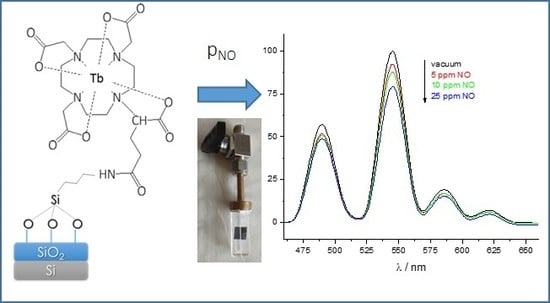
Silicon Wafer Functionalization with a Luminescent Tb(III) Coordination Complex: Synthesis, Characterization, and Application to the Optical Detection of NO in the Gas Phase
Abstract
:1. Introduction
2. Results and Discussion
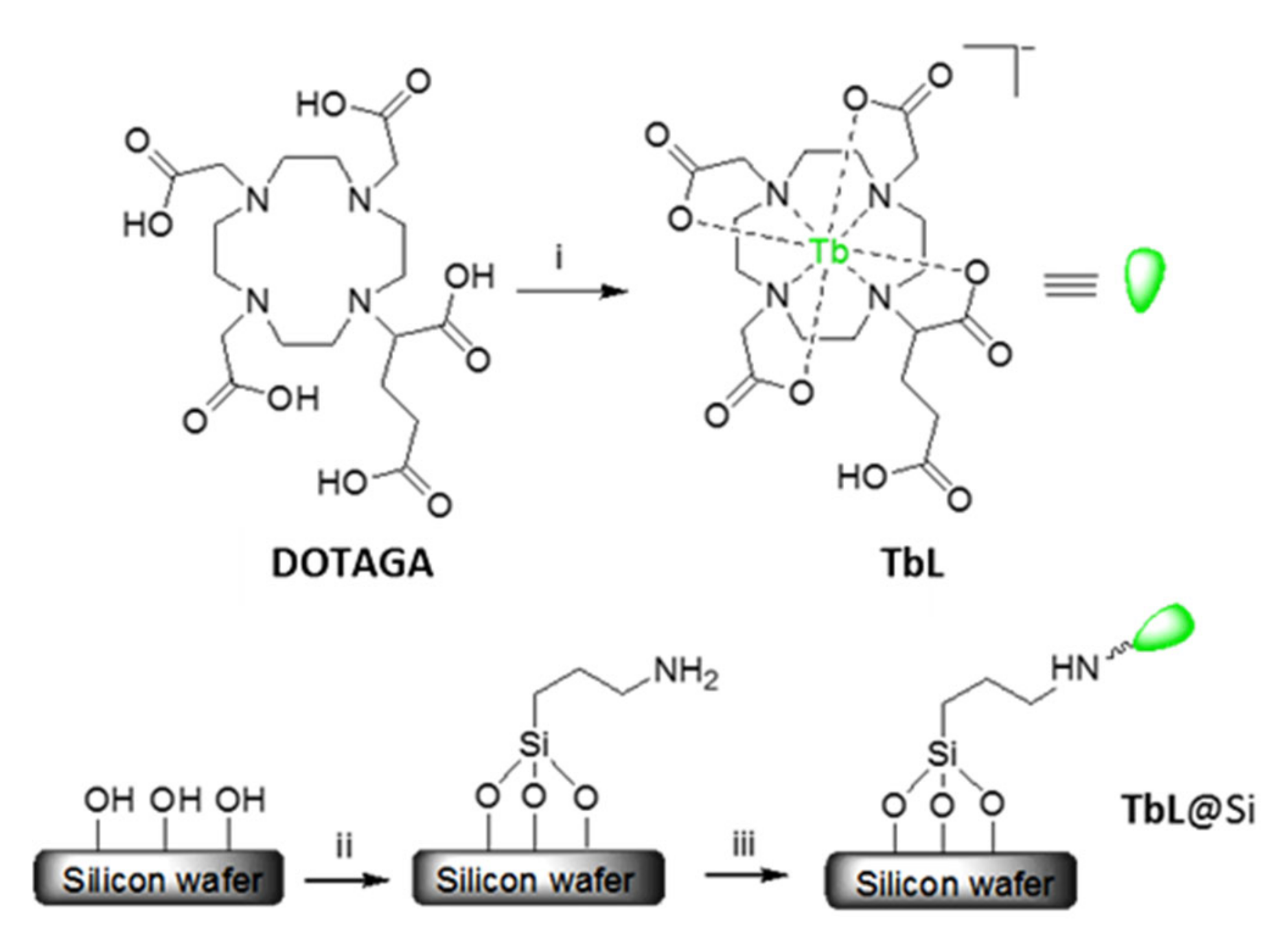
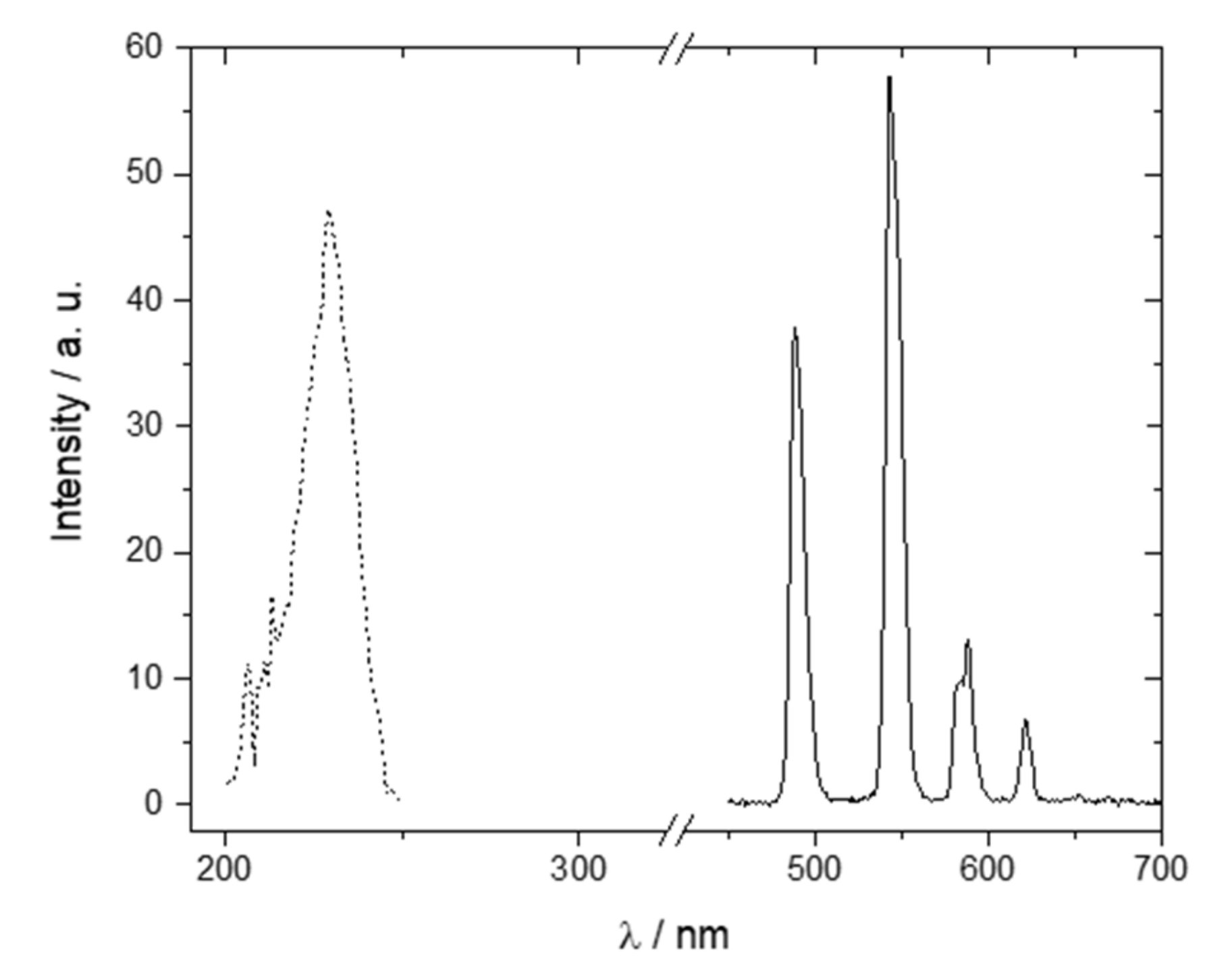
2.1. Tb-DOTAGA Complex Synthesis and Spectroscopic Characterization
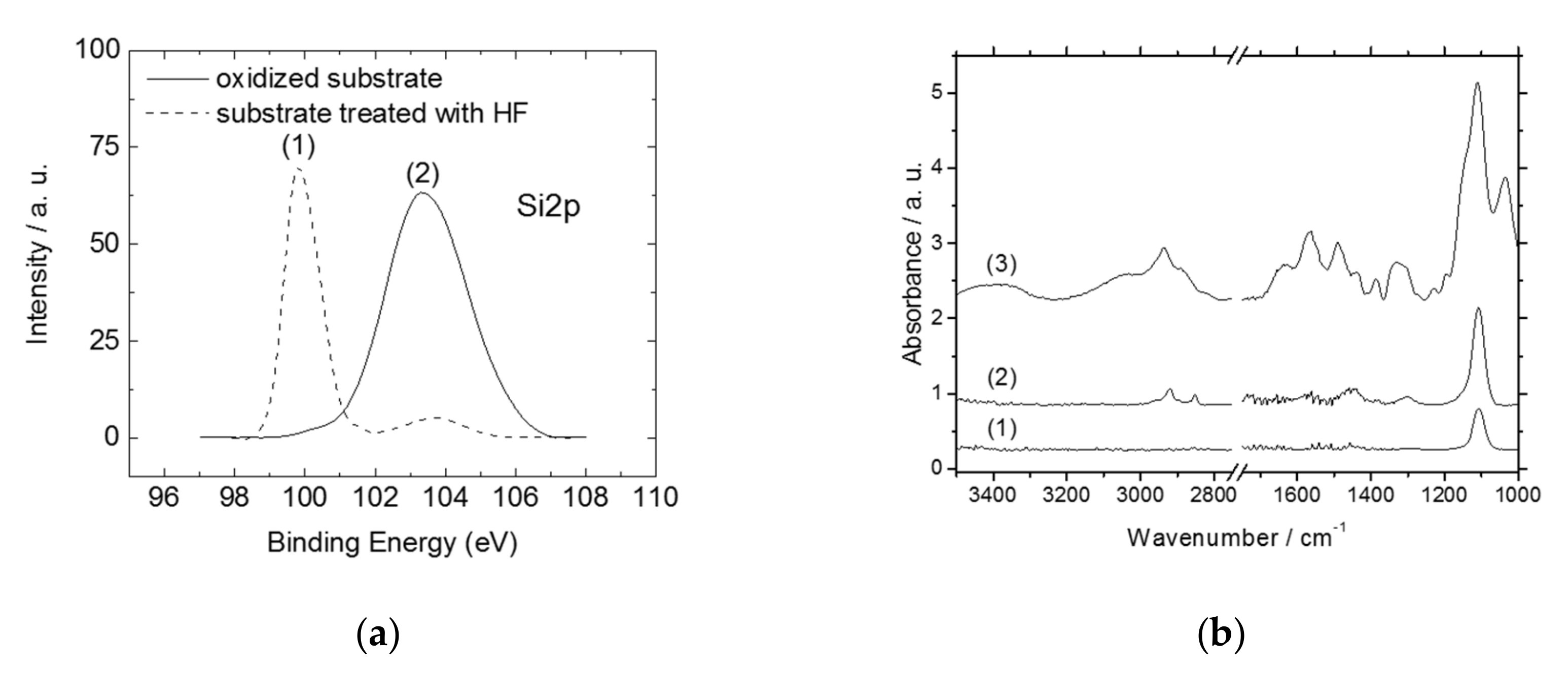
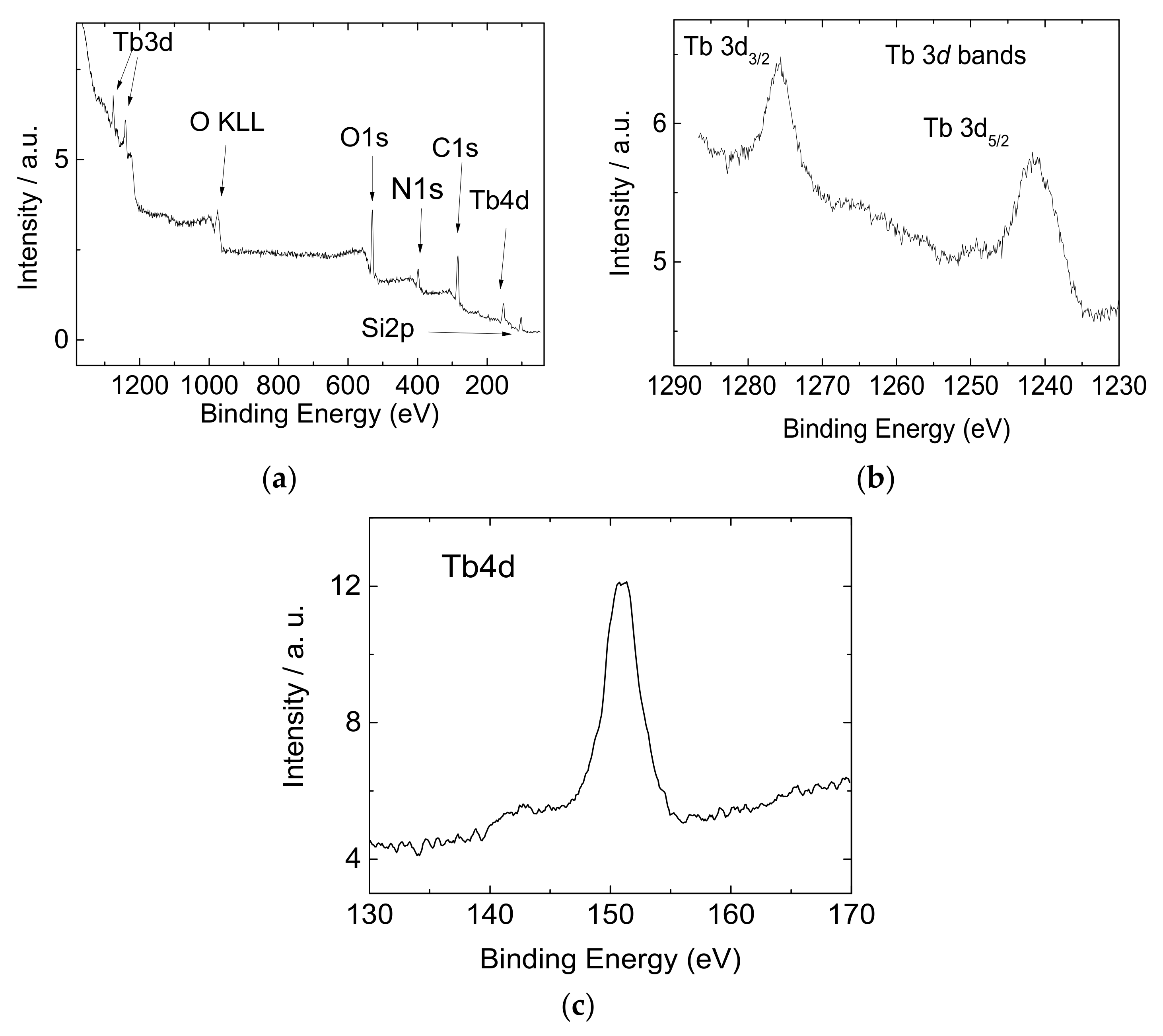
2.2. Functionalization and Characterization of the Silicon Wafers
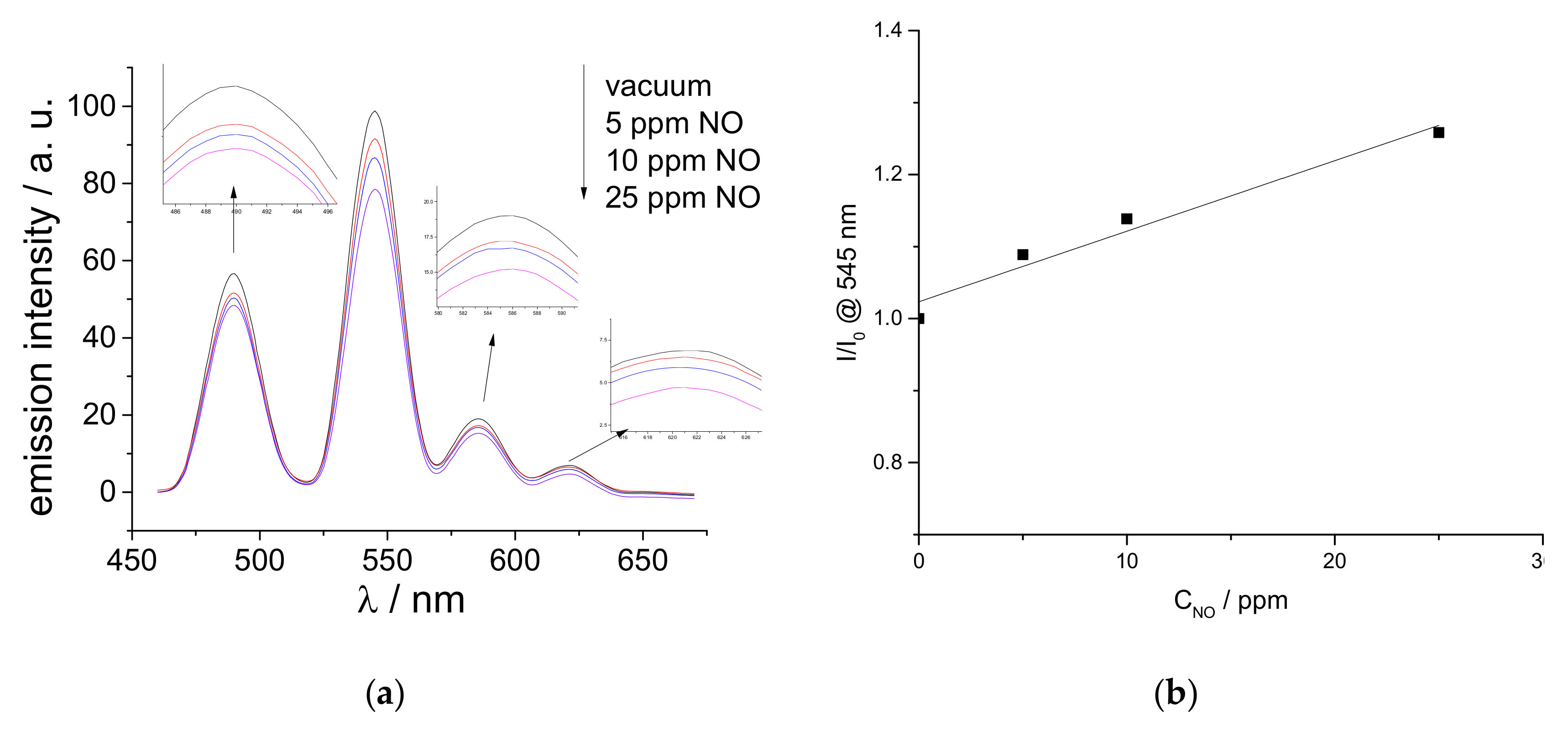
2.3. Luminescent Properties of the Functionalized Silicon Wafers
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
3.3. X-Ray Photoelectron Spectroscopy
3.4. Fluorescence Spectroscopy and Gas Dilution
3.5. Synthesis of TbL
3.6. Preparation of Silicon Wafers
3.7. Silicon Wafer Functionalization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: from mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Yoshimura, T. Bioimaging of Nitric Oxide. Chem. Rev. 2002, 102, 1235–1270. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Analytical Chemistry of Nitric Oxide. Annu. Rev. Anal. Chem. 2009, 2, 409–433. [Google Scholar] [CrossRef] [Green Version]
- Souto, J.; Rodríguez, M.L.; Desaja, J.A.; Aroca, R. Langmuir-Blodgett films of lanthanide bysphthalocyanines: applications as gas sensors. Int. J. Electron. 1994, 76, 763–769. [Google Scholar] [CrossRef]
- Liu, S.; Volkmer, D.; Kurth, D.G. Smart Polyoxometalate-Based Nitrogen Monoxide Sensors. Anal. Chem. 2004, 76, 4579–4582. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, M.; Bernasek, S.L.; Schwartz, J. Highly Sensitive Nitric Oxide Detection Using X-ray Photoelectron Spectroscopy. J. Am. Chem. Soc. 2007, 129, 6980–6981. [Google Scholar] [CrossRef]
- Victor, E.; Kim, S.; Lippard, S.J. Synthesis of Bis(imidazole) Metal Complexes and Their Use in Rapid NO Detection and Quantification Devices. Inorg. Chem. 2014, 53, 12809–12821. [Google Scholar] [CrossRef]
- Lim, M.H.; Lippard, S.J. Metal-Based Turn-On Fluorescent Probes for Sensing Nitric Oxide. Acc. Chem. Res. 2007, 40, 41–51. [Google Scholar] [CrossRef]
- Kim, J.-H.; Heller, D.A.; Jin, H.; Barone, P.W.; Song, C.; Zhang, J.; Trudel, L.J.; Wogan, G.N.; Tannenbaum, S.R.; Strano, M.S. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat. Chem. 2009, 1, 473–481. [Google Scholar] [CrossRef]
- Robinson, J.K.; Bollinger, M.J.; Birks, J.W. Luminol/H2O2 Chemiluminescence Detector for the Analysis of Nitric Oxide in Exhaled Breath. Anal. Chem. 1999, 71, 5131–5136. [Google Scholar] [CrossRef]
- Harper, J.; Sailor, M.J. Detection of Nitric Oxide and Nitrogen Dioxide with Photoluminescent Porous Silicon. Anal. Chem. 1996, 68, 3713–3717. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Dennison, G.H.; Johnston, M.R. Mechanistic Insights into the Luminescent Sensing of Organophosphorus Chemical Warfare Agents and Simulants Using Trivalent Lanthanide Complexes. Chem. Eur. J. 2015, 21, 6328–6338. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Yan, B. Eu(III)-Functionalized MIL-124 as Fluorescent Probe for Highly Selectively Sensing Ions and Organic Small Molecules Especially for Fe(III) and Fe(II). ACS Appl. Mater. Interfaces 2015, 7, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Su, S.; Yang, W.; Song, S.; Zhang, H. One-dimensional channel-structured Eu-MOF for sensing small organic molecules and Cu2+ ion. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, S.-R.; Du, D.-Y.; Qin, J.-S.; Bao, S.-J.; Li, J.; Su, Z.-M.; He, W.-W.; Fu, Q.; Lan, Y.-Q. Stable Luminescent Metal–Organic Frameworks as Dual-Functional Materials to Encapsulate Ln3+ Ions for White-Light Emission and to Detect Nitroaromatic Explosives. Inorg. Chem. 2015, 54, 3290–3296. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, R.; Ye, Z.; Dai, Z.; An, H.; Yuan, J. Lanthanide Complex-Based Luminescent Probes for Highly Sensitive Time-Gated Luminescence Detection of Hypochlorous Acid. Anal. Chem. 2012, 84, 10785–10792. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, W.; Ye, Z.; Wang, G.; Yuan, J. A europium(III) chelate as an efficient time-gated luminescent probe for nitric oxide. Chem. Commun. 2011, 47. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: a review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef]
- Ibarra, I.A.; Hesterberg, T.W.; Chang, J.-S.; Yoon, J.W.; Holliday, B.J.; Humphrey, S.M. Molecular sensing and discrimination by a luminescent terbium–phosphine oxide coordination material. Chem. Commun. 2013, 49, 7156–7158. [Google Scholar] [CrossRef]
- Dou, Z.; Yu, J.; Cui, Y.; Yang, Y.; Wang, Z.; Yang, D.; Qian, G. Luminescent Metal–Organic Framework Films as Highly Sensitive and Fast-Response Oxygen Sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef] [PubMed]
- Kielar, F.; Tei, L.; Terreno, E.; Botta, M. Large Relaxivity Enhancement of Paramagnetic Lipid Nanoparticles by Restricting the Local Motions of the Gd III Chelates. J. Am. Chem. Soc. 2010, 132, 7836–7837. [Google Scholar] [CrossRef] [PubMed]
- Henig, J.; Toóth, E.; Engelmann, J.; Gottschalk, S.; Mayer, H.A. Macrocyclic Gd 3+ Chelates Attached to a Silsesquioxane Core as Potential Magnetic Resonance Imaging Contrast Agents: Synthesis, Physicochemical Characterization, and Stability Studies. Inorg. Chem. 2010, 49, 6124–6138. [Google Scholar] [CrossRef]
- Rigaux, G.; Roullin, V.G.; Cadiou, C.; Portefaix, C.; Van Gulick, L.; Bœuf, G.; Andry, M.C.; Hoeffel, C.; Vander Elst, L.; Laurent, S.; et al. A new magnetic resonance imaging contrast agent loaded into poly(lacide-co-glycolide) nanoparticles for long-term detection of tumors. Nanotechnology 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.S.; Kaneshige, K.R.; Strong, J.S.; Tan, K.; von Bremen, H.F.; Mogul, R. Effects of terbium chelate structure on dipicolinate ligation and the detection of Bacillus spores. J. Inorg. Biochem. 2011, 105, 1580–1588. [Google Scholar] [CrossRef]
- Moreau, J.; Guillon, E.; Pierrard, J.-C.; Rimbault, J.; Port, M.; Aplincourt, M. Complexing Mechanism of the Lanthanide Cations Eu3+, Gd3+, and Tb(III) with 1,4,7,10-Tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane (dota)—Characterization of Three Successive Complexing Phases: Study of the Thermodynamic and Structural Properties of the Complexes by Potentiometry, Luminescence Spectroscopy and EXAFS. Chem. Eur. J. 2004, 10, 5218–5232. [Google Scholar] [PubMed]
- Bell, F.G.; Ley, L. Photoemission study of SiOx (0 ≤ x ≤ 2) alloys. Phys. Rev. B 1988, 37, 8383–8393. [Google Scholar] [CrossRef]
- Aureau, D.; Varin, Y.; Roodenko, K.; Seitz, O.; Pluchery, O.; Chabal, Y.J. Controlled Deposition of Gold Nanoparticles on Well-Defined Organic Monolayer Grafted on Silicon Surfaces. J. Phys. Chem. C 2010, 114, 14180–14186. [Google Scholar] [CrossRef]
- Armelao, L.; Belli Dell’Amico, D.; Bellucci, L.; Bottaro, G.; Labella, L.; Marchetti, F.; Samaritani, S. Smart Grafting of Lanthanides onto Silica via N,N-Dialkylcarbamato Complexes. Inorg. Chem. 2016, 55, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, Y.; Kirillov, A.M.; Liu, L.; Yu, M.; Liu, W.; Tang, Y. A ratiometric fluorescent nanoprobe based on terbium functionalized carbon dots for highly sensitive detection of an anthrax biomarker. Chem. Commun. 2015, 51, 5036–5039. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.; Aime, S.; Botta, M.; Howard, J.A.K.; Moloney, J.M.; Navet, M.; Parker, D.; Port, M.; Rousseaux, O. Correlation of Water Exchange Rate with Isomeric Composition in Diastereoisomeric Gadolinium Complexes of Tetra(carboxyethyl)dota and Related Macrocyclic Ligands. J. Am. Chem. Soc. 2000, 122, 9781–9792. [Google Scholar] [CrossRef]
- Vlad, A.; Zaltariov, M.-F.; Shova, S.; Cazacu, M.; Avadanei, M.; Soroceanu, A.; Samoila, P. New Zn(II) and Cu(II) complexes with in situ generated N2O2 siloxane Schiff base ligands. Polyhedron 2016, 115, 76–85. [Google Scholar] [CrossRef]
- Tiseanu, C.; Kumke, M.U.; Parvulescu, V.I.; Gessner, A.; Gagea, B.C.; Martens, J.A. Photoluminescence Response of Terbium-Exchanged MFI-Type Materials to Si/Al Ratio, Texture, and Hydration State. J. Phys. Chem. B 2006, 110, 25707–25715. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Tatsumi, Y.; Akimoto, I.; Osaki, S.; Doi, M.; Kimura, K. Fluorescent silica nanoparticles modified chemically with terbium complexes as potential bioimaging probes: their fluorescence and colloidal properties in water. New J. Chem. 2015, 39, 1452–1458. [Google Scholar] [CrossRef]
- Sailor, M.J.; Wu, E.C. Photoluminescence-Based Sensing with Porous Silicon Films, Microparticles, and Nanoparticles. Adv. Funct. Mater. 2009, 19, 3195–3208. [Google Scholar] [CrossRef]
- Richardson, F.S. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 1982, 82, 541–552. [Google Scholar] [CrossRef]
- Kim, K.J.; Jang, J.S.; Moon, D.W. Accurate determination of the surface normal for the reliable measurement of ultra-thin SiO2 thickness by X-ray Photoelectron Spectroscopy. Metrologia 2006, 43, L28–L32. [Google Scholar] [CrossRef]
- Seah, M.P.; White, R. Ultrathin SiO2 on Si: III mapping the layer thickness efficiently by XPS. Surf. Interface Anal. 2002, 33, 960–963. [Google Scholar] [CrossRef]
- Cole, D.A.; Shallenberger, J.R.; Novak, S.W.; Moore, R.L.; Edgell, M.J.; Smith, S.P.; Hitzman, C.J.; Kirchhoff, J.F.; Principe, E.; Nieveen, W.; et al. SiO2 thickness determination by X-ray Photoelectron Spectroscopy, Auger electron spectroscopy, secondary ion mass spectrometry, Rutherford backscattering, transmission electron microscopy, and ellipsometry. J. Vac. Sci. Technol. B Microelectron Nanom. Struct. 2000, 18. [Google Scholar] [CrossRef]
Sample Availability: Complexes (TbL) used in the study are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahuleyan, B.K.; Toussaint, K.; Rinnert, H.; Vallon, R.; Molinari, M.; Chuburu, F.; Cadiou, C. Silicon Wafer Functionalization with a Luminescent Tb(III) Coordination Complex: Synthesis, Characterization, and Application to the Optical Detection of NO in the Gas Phase. Molecules 2019, 24, 1914. https://doi.org/10.3390/molecules24101914
Bahuleyan BK, Toussaint K, Rinnert H, Vallon R, Molinari M, Chuburu F, Cadiou C. Silicon Wafer Functionalization with a Luminescent Tb(III) Coordination Complex: Synthesis, Characterization, and Application to the Optical Detection of NO in the Gas Phase. Molecules. 2019; 24(10):1914. https://doi.org/10.3390/molecules24101914
Chicago/Turabian StyleBahuleyan, Bijal K., Kathleen Toussaint, Hervé Rinnert, Raphaël Vallon, Michaël Molinari, Françoise Chuburu, and Cyril Cadiou. 2019. "Silicon Wafer Functionalization with a Luminescent Tb(III) Coordination Complex: Synthesis, Characterization, and Application to the Optical Detection of NO in the Gas Phase" Molecules 24, no. 10: 1914. https://doi.org/10.3390/molecules24101914
APA StyleBahuleyan, B. K., Toussaint, K., Rinnert, H., Vallon, R., Molinari, M., Chuburu, F., & Cadiou, C. (2019). Silicon Wafer Functionalization with a Luminescent Tb(III) Coordination Complex: Synthesis, Characterization, and Application to the Optical Detection of NO in the Gas Phase. Molecules, 24(10), 1914. https://doi.org/10.3390/molecules24101914