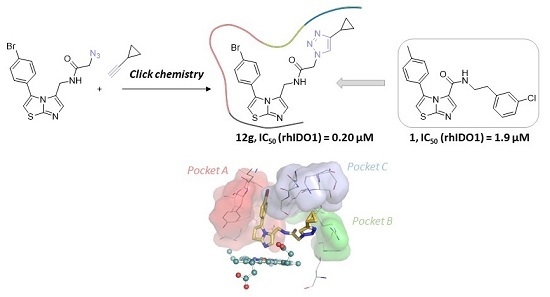
Synthesis, Docking and Biological Evaluation of a Novel Class of Imidazothiazoles as IDO1 Inhibitors
Abstract
1. Introduction
2. Results
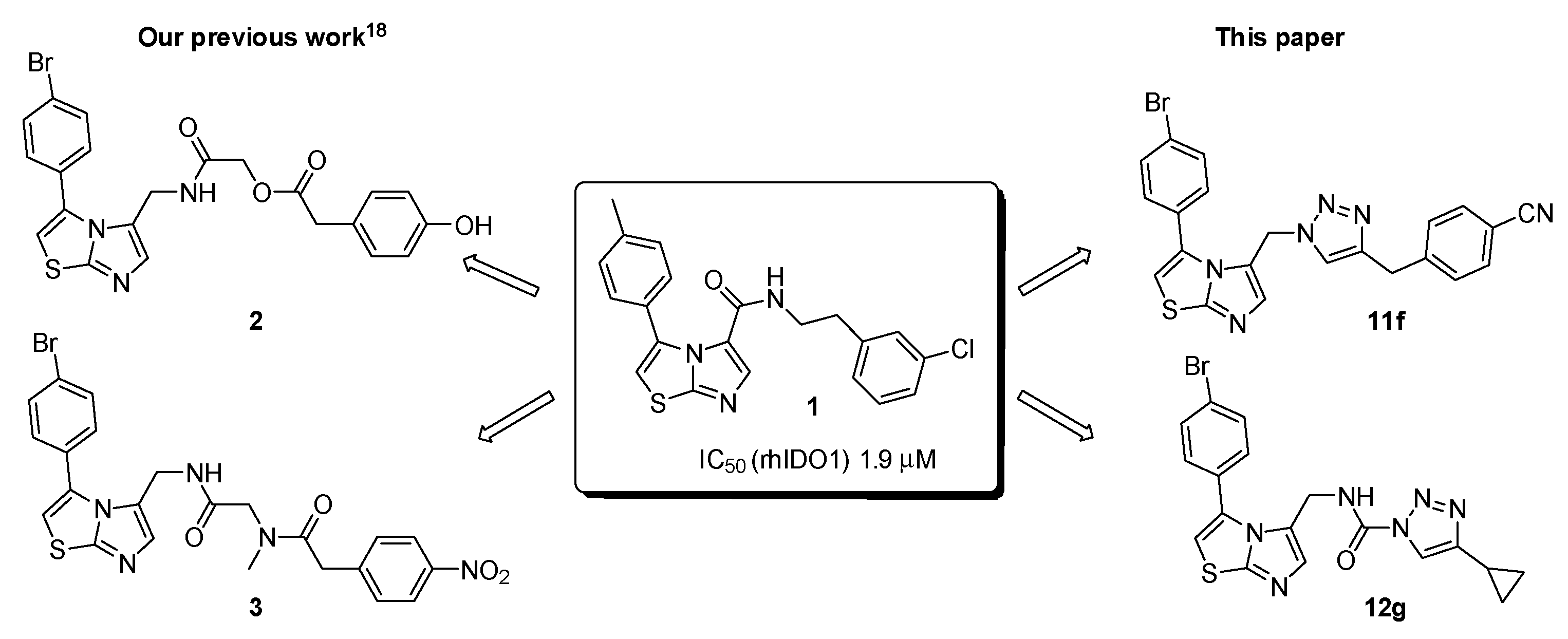
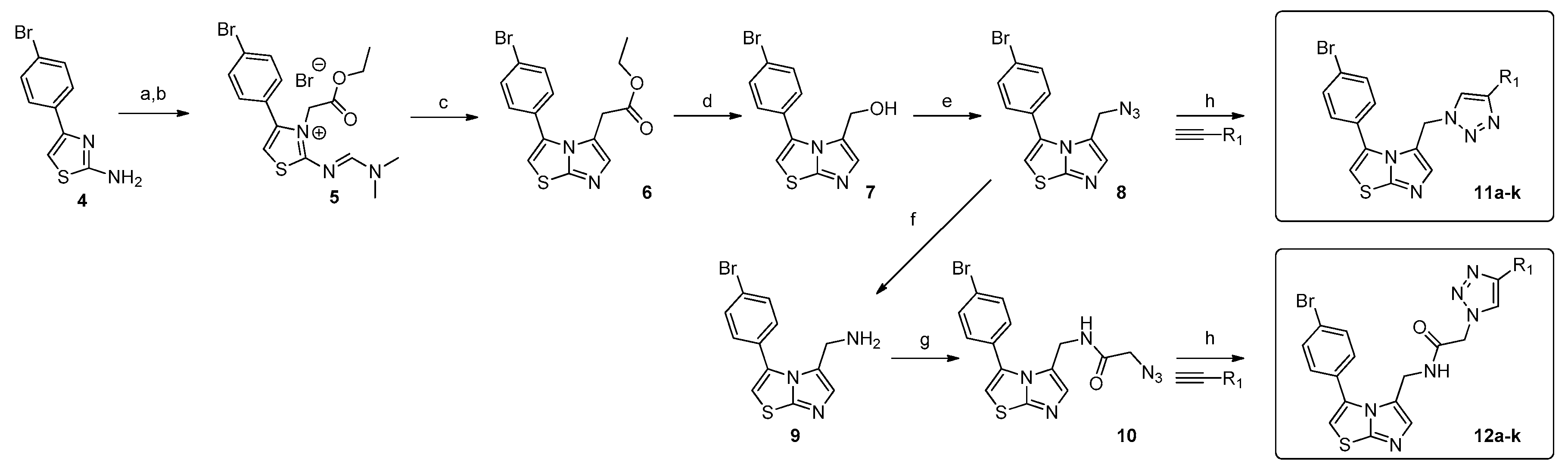
2.1. Chemistry
2.2. Biological Evaluation
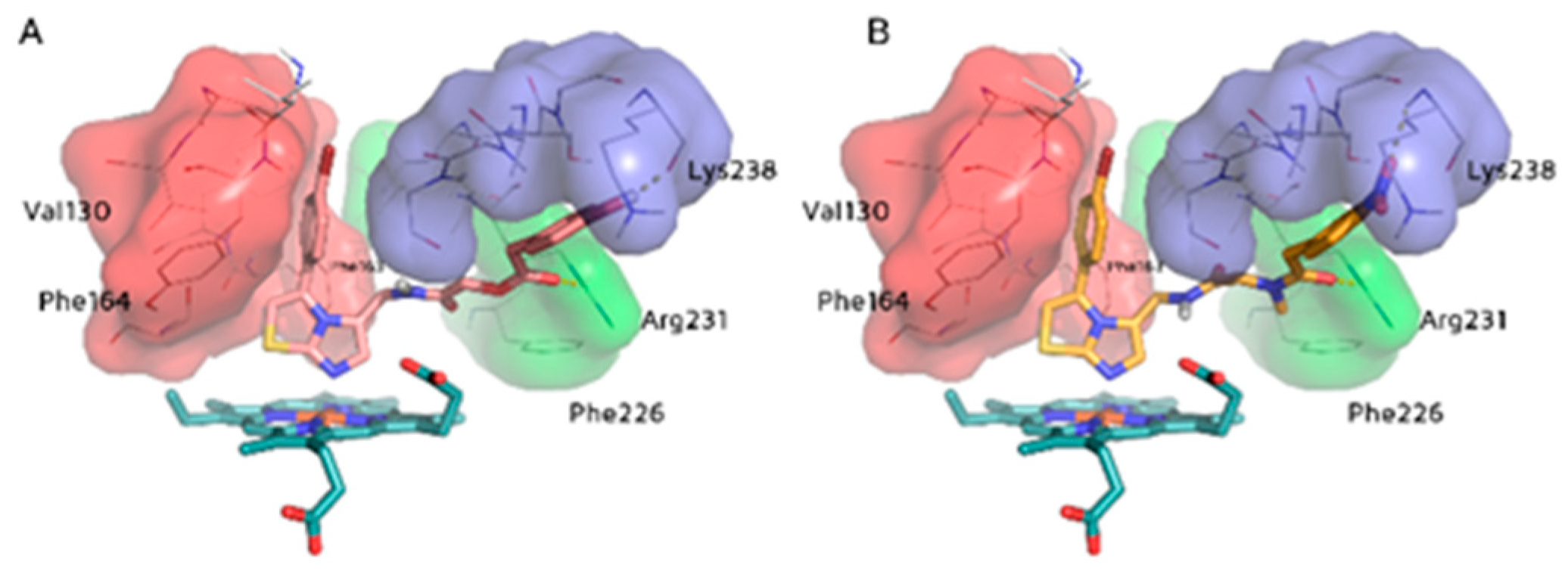
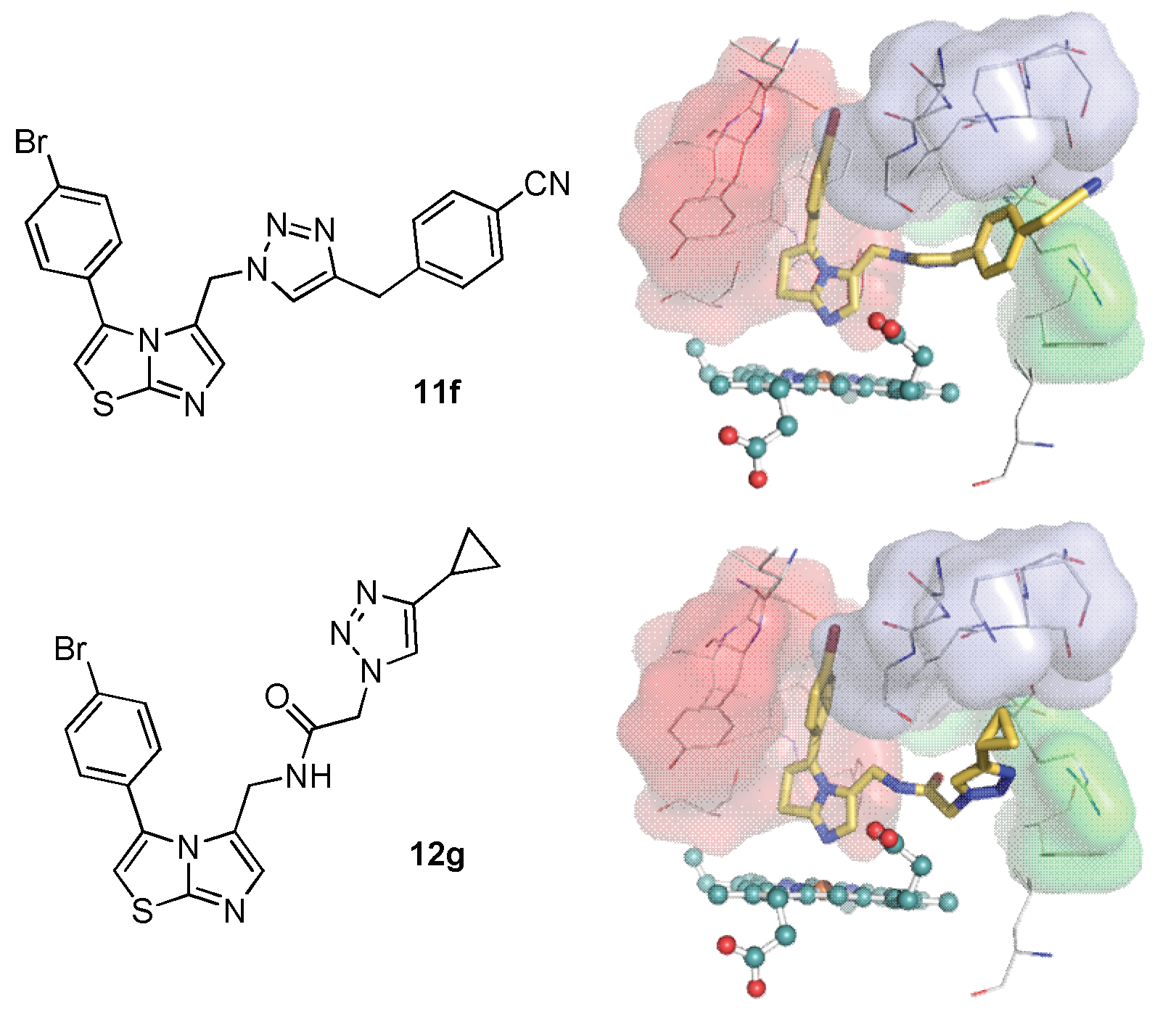
2.3. Molecular Docking Study
3. Materials and Methods
3.1. Chemistry
3.1.1. General Chemistry
3.1.2. Synthesis of 2-azido-N-((3-(4-bromophenyl)imidazo[2,1-b]thiazol-5-yl)methyl)acetamide, (10)
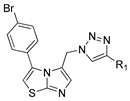
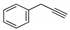
3.1.3. General Procedure for the Synthesis of Compounds 11a–11k and 12a–12k
3.1.4. Characterization of Compounds 11a–11k and 12a–12k
3.2. Biology: rhIDO1 Enzymatic Assay
3.3. Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Platten, M.; Wick, W.; Van den Eynde, B.J. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012, 72, 5435–5440. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-dioxygenase Pathways of Pathogenic Inflammation and Immune Escape in Cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Uberall, F.; Fuchs, D. The Potential of Targeting Indoleamine 2,3-dioxygenase for Cancer Treatment. Expert Opin. Ther. Targets 2015, 19, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Mondal, A.; Dey, S.; Laury-Kleintop, L.D.; Muller, A.J. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive ‘Cold’ Tumors ‘Hot’. Trends Cancer 2018, 4, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Smith, C.; DuHadaway, J.B.; Sutanto-Ward, E.; Prendergast, G.C.; Bravo-Nuevo, A.; Muller, A.J. IDO1 is an Integral Mediator of Inflammatory Neovascularization. EBioMedicine 2016, 14, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: Results of the phase 3 ECHO-301/KEYNOTE-252 study. J. Clin. Oncol. 2018, 36, 108. [Google Scholar] [CrossRef]
- Garber, K. A New Cancer Immunotherapy Suffers a Setback. Science 2018, 360, 588. [Google Scholar] [CrossRef]
- Tojo, S.; Kohno, T.; Tanaka, T.; Kamioka, S.; Ota, Y.; Ishii, T.; Kamimoto, K.; Asano, S.; Isobe, Y. Crystal Structures and Structure-Activity Relationships of Imidazothiazole Derivatives as IDO1 Inhibitors. ACS Med. Chem. Lett. 2014, 5, 1119–1123. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Zarganes-Tzizikas, T.; Dömling, A. Modern Multicomponent Reactions for Better Drug Syntheses. Org. Chem. Front. 2014, 1, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click Chemistry Reactions in Medicinal Chemistry: Applications Of the 1,3-Dipolar Cycloaddition Between Azides and Alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Riva, B.; Griglio, A.; Serafini, M.; Cordero-Sanchez, C.; Aprile, S.; Di Paola, R.; Gugliandolo, E.; Alansary, D.; Biocotino, I.; Lim, D.; et al. Pyrtriazoles, a Novel Class of Store-Operated Calcium Entry Modulators: Discovery, Biological Profiling, and in Vivo Proof-of-Concept Efficacy in Acute Pancreatitis. J. Med. Chem. 2018, 61, 9756–9783. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Griglio, A.; Aprile, S.; Seiti, F.; Travelli, C.; Pattarino, F.; Grosa, G.; Sorba, G.; Genazzani, A.A.; Gonzalez-Rodriguez, S.; et al. Targeting Transient Receptor Potential Vanilloid 1 (TRPV1) Channel Softly: The Discovery of Passerini Adducts as a Topical Treatment for Inflammatory Skin Disorders. J. Med. Chem. 2018, 61, 4436–4455. [Google Scholar] [CrossRef] [PubMed]
- Pirali, T.; Ciraolo, E.; Aprile, S.; Massarotti, A.; Berndt, A.; Griglio, A.; Serafini, M.; Mercalli, V.; Landoni, C.; Campa, C.C.; et al. Identification of a Potent Phosphoinositide 3-Kinase Pan Inhibitor Displaying a Strategic Carboxylic Acid Group and Development of Its Prodrugs. ChemMedChem 2017, 12, 1542–1554. [Google Scholar] [CrossRef]
- Fallarini, S.; Massarotti, A.; Gesù, A.; Giovarruscio, S.; Coda Zabetta, G.; Bergo, R.; Giannelli, B.; Brunco, A.; Lombardi, G.; Sorba, G.; et al. In Silico-Driven Multicomponent Synthesis Of 4,5- and 1,5-Disubstituted Imidazoles as Indoleamine 2,3-Dioxygenase Inhibitors. Med. Chem. Commun. 2016, 7, 409–419. [Google Scholar] [CrossRef]
- Griglio, A.; Torre, E.; Serafini, M.; Bianchi, A.; Schmid, R.; Coda Zabetta, G.; Massarotti, A.; Sorba, G.; Pirali, T.; Fallarini, S. A Multicomponent Approach in the Discovery of Indoleamine 2,3-Dioxygenase 1 Inhibitors: Synthesis, Biological Investigation and Docking Studies. Bioorg. Med. Chem. Lett. 2018, 28, 651–657. [Google Scholar] [CrossRef]
- Röhrig, U.F.; Majjigapu, S.R.; Grosdidier, A.; Bron, S.; Stroobant, V.; Pilotte, L.; Colau, D.; Vogel, P.; Van den Eynde, B.J.; Zoete, V.; et al. Rational Design of 4-Aryl-1,2,3-Triazoles for Indoleamine 2,3-Dioxygenase 1 Inhibition. J. Med. Chem. 2012, 55, 5270–5290. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, U.F.; Majiigapu, S.R.; Caldelari, D.; Dilek, N.; Reichenbach, P.; Ascencao, K.; Irving, M.; Coukos, G.; Vogel, P.; Zoete, V.; et al. 1,2,3-Triazoles as Inhibitors Of Indoleamine 2,3-Dioxygenase 2 (IDO2). Bioorg. Med. Chem. Lett. 2016, 26, 4330–4333. [Google Scholar] [CrossRef]
- Alexandre, J.A.; Swan, M.K.; Latchem, M.J.; Boyall, D.; Pollard, J.R.; Hughes, S.W.; Westcott, J. New 4-Amino-1,2,3-Triazole Inhibitors of Indoleamine 2,3-Dioxygenase Form a Long-Lived Complex with the Enzyme and Display Exquisite Cellular Potency. ChemBioChem 2018, 19, 552–561. [Google Scholar] [CrossRef]
- OMEGA, version 2.4.6, OpenEye Scientific Software, Santa Fe, N.M. Available online: http://www.eyesopen.com (accessed on 3 March 2018).
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.C.D.; Nicholls, A. Conformer Generation with OMEGA: Learning from the Data Set and the Analysis of Failures. J. Chem. Inf. Model. 2012, 52, 2919–2936. [Google Scholar] [CrossRef] [PubMed]
- FRED, version 3.0.0, OpenEye Scientific Software, Santa Fe, N.M. Available online: http://www.eyesopen.com (accessed on 3 March 2018).
- McGann, M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.W.; Leaver-Fay, A.; Chen, V.B.; Block, J.N.; Kapral, G.J.; Wang, X.; Murray, L.W.; Arendall, W.B.; Snoeyink, J.; Richardson, J.S.; et al. MolProbity: All-Atom Contacts and Structure Validation for Proteins and Nucleic Acids. Nucleic Acids Res. 2007, 35, 375–383. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System, version 1.3, Schrödinger LLC. 2010. Available online: https://pymol.org/2/ (accessed on 3 March 2018).
Sample Availability: Samples of the compounds 11f and 12g are available from the authors. |
 |  | ||||
|---|---|---|---|---|---|
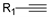 | Cpd, Yield (%) | Enzymatic Assay Inhibition (%) at 1 µM a | Cpd, Yield (%) | Enzymatic Assay Inhibition (%) at 1 µM a | |
 | a | 11a, 77% | 30 ± 6.7 | 12a, 81% | 63 ± 4.3 |
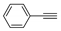 | b | 11b, 51% | 35 ± 2.7 | 12b, 77% | 61 ± 5.5 |
 | c | 11c, 87% | 57 ± 13 | 12c, 46% | 5 ± 1.2 |
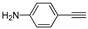 | d | 11d, 43% | 17 ± 1.2 | 12d, 83% | 56 ± 4.7 |
 | e | 11e, 83% | 28 ± 1.5 | 12e, 76% | 50 ± 4.5 |
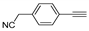 | f | 11f, 76% | 74 ± 5.8 | 12f, 68% | 55 ± 7.2 |
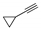 | g | 11g, 44% | 61 ± 7.1 | 12g, 46% | 70 ± 9.2 |
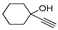 | h | 11h, 68% | 35 ± 2.4 | 12h, 26% | 63 ± 4.1 |
 | i | 11i, 45% | 0 | 12i, 82% | 45 ± 2.8 |
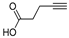 | j | 11j, 41% | 81 ± 7.9 | 12j, 42% | 33 ± 4.5 |
 | k | 11k, 48% | 62 ± 5.6 | 12k, 55% | 50 ± 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafini, M.; Torre, E.; Aprile, S.; Massarotti, A.; Fallarini, S.; Pirali, T. Synthesis, Docking and Biological Evaluation of a Novel Class of Imidazothiazoles as IDO1 Inhibitors. Molecules 2019, 24, 1874. https://doi.org/10.3390/molecules24101874
Serafini M, Torre E, Aprile S, Massarotti A, Fallarini S, Pirali T. Synthesis, Docking and Biological Evaluation of a Novel Class of Imidazothiazoles as IDO1 Inhibitors. Molecules. 2019; 24(10):1874. https://doi.org/10.3390/molecules24101874
Chicago/Turabian StyleSerafini, Marta, Enza Torre, Silvio Aprile, Alberto Massarotti, Silvia Fallarini, and Tracey Pirali. 2019. "Synthesis, Docking and Biological Evaluation of a Novel Class of Imidazothiazoles as IDO1 Inhibitors" Molecules 24, no. 10: 1874. https://doi.org/10.3390/molecules24101874
APA StyleSerafini, M., Torre, E., Aprile, S., Massarotti, A., Fallarini, S., & Pirali, T. (2019). Synthesis, Docking and Biological Evaluation of a Novel Class of Imidazothiazoles as IDO1 Inhibitors. Molecules, 24(10), 1874. https://doi.org/10.3390/molecules24101874