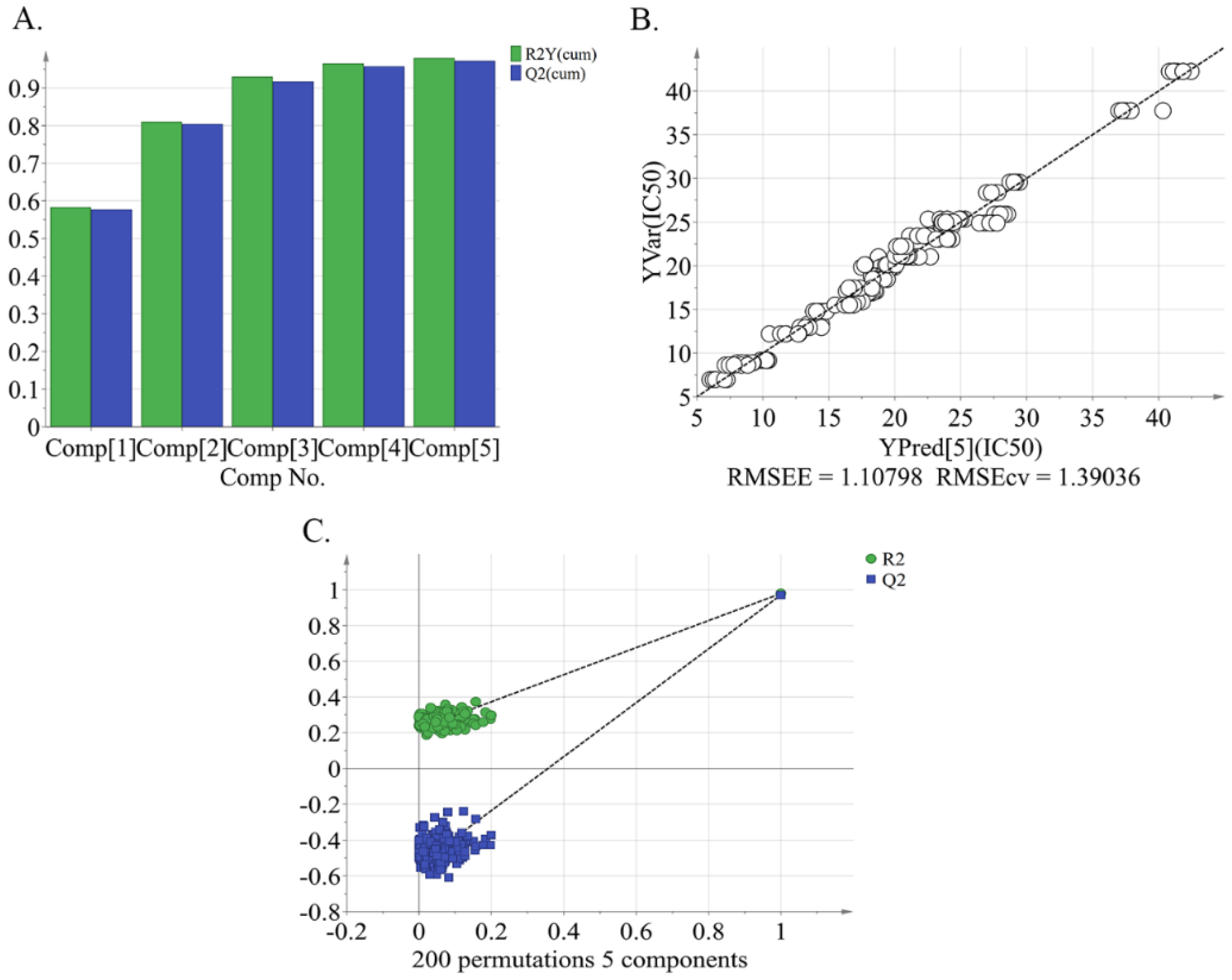
2.2. Relation between Metabolite Profiling and Alpha-glucosidase Inhibitory Activity
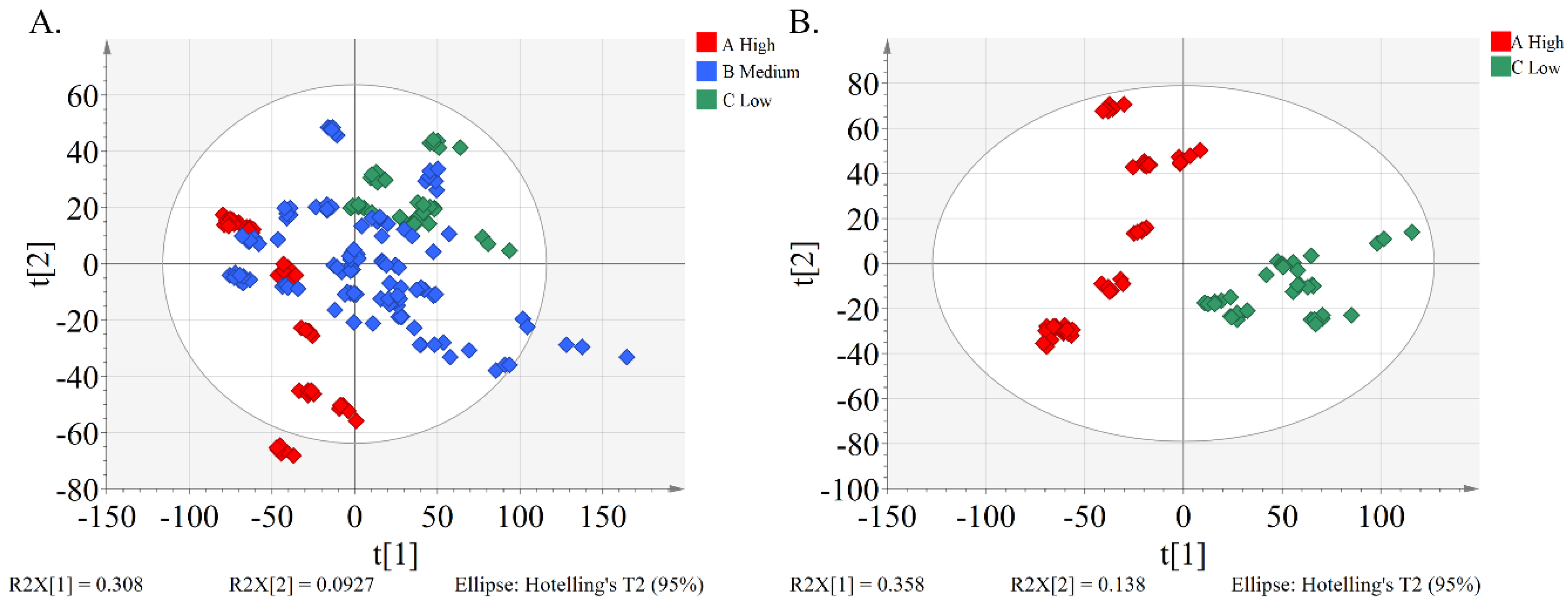
2.3. Metabolic Phenotype Differences between the High and Low Alpha-glucosidase Inhibitory Activity Groups
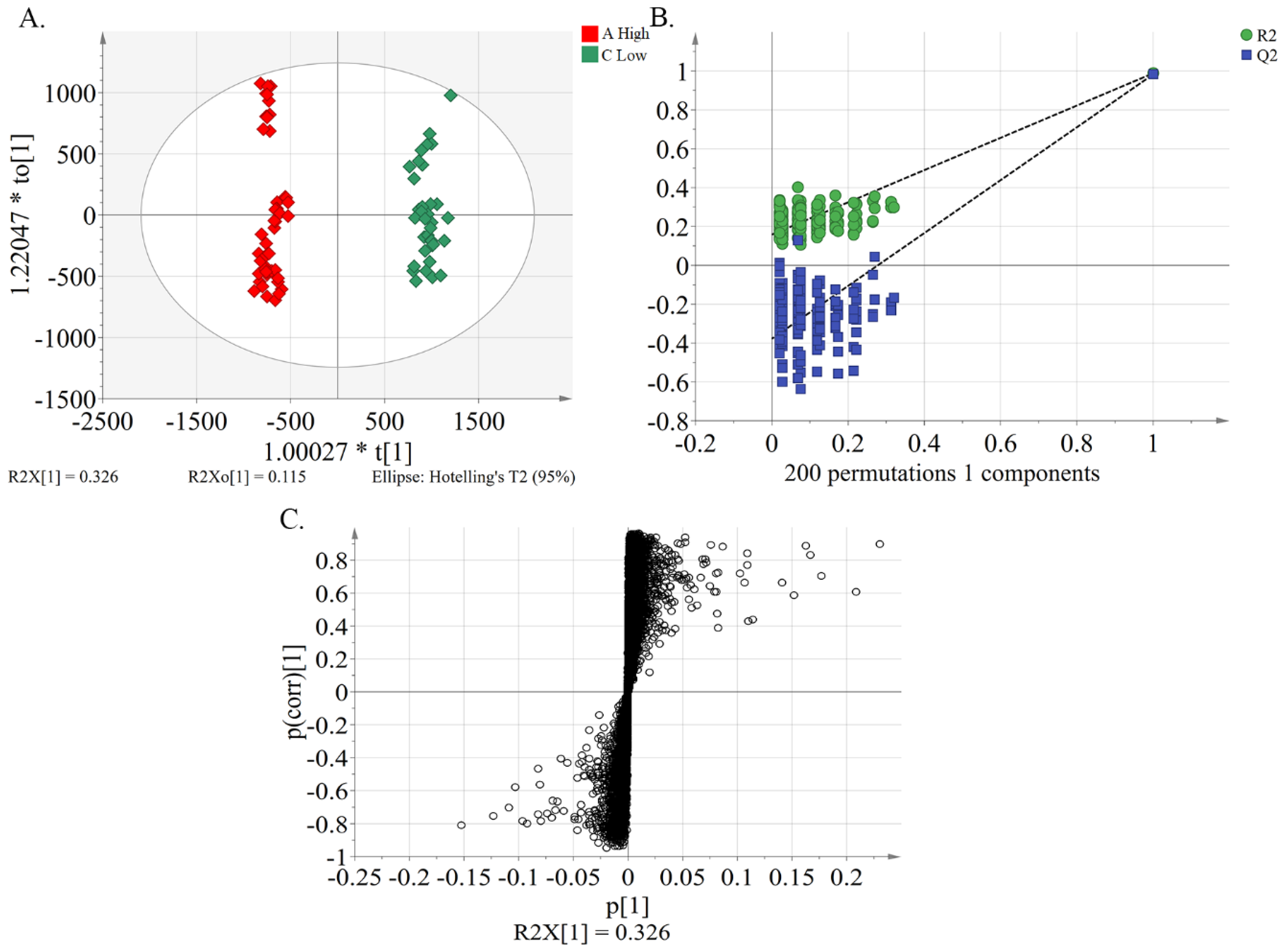
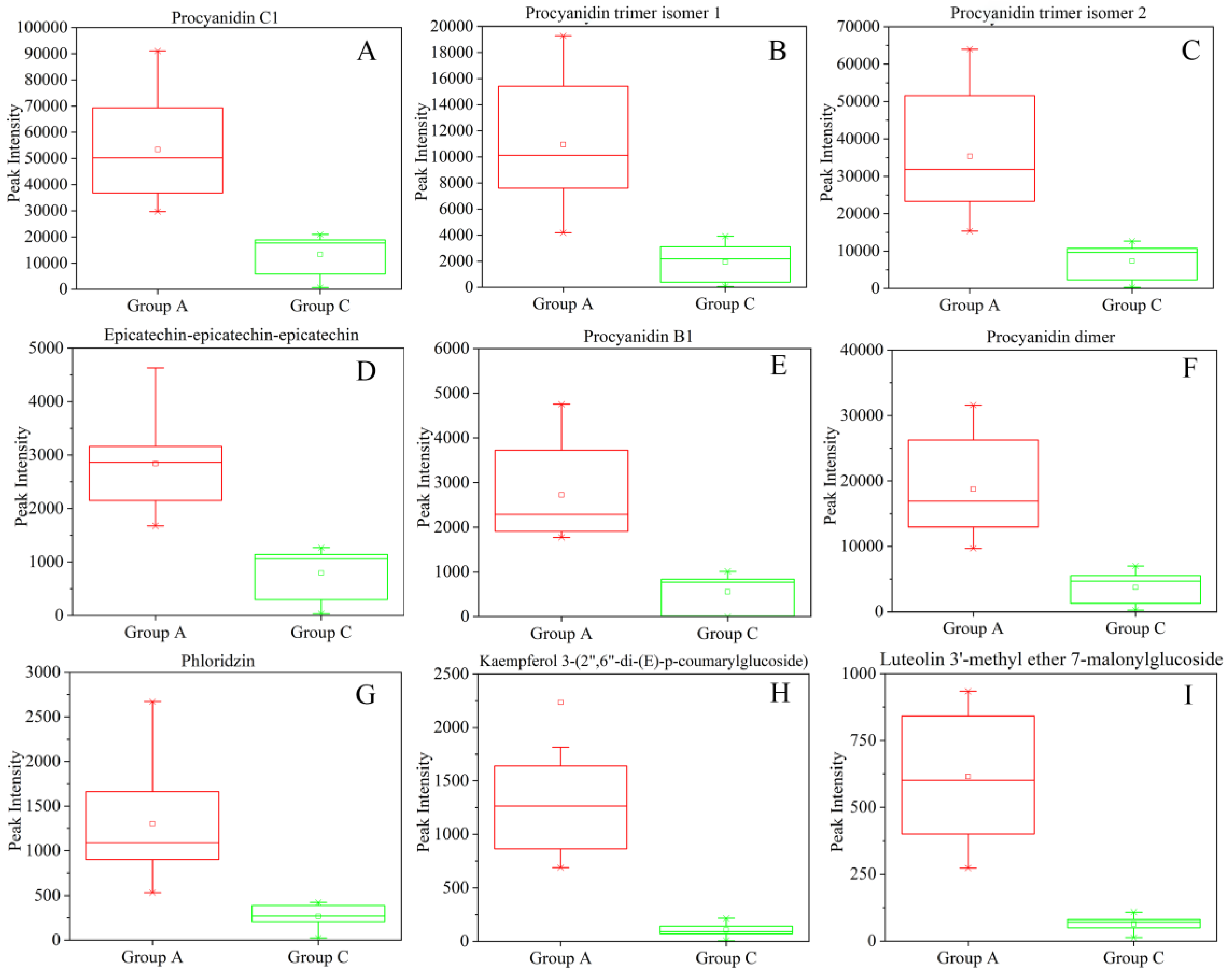
2.4. Identification of Characteristic Compounds and Marker Compounds in Peach Pulp Extract Differentiating the High and Low Alpha-glucosidase Inhibitory Activity
3. Materials and Methods
3.1. Fruit Materials
3.2. Chemicals and Reagents
3.3. Preparation of Peach Pulp Extracts
3.4. Total Phenolics, Total Flavonoids, Total Anthocyanins, and Total Procyanidins Contents
3.5. Determination of Alpha-glucosidase Inhibitory Activity
3.6. Condition for Metabolomic Analysis
3.7. Data Processing, Statistical Analysis, and Metabolite Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, S.L.; Hickey, M.S. Regulation of Insulin Action by Diet and Exercise. J. Equine. Vet. Sci. 2009, 29, 274–284. [Google Scholar] [CrossRef]
- WHO, 10 facts on diabetes. Available online: https://www.who.int/features/factfiles/diabetes/zh/ (accessed on 27 February. 2019).
- Ma, R.C.W. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018, 61, 1249–1260. [Google Scholar] [CrossRef]
- Safabakhsh, M.; Koohdani, F.; Bagheri, F.; Siassi, F.; Khajehnasiri, F.; Sotoudeh, G. Fruit and vegetable intake and pre-diabetes: A case-control study. Eur. J. Nutr. 2018, 57, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Du, H.D.; Li, L.M.; Bennett, D.; Guo, Y.; Turnbull, I.; Yang, L.; Bragg, F.; Bian, Z.; Chen, Y.P.; Chen, J.S.; et al. Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: A 7-y prospective study of 0.5 million Chinese adults. PloS Med. 2017, 14, e1002279. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanan, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as -Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziolkiewicz, M. In Vitro Inhibitory Effect on Digestive Enzymes and Antioxidant Potential of Commonly Consumed Fruits. J. Agr. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.; Timon, B. Origin and dissemination of peach. Hortic. Rev. 1995, 17, 334–379. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/zh/#data/QC (accessed on 30 April 2019).
- Noratto, G.; Martino, H.S.; Simbo, S.; Byrne, D.; Mertens-Talcott, S.U. Consumption of polyphenol-rich peach and plum juice prevents risk factors for obesity-related metabolic disorders and cardiovascular disease in Zucker rats. J. Nutr. Biochem. 2015, 26, 633–641. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Rodríguez-González, S.; Pérez-Ramírez, I.F.; Loarca-Piña, G.; Amaya-Llano, S.; Gallegos-Corona, M.A.; Reynoso-Camacho, R. Juice by-products as a source of dietary fibre and antioxidants and their effect on hepatic steatosis. J. Funct. Foods 2015, 17, 93–102. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, S.; Perez-Ramirez, I.F.; Amaya-Cruz, D.M.; Gallegos-Corona, M.A.; Ramos-Gomez, M.; Mora, O.; Reynoso-Camacho, R. Polyphenol-rich peach (Prunus persica L.) by-product exerts a greater beneficial effect than dietary fiber-rich by-product on insulin resistance and hepatic steatosis in obese rats. J. Funct. Foods 2018, 45, 58–66. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdylo, A.; Laskowski, P. Inhibitory Potential against Digestive Enzymes Linked to Obesity and Type 2 Diabetes and Content of Bioactive Compounds in 20 Cultivars of the Peach Fruit Grown in Poland. Plant Food Hum. Nutr. 2018, 73, 314–320. [Google Scholar] [CrossRef]
- Bento, C.; Goncalves, A.C.; Silva, B.; Silva, L.R. Assessing the phenolic profile, antioxidant, antidiabetic and protective effects against oxidative damage in human erythrocytes of peaches from Fundao. J. Funct. Foods 2018, 43, 224–233. [Google Scholar] [CrossRef]
- Xian, F.; Hendrickson, C.L.; Marshall, A.G. High Resolution Mass Spectrometry. Anal. Chem. 2012, 84, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, O.; Fu, H.; Ouyang, L.; Gong, X.; Meng, P.; Wang, Z.; Dai, M.; Guo, X.; Wang, Y. UPLC–Q-TOF/MS-based untargeted metabolomics coupled with chemometrics approach for Tieguanyin tea with seasonal and year variations. Food Chem. 2019, 283, 73–82. [Google Scholar] [CrossRef]
- Jing, J.; Shi, Y.; Zhang, Q.; Wang, J.; Ruan, J. Prediction of Chinese green tea ranking by metabolite profiling using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS). Food Chem. 2017, 221, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, W.N.; Yin, X.R.; Su, M.S.; Sun, C.D.; Li, X.; Chen, K.S. Phenolic Composition and Antioxidant Properties of Different Peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Nunez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive Compounds and Antioxidant Activity of Pulp, Peel and Seed Ethanolic Extracts. Plant Food Hum. Nutr. 2015, 70, 331–337. [Google Scholar] [CrossRef]
- Abidi, W.; Jimenez, S.; Moreno, M.A.; Gogorcena, Y. Evaluation of Antioxidant Compounds and Total Sugar Content in a Nectarine [Prunus persica (L.) Batsch] Progeny. Int. J. Mol. Sci. 2011, 12, 6919–6935. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin-Ciocalteu reagent. J. Agr. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agr. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agr. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agr. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Liao, X.X.; Greenspan, P.; Pegg, R.B. Characterizing the phenolic constituents and antioxidant capacity of Georgia peaches. Food Chem. 2019, 271, 345–353. [Google Scholar] [CrossRef]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Merillon, J.M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdylo, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2019, 23, 1–15. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemometr. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Soreide, K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J. Clin. Pathol. 2009, 62, 1–5. [Google Scholar] [CrossRef]
- Xia, J.G.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agr. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadi, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitaminol. 2007, 53, 166–173. [Google Scholar] [CrossRef]
- Iwai, K.; Kim, M.Y.; Onodera, A.; Matsue, H. alpha-glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J. Agr. Food Chem. 2006, 54, 4588–4592. [Google Scholar] [CrossRef]
- Zhang, L.L.; Han, L.; Yang, S.Y.; Meng, X.M.; Ma, W.F.; Wang, M. The mechanism of interactions between flavan-3-ols against a-glucosidase and their in vivo antihyperglycemic effects. Bioorg. Chem. 2019, 85, 364–372. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young apple polyphenols postpone starch digestion in vitro and in vivo. J. Funct. Foods 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Schäfer, A.; Högger, P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol®) effectively inhibit α-glucosidase. Diabetes. Res. Clin. Pr. 2007, 77, 41–46. [Google Scholar] [CrossRef]
- Lin, H.; Lingling, Z.; Wenfang, M.; Ding, L.; Rujie, S.; Min, W. Proanthocyanidin B2 attenuates postprandial blood glucose and its inhibitory effect on alpha-glucosidase: Analysis by kinetics, fluorescence spectroscopy, atomic force microscopy and molecular docking. Food Funct. 2018, 9, 4673–4682. [Google Scholar]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Wen, X.; Lv, Q.; Xu, C.; Sun, C.; Li, X. Purification of Flavonoids from Chinese Bayberry (Morella rubra Sieb. et Zucc.) Fruit Extracts and alpha-Glucosidase Inhibitory Activities of Different Fractionations. Molecules 2016, 21, 1148. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- ChunHua, Z.; ChongDe, S.U.N.; Li, X. Study on Method for Flavonoids Determining of Plant Rich in Chlorogenic Acid. Plant Physiol. Commun. 2007, 43, 902–904. [Google Scholar]
- Cao, J.P.; Jiang, Q.; Lin, J.Y.; Li, X.; Sun, C.D.; Chen, K.S. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.X.; Fan, E.; Ji, H.P.; Kou, Y. Determination of Total Procyanidins in Selected Chocolate and Confectionery Products Using DMAC. J. Aoac. Int. 2010, 93, 89–96. [Google Scholar]
Sample Availability: Samples of the 33 cultivars of peach fruits in this study are available from the authors. |
| Cultivars | Total Phenolics | Total Flavonoids | Total Anthocyanins | Total Procyanidins | IC50* |
|---|---|---|---|---|---|
| Bai Hua | 1034.02 ± 21.32 h | 5.76 ± 0.22 fghi | 6.19 ± 1.97 jk | 711.8 ± 13.62 i | 20.99 ± 1.06 efghij |
| Chun Mei | 660.39 ± 17.9 k | 10.46 ± 1.2 de | 13.86 ± 0.25 defgh | 484.33 ± 7.79 k | 19.8 ± 0.85 dfghij |
| Feng Bai | 1805.68 ± 51.07 c | 6.39 ± 0.21 fghi | 5.86 ± 0.91 jk | 1869.18 ± 38.6 b | 9.18 ± 0.98 ab |
| Gui Fei | 1163.96 ± 14.24 g | 8.25 ± 0.37 efgh | 15.22 ± 0.64 def | 1066.69 ± 35.9 g | 12.93 ± 0.63 abcd |
| Qiu Yue | 822.26 ± 23.09 i | 5.88 ± 0.75 fghi | 6.84 ± 1.44 ijk | 257.19 ± 29.63 o | 20.11 ± 1.25 efghij |
| Tai Nong 2 | 670.26 ± 19.21 k | 7.52 ± 0.17 efghi | 21.72 ± 1.12 c | 161.3 ± 7.05 p | 25.36 ± 1.02 jkl |
| Ye Sheng Tao | 1299.55 ± 19.62 f | 6.89 ± 1.71 efg | 17.62 ±1.04 cd | 1223.82 ± 15.08 e | 12.15 ± 0.44 abc |
| Yu Bai | 2386.38 ± 18.44 a | 16.74 ± 2.03 c | 5.38 ± 0.98 jk | 2643.68 ± 23.17 a | 6.92 ± 0.15 a |
| Cheng Xiang | 414.81 ± 6.54 no | 5.2 ± 0.25 ghi | 14.95 ± 0.64 def | 300.87 ± 6.66 no | 23.04 ± 2.91 ghijk |
| Dalian 12-28 | 1014.28 ± 12.03 h | 7.91 ± 0.46 efghi | 14.37 ± 1.39 defg | 799.33 ± 10.49 h | 15.86 ± 0.78 cdef |
| Dalian 1-49 | 641.18 ± 17.07 k | 5.03 ± 0.4 ghi | 8.99 ± 0.48 hijk | 463.28 ± 12.84 kl | 16.85 ± 1.12 cdefg |
| Jin Xiang | 793.35 ± 11.63 ij | 4.35 ± 0.27 i | 10.49 ± 1.03 fghij | 716.44 ± 27.63 i | 21.14 ± 0.26 efghij |
| Long 1-2-4 | 1055.32 ± 29.97 h | 13.23 ± 1.36 d | 16.22 ± 1.67 de | 715.96 ± 13.49 i | 18.54 ± 0.4 cdefghi |
| Zheng Huang 3 | 450.35 ± 83.24 lm | 7.52 ± 0.46 efghi | 15.53 ± 2.89 cd | 362.74 ± 8.65 mn | 29.6 ± 2.03 l |
| Hei Tao | 624.44 ± 17.92 kl | 20.02 ± 1.36 b | 17.58 ± 1.93 cd | 707.05 ± 24.5 i | 20.35 ± 1.48 cdefgh |
| Tianjin Shui Mi | 1976.39 ± 63.74 b | 18.95 ± 1.32 bc | 138.16 ± 5.05 a | 1931.5 ± 73.41 b | 8.91 ± 1.02 ab |
| Wuhan Da Hong Pao | 1278.43 ± 80.13 f | 9.38 ± 0.22 ef | 86.88 ± 2.15 b | 305.19 ± 4.46 no | 24.5 ± 0.87 ijkl |
| Mao Tao | 1619.42 ± 32.38 d | 35.51 ± 3.76 a | 17.74 ± 0.97 cd | 1176.83 ± 37.64 ef | 8.64 ± 0.16 ab |
| 88-4-25 | 1331.91 ± 41.56 f | 8.03 ± 0.29 efghi | 18.45 ± 1.04 cd | 1126.83 ± 25.3 fg | 14.77 ± 1.13 bcde |
| Hong Shan Hu | 346.88 ± 10.73 o | 4.97 ± 0.49 ghi | 5.73 ± 1.83 ijk | 239.01 ± 5.36 o | 28.32 ± 1.78 kl |
| Huyou 002 | 440.35 ± 20.01 no | 6.5 ± 0.24 fghi | 8.39 ± 1.2 ijk | 241.86 ± 19.3 o | 37.75 ± 2.14 m |
| Zao You Tao | 203.46 ± 7.59 p | 7.97 ± 0.55 efghi | 9.26 ± 0.56 ghijk | 25 ± 1.65 q | 24.95 ± 0.63 ijkl |
| Huyou 018 | 650.7 ± 12.57 k | 10.29 ± 1.42 de | 7.86 ± 0.96 ijk | 504.84 ± 12.93 k | 23.39 ± 0.54 hijkl |
| Shuang Xi Hong | 63.61 ± 5.15 q | 7.4 ± 1.5 efghi | 1.51 ± 0.37 l | 0.43 ± 1.15 q | 42.28 ± 3.81 m |
| Zhong Nong Jin Hui | 201.13 ± 8.04 p | 5.99 ± 0.45 fghi | 6.23 ± 0.68 jk | 129.33 ± 7.13 p | 24.87 ± 0.69 ijkl |
| Hu 238 | 1494.97 ± 41.42 e | 6.27 ± 0.41 fghi | 4.13 ± 0.86 k | 1355.68 ± 26.44 d | 13.27 ± 0.48 bcd |
| Pan Tao Huang Hou | 482.64 ± 17.23 mn | 6.5 ± 0.63 fghi | 9.58 ± 1.66 ghij | 298.51 ± 12.31 no | 20.99 ± 0.51 efghij |
| Ying Ri Er Pan Tao | 662.58 ± 9.71 k | 7.01 ± 0.36 efghi | 10.38 ± 1 fghij | 404.79 ± 14 lm | 15.5 ± 1.61 cde |
| Dalian 4-35 | 722.36 ± 10.04 jk | 5.88 ± 0.15 fghi | 10.13 ± 1.17 fghij | 612.11 ± 11.79 j | 22.17 ± 0.4 fghijk |
| Zao Huang Pan Tao | 362.95 ± 8.11 o | 4.46 ± 0.44 hi | 11.67 ± 1.09 efghi | 256.62 ± 11.81 o | 30.41 ± 0.32 jkl |
| Jin Xia You Pan | 397.26 ± 24.92 no | 7.23 ± 0.31 efghi | 11.94 ± 0.35 efghi | 796.12 ± 19.61 h | 18.48 ± 1.02 cdefghi |
| Long You Pan Tao | 1469.05 ± 53.34 e | 13.57 ± 1.72 d | 18.82 ± 1.15 cd | 1494.45 ± 31.09 c | 17.4 ± 2.35 cdefgh |
| Zhong You Pan 2 | 404.44 ± 48.7 no | 4.63 ± 0.28 hi | 16.17 ± 0.56 de | 684.65 ± 29.94 i | 18.78 ± 0.14 defghi |
| Component | VIP a | Class | Identification Level b | r c |
|---|---|---|---|---|
| Procyanidin C1 | 1.73 | Proanthocyanidins | (i) | –0.721 * |
| Procyanidin B1 | 1.71 | Proanthocyanidins | (i) | –0.719 * |
| Epicatechin-epicatechin-epicatechin | 1.68 | Proanthocyanidins | (iii) | –0.678 * |
| Procyanidin dimer | 1.66 | Proanthocyanidins | (iii) | –0.722 * |
| Procyanidin trimer isomer 2 | 1.66 | Proanthocyanidins | (iii) | –0.692 * |
| Procyanidin trimer isomer 1 | 1.61 | Proanthocyanidins | (iii) | –0.695 * |
| 8,8’-Methylenebiscatechin | 1.59 | Catechins | (ii) | –0.583 * |
| Procyanidin B2 | 1.53 | Proanthocyanidins | (i) | –0.615 * |
| Prunus inhibitor b | 1.47 | Proanthocyanidins | (ii) | –0.633 * |
| Catechin | 1.43 | Catechins | (i) | –0.527 * |
| Prunin | 1.39 | Flavanones | (ii) | –0.355 * |
| Phloridzin | 1.38 | Dihydrochalcones | (i) | –0.618 * |
| Naringenin | 1.35 | Flavanones | (ii) | –0.365 * |
| Neochlorogenic acid | 1.25 | Quinic acids and derivatives | (i) | –0.360 * |
| 3-O-Feruloylquinic acid | 1.20 | Quinic acids and derivatives | (iii) | –0.511 * |
| Caffeoylquinic acid | 1.15 | Quinic acids and derivatives | (iii) | –0.446 * |
| Chlorogenic acid | 1.13 | Quinic acids and derivatives | (i) | –0.465 * |
| Component | VIP a | P-Value b | FC c | Class | Identification Level d |
|---|---|---|---|---|---|
| Procyanidin C1 | 12.88 | 2.55E-20 | 4.00 | Proanthocyanidins | (i) |
| Procyanidin trimer isomer 2 | 10.39 | 1.10E-16 | 4.81 | Proanthocyanidins | (iii) |
| Catechin | 9.25 | 4.04E-13 | 2.50 | Catechins | (i) |
| Procyanidin dimer | 7.84 | 6.07E-20 | 5.00 | Proanthocyanidins | (iii) |
| Procyanidin trimer isomer 1 | 5.91 | 6.57E-18 | 5.62 | Proanthocyanidins | (iii) |
| Procyanidin B2 | 5.82 | 6.25E-12 | 3.11 | Proanthocyanidins | (i) |
| Cynaroside A | 3.34 | 4.39E-07 | 2.13 | Guaianolides | (ii) |
| Quercetin 3-glucoside | 3.00 | 4.11E-02 | 5.89 | Flavonols | (i) |
| Procyanidin B1 | 2.98 | 1.37E-20 | 4.91 | Proanthocyanidins | (i) |
| Epicatechin-epicatechin-epicatechin | 2.90 | 3.19E-20 | 3.56 | Proanthocyanidins | (iii) |
| Aucubin | 2.87 | 9.58E-06 | 4.57 | Iridoid O-glycosides | (ii) |
| Ptelatoside B | 2.80 | 2.20E-06 | 4.91 | Phenolic glycosides | (ii) |
| (1RS,2RS)-Guaiacylglycerol 1-glucoside | 2.29 | 8.91E-06 | 3.67 | O-glycosyl compounds | (ii) |
| Kaempferol 3-(2",6"-di-(E)-p-coumarylglucoside) | 2.25 | 2.02E-05 | 21.22 | Flavone | (ii) |
| Phloridzin | 1.99 | 6.34E-16 | 4.88 | Dihydrochalcones | (i) |
| Xanthotoxol glucoside | 1.93 | 2.37E-21 | 2.82 | Coumarin glycosides | (ii) |
| Prunitrin | 1.85 | 1.51E-06 | 4.70 | Isoflavone | (ii) |
| 3-O-Feruloylquinic acid | 1.75 | 4.76E-05 | 3.01 | Quinic acids derivatives | (ii) |
| Epifisetinidol (4b->8) catechin | 1.54 | 7.27E-11 | 8.78 | Proanthocyanidins | (ii) |
| Luteolin 3’-methyl ether 7-malonylglucoside | 1.52 | 7.95E-23 | 9.53 | Flavone | (ii) |
| Naringenin | 1.51 | 7.60E-04 | 5.84 | Flavanones | (ii) |
| Prunus inhibitor b | 1.16 | 2.78E-15 | 3.30 | Proanthocyanidins | (ii) |
| Quercetin 3-galactoside | 1.12 | 1.16E-02 | 3.16 | Flavonols | (i) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Su, M.; Du, J.; Zhou, H.; Li, X.; Li, X.; Ye, Z. Comparison of Phytochemical Differences of the Pulp of Different Peach [Prunus persica (L.) Batsch] Cultivars with Alpha-Glucosidase Inhibitory Activity Variations in China Using UPLC-Q-TOF/MS. Molecules 2019, 24, 1968. https://doi.org/10.3390/molecules24101968
Zhang X, Su M, Du J, Zhou H, Li X, Li X, Ye Z. Comparison of Phytochemical Differences of the Pulp of Different Peach [Prunus persica (L.) Batsch] Cultivars with Alpha-Glucosidase Inhibitory Activity Variations in China Using UPLC-Q-TOF/MS. Molecules. 2019; 24(10):1968. https://doi.org/10.3390/molecules24101968
Chicago/Turabian StyleZhang, Xianan, Mingshen Su, Jihong Du, Huijuan Zhou, Xiongwei Li, Xin Li, and Zhengwen Ye. 2019. "Comparison of Phytochemical Differences of the Pulp of Different Peach [Prunus persica (L.) Batsch] Cultivars with Alpha-Glucosidase Inhibitory Activity Variations in China Using UPLC-Q-TOF/MS" Molecules 24, no. 10: 1968. https://doi.org/10.3390/molecules24101968
APA StyleZhang, X., Su, M., Du, J., Zhou, H., Li, X., Li, X., & Ye, Z. (2019). Comparison of Phytochemical Differences of the Pulp of Different Peach [Prunus persica (L.) Batsch] Cultivars with Alpha-Glucosidase Inhibitory Activity Variations in China Using UPLC-Q-TOF/MS. Molecules, 24(10), 1968. https://doi.org/10.3390/molecules24101968





