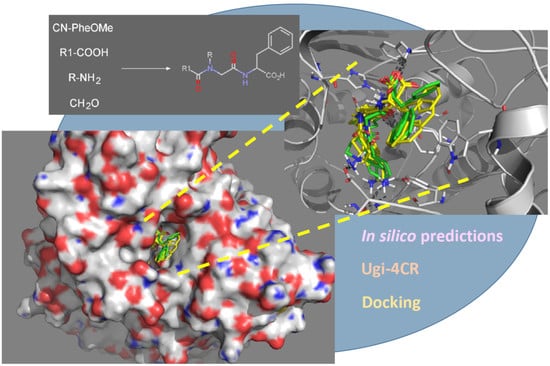
Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors
Abstract
1. Introduction
2. Results
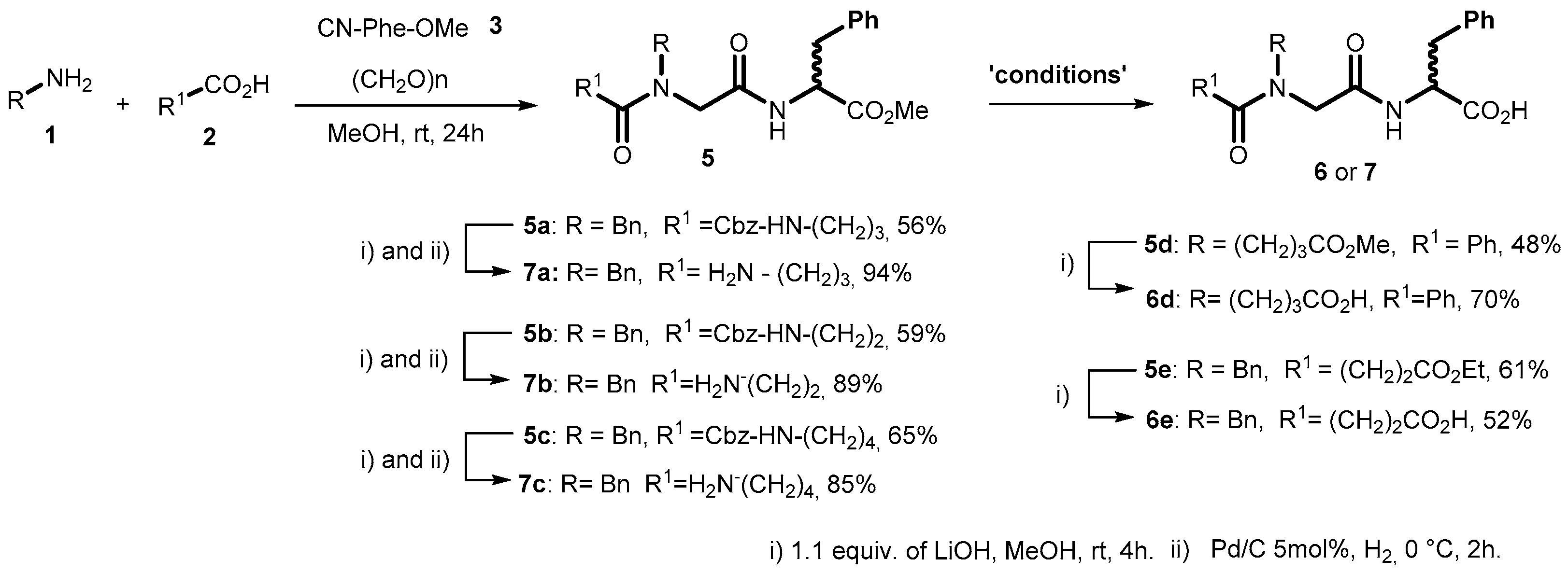
2.1. Synthesis of diN-Substituted Glycyl-Phenylalanine (diNsGF) Derivatives
2.2. Predictions of the AchE Inhibitory Activities by Using Two-Dimensional (2D) Autocorrelation Models
2.3. Results of the AChE and BuChE Inhibitory Activities
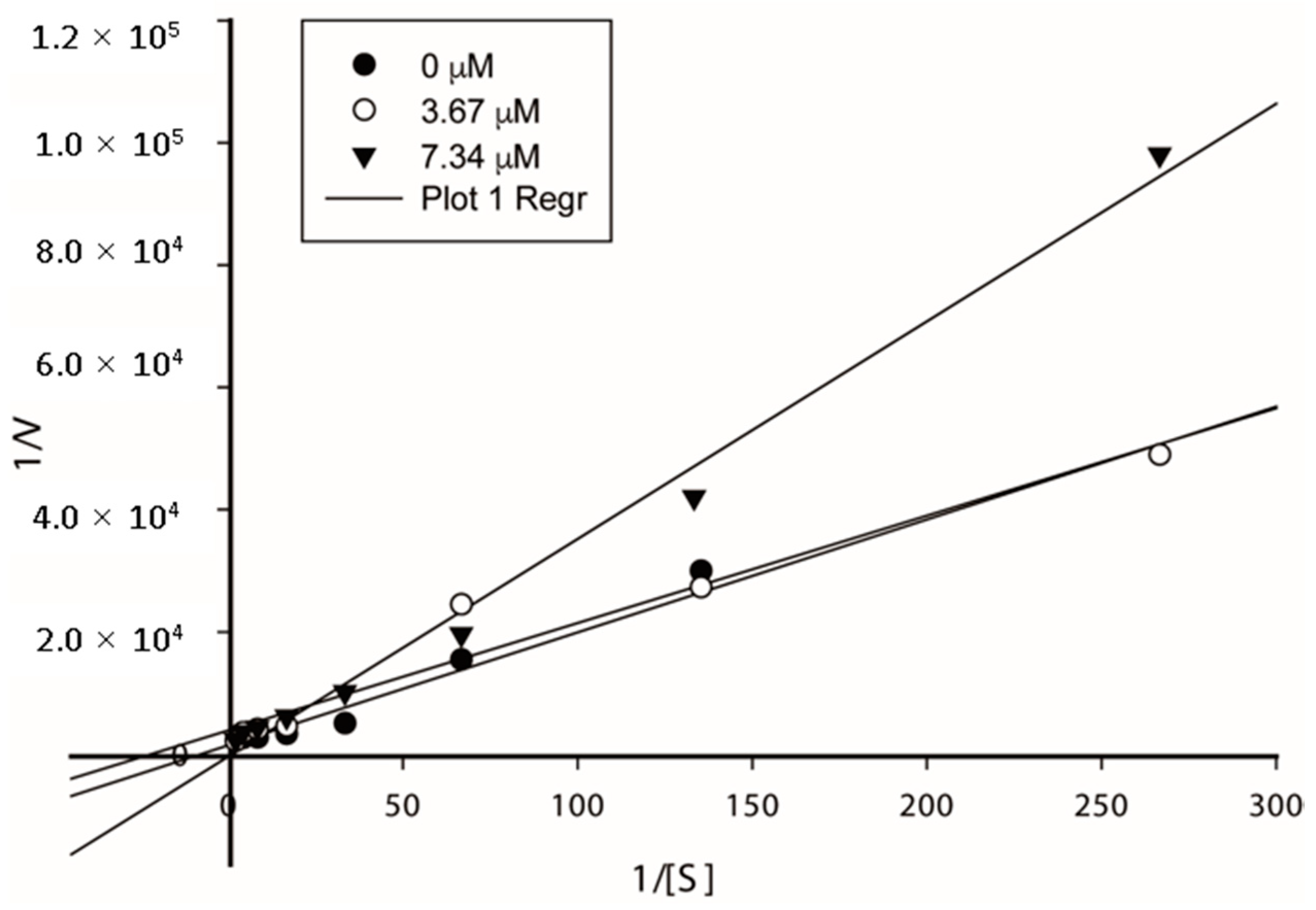
2.4. Results of the Kinetic Assays
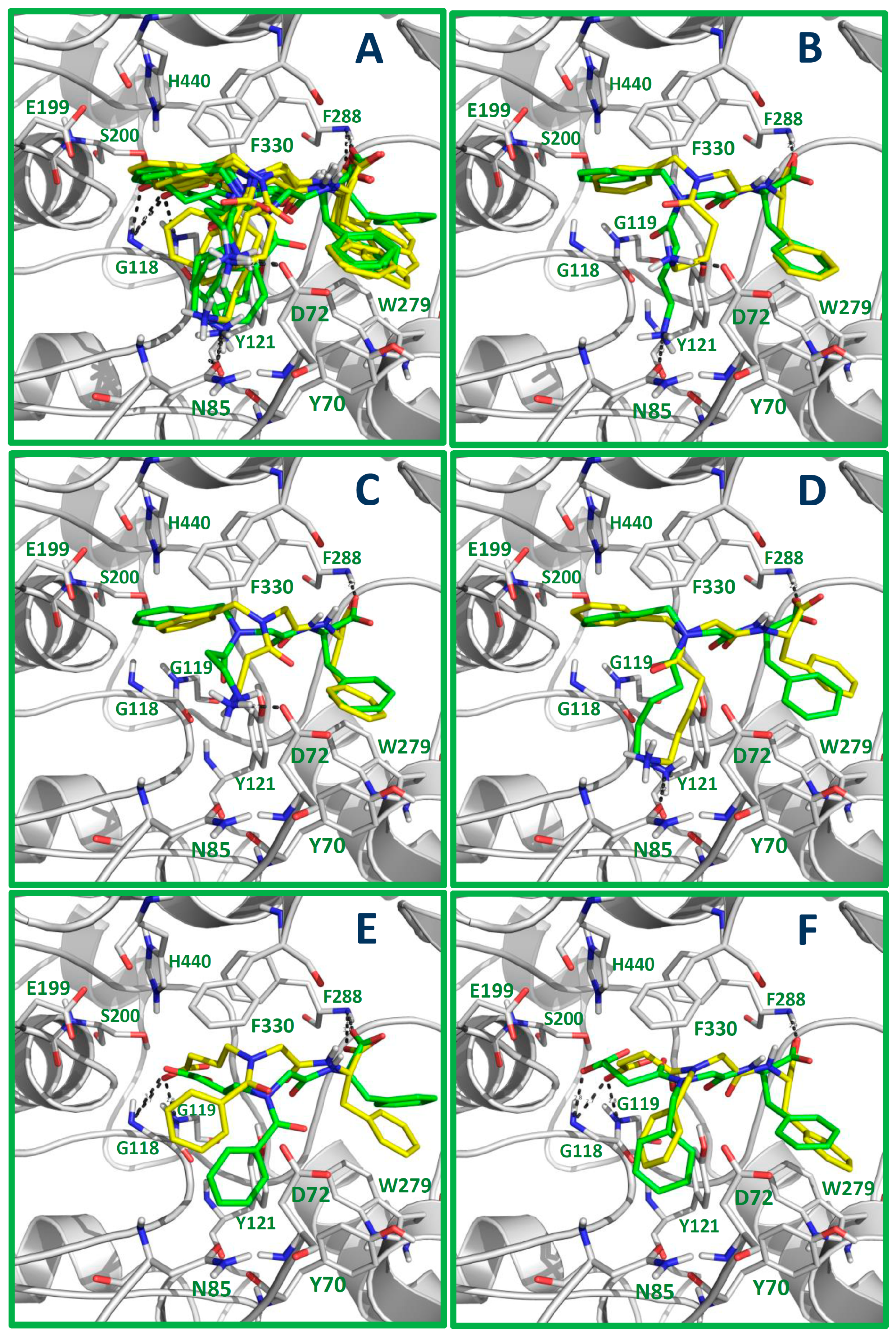
2.5. Docking Results
3. Materials and Methods
3.1. Preparation of Compounds
3.2. Computational Predictions of Bioactivities
3.3. AChE and BuChE Assays
3.4. Kinetic Assays
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Slobbe, P.; Ruijter, E.; Orru, R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. Med. Chem. Commun. 2012, 3, 1189–1218. [Google Scholar] [CrossRef]
- Ahmadi, T.; Mohammadi Ziarani, G.; Gholamzadeh, P.; Mollabagher, H. Recent advances in asymmetric multicomponent reactions (AMCRs). Tetrahedron Asymmetry 2017, 28, 708–724. [Google Scholar] [CrossRef]
- Ghabraie, E.; Balalaie, S.; Mehrparvar, S.; Rominger, F. Synthesis of functionalized β-lactams and pyrrolidine-2,5-diones through a metal-free sequential Ugi-4CR/cyclization reaction. J. Org. Chem. 2014, 79, 7926–7934. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.C.N.; Neves Filho, R.A.W.; Westermann, B.; Heinke, R.; Wessjohann, L.A. Synthesis of antibacterial 1,3-diyne-linked peptoids from an Ugi-4CR/Glaser coupling approach. Beilstein J. Org. Chem. 2015, 11, 25–30. [Google Scholar] [CrossRef]
- Borah, P.; Borah, J.M.; Chowdhury, P. Microwave (MW) irradiated Ugi four-component reaction (Ugi-4CR): Expedited synthesis of steroid-amino acid conjugatesA novel class of hybrid compounds. Steroids 2015, 98, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Polindara-García, L.A.; Montesinos-Miguel, D.; Vazquez, A. An efficient microwave-assisted synthesis of cotinine and iso-cotinine analogs from an Ugi-4CR approach. Org. Biomol. Chem. 2015, 13, 9065–9071. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, M.; Zarezadeh, N.; Abbasi, A. One-pot tandem Ugi-4CR/S(N)Ar approach to highly functionalized quino [2,3-b][1,5]benzoxazepines. Mol. Divers. 2016, 20, 483–495. [Google Scholar] [CrossRef]
- Van der Poorten, O.; Van Den Hauwe, R.; Hollanders, K.; Maes, B.U.W.; Tourwé, D.; Jida, M.; Ballet, S. Rapid construction of substituted 3-amino-1,5-benzothiazepin-4(5H)-one dipeptide scaffolds through an Ugi-4CR-Ullmann cross-coupling sequence. Org. Biomol. Chem. 2018, 16, 1242–1246. [Google Scholar] [CrossRef]
- Bauer, S.M.; Armstrong, R.W. Total Synthesis of Motuporin (Nodularin-V). J. Am. Chem. Soc. 1999, 121, 6355–6366. [Google Scholar] [CrossRef]
- Bayer, T.; Riemer, C.; Kessler, H. A new strategy for the synthesis of cyclopeptides containing diaminoglutaric acid. J. Pept. Sci. 2001, 7, 250–261. [Google Scholar] [CrossRef]
- Bonne, D.; Dekhane, M.; Zhu, J. Modulating the reactivity of α-Isocyanoacetates: Multicomponent synthesis of 5-Methoxyoxazoles and Furopyrrolones. Angew. Chem. Int. Ed. 2007, 46, 2485–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhdanko, A.G.; Nenajdenko, V.G. Nonracemizable isocyanoacetates for multicomponent reactions. J. Org. Chem. 2009, 74, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Zhdanko, A.G.; Gulevich, A.V.; Nenajdenko, V.G. One-step synthesis of N-acetylcysteine and glutathione derivatives using the Ugi reaction. Tetrahedron 2009, 65, 4692–4702. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Yan, B. Use of cyclohexylisocyanide and methyl 2-isocyanoacetate as convertible isocyanides for microwave-assisted fluorous synthesis of 1,4-benzodiazepine-2,5-dione library. J. Comb. Chem. 2010, 12, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S. The building block approach to unusual α-amino acid derivatives and peptides. Acc. Chem. Res. 2003, 36, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, S.; Torroba, T. The use of the Ugi four-component condensation. Nat. Protoc. 2007, 2, 632–639. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, A.F.; Rivera, D.G.; Ferreira, M.A.B.; Corrêa, A.G.; Paixão, M.W. Multicomponent combinatorial development and conformational analysis of prolyl peptide–peptoid hybrid catalysts: Application in the direct asymmetric michael addition. J. Org. Chem. 2013, 78, 10221–10232. [Google Scholar] [CrossRef]
- Velázquez-Libera, J.L.; Caballero, J.; Toropova, A.P.; Toropov, A.A. Estimation of 2D autocorrelation descriptors and 2D Monte Carlo descriptors as a tool to build up predictive models for acetylcholinesterase (AChE) inhibitory activity. Chemom. Intell. Lab. Syst. 2019, 184, 14–21. [Google Scholar] [CrossRef]
- Eriksson, L.; Jaworska, J.; Worth, A.P.; Cronin, M.T.D.; McDowell, R.M.; Gramatica, P. Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ. Health Perspect. 2003, 111, 1361–1375. [Google Scholar] [CrossRef]
- Sakuratani, Y.; Kasai, K.; Noguchi, Y.; Yamada, J. Comparison of Predictivities of Log P Calculation Models Based on Experimental Data for 134 Simple Organic Compounds. QSAR Comb. Sci. 2007, 26, 109–116. [Google Scholar] [CrossRef]
- Souza, E.S.; Zaramello, L.; Kuhnen, C.A.; Junkes, B.D.S.; Yunes, R.A.; Heinzen, V.E.F. Estimating the octanol/water partition coefficient for aliphatic organic compounds using semi-empirical electrotopological index. Int. J. Mol. Sci. 2011, 12, 7250–7264. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.J.; Xia, K.; Zhang, W.; Xu, X.J. ADME evaluation in drug discovery. 4. Prediction of aqueous solubility based on atom contribution approach. J. Chem. Inf. Comput. Sci. 2004, 44, 266–275. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Zhang, W.; Xu, X. ADME evaluation in drug discovery. 7. Prediction of oral absorption by correlation and classification. J. Chem. Inf. Model. 2007, 47, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lian, G.; Han, L. Prediction of human skin permeability using artificial neural network (ANN) modeling. Acta Pharmacol. Sin. 2007, 28, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 2001, 6, 357–366. [Google Scholar] [CrossRef]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- De la Torre, P.; Astudillo-Saavedra, L.; Caballero, J.; Quiroga, J.; Alzate-Morales, J.; Cabrera, M.; Trilleras, J. A novel class of selective acetylcholinesterase inhibitors: Synthesis and evaluation of (E)-2-(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles. Molecules 2012, 17, 12072–12085. [Google Scholar] [CrossRef]
- Ambure, P.; Kar, S.; Roy, K. Pharmacophore mapping-based virtual screening followed by molecular docking studies in search of potential acetylcholinesterase inhibitors as anti-Alzheimer’s agents. BioSystems 2014, 116, 10–20. [Google Scholar] [CrossRef]
- Guo, F.; Chang, D.; Lai, G.; Zhu, T.; Xiong, S.; Wang, S.; Wang, Z. Enantioselective and regioselective friedel–crafts alkylation of pyrroles with nitroalkenes catalyzed by a tridentate schiff base–copper complex. Chem. A Euro. J. 2011, 17, 11127–11130. [Google Scholar] [CrossRef]
- Monge, D.; Jensen, K.L.; Marín, I.; Jørgensen, K.A. Synthesis of 1,2,4-triazolines: Base-catalyzed hydrazination/cyclization cascade of α-isocyano esters and amides. Org. Lett. 2011, 13, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Amata, E.; Bland, N.D.; Campbell, R.K.; Pollastri, M.P. Evaluation of pyrrolidine and pyrazolone derivatives as inhibitors of trypanosomal phosphodiesterase B1 (TbrPDEB1). Tetrahedron Lett. 2015, 56, 2832–2835. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Tsuritani, T.; Yasukata, T. A Mild method for the synthesis of carbamate-protected guanidines using the burgess reagent. Org. Lett. 2014, 16, 1868–1871. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Caballero, J.; Helguera, A.; Garriga, M.; González, G.; Fernández, M. 2D Autocorrelation modelling of the inhibitory activity of cytokinin-derived cyclin-dependent kinase inhibitors. Bull. Math. Biol. 2006, 68, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Tundidor-Camba, A.; Caballero, J.M. 2D Autocorrelation modeling of the activity of trihalobenzocycloheptapyridine analogues as farnesyl protein transferase inhibitors. Mol. Simul. 2005, 31, 575–584. [Google Scholar] [CrossRef]
- Greenblatt, H.M.; Guillou, C.; Guénard, D.; Argaman, A.; Botti, S.; Badet, B.; Thal, C.; Silman, I.; Sussman, J.L. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: Implications for structure-based drug design. J. Am. Chem. Soc. 2004, 126, 15405–15411. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, P.; Millard, C.B.; Harel, M.; Dvir, H.; Enz, A.; Sussman, J.L.; Silman, I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry 2002, 41, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD001190. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD005593. [Google Scholar] [CrossRef]
- Jackson, S.; Ham, R.J.; Wilkinson, D. The safety and tolerability of donepezil in patients with Alzheimer’s disease. Br. J. Clin. Pharmacol. 2004, 58, 1–8. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom Force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 7a, 7b, 7c, 6d, and 6e are available from the authors. |
| Entry | IC50 ± SEM (µM) AChE | IC50 (µM) BuChE | Selectivity | QPlogPo/w 2 | QPlogS 2 | QPlogKp 2 | Human Oral Absorption 2 |
|---|---|---|---|---|---|---|---|
| 7a | 3.67 ± 0.8 | 243.84 ± 4.6 | 66.44 | −0.527 | −2.127 | −5.078 | 2 |
| 7b | 41.31 ± 2.3 | 461.49 ± 6.2 | 11.17 | 0.235 | −2.639 | −4.401 | 2 |
| 7c | 12.56 ± 1.6 | 636.72 ± 3.7 | 50.69 | −0.104 | −2.775 | −5.071 | 2 |
| 6d | 10.64 ± 1.5 | 301.77 ± 5.8 | 28.36 | 2.873 | −3.249 | −3.541 | 1 |
| 6e | 304.87 ± 5.7 | 135.85 ± 2.9 | 0.45 | 2.246 | −2.072 | −2.696 | 2 |
| Galantamine | 0.57 ± 0.1 | 7.96 ± 0.8 | 13.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prent-Peñaloza, L.; De la Torre, A.F.; Velázquez-Libera, J.L.; Gutiérrez, M.; Caballero, J. Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors. Molecules 2019, 24, 189. https://doi.org/10.3390/molecules24010189
Prent-Peñaloza L, De la Torre AF, Velázquez-Libera JL, Gutiérrez M, Caballero J. Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors. Molecules. 2019; 24(1):189. https://doi.org/10.3390/molecules24010189
Chicago/Turabian StylePrent-Peñaloza, Luis, Alexander F. De la Torre, José L. Velázquez-Libera, Margarita Gutiérrez, and Julio Caballero. 2019. "Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors" Molecules 24, no. 1: 189. https://doi.org/10.3390/molecules24010189
APA StylePrent-Peñaloza, L., De la Torre, A. F., Velázquez-Libera, J. L., Gutiérrez, M., & Caballero, J. (2019). Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors. Molecules, 24(1), 189. https://doi.org/10.3390/molecules24010189