Antioxidant Mechanisms of Echinatin and Licochalcone A
Abstract
1. Introduction
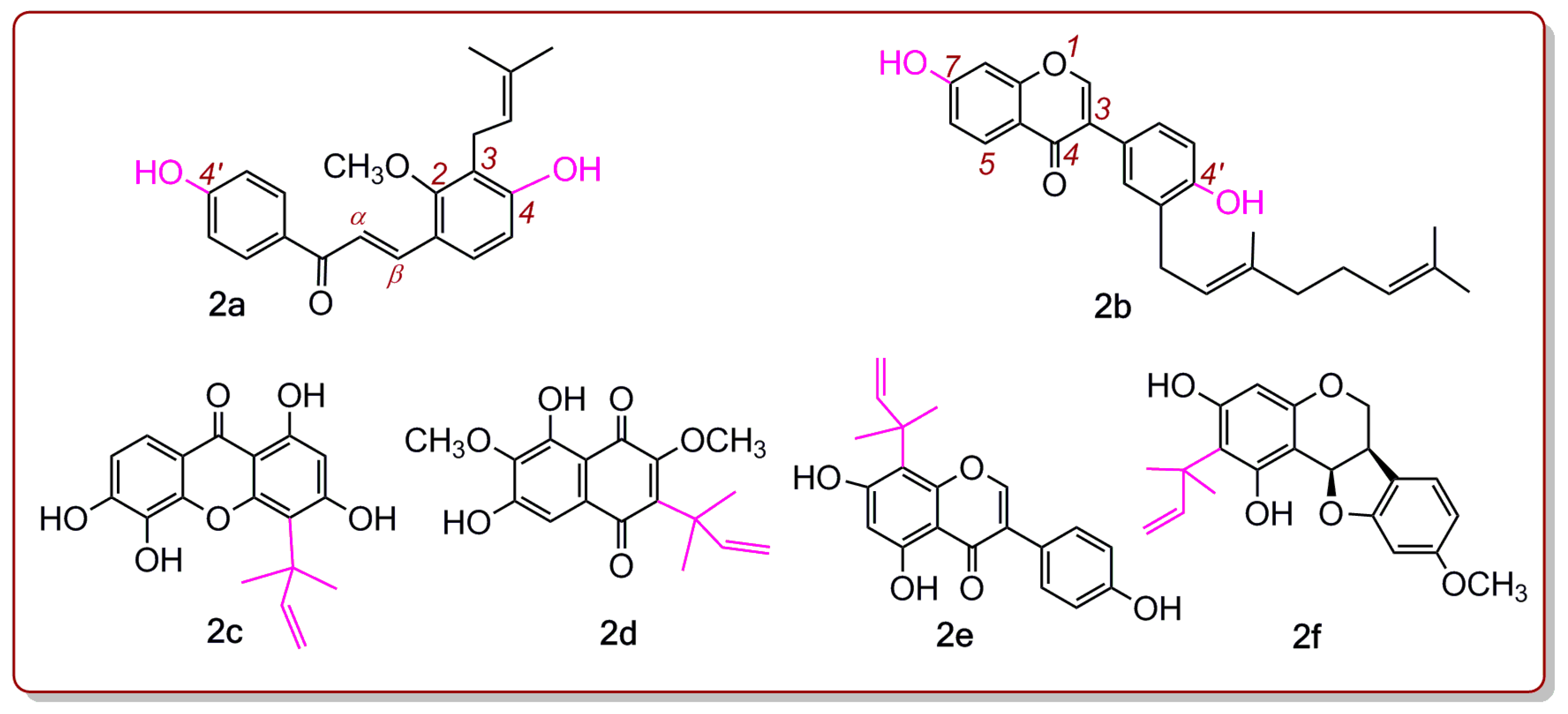
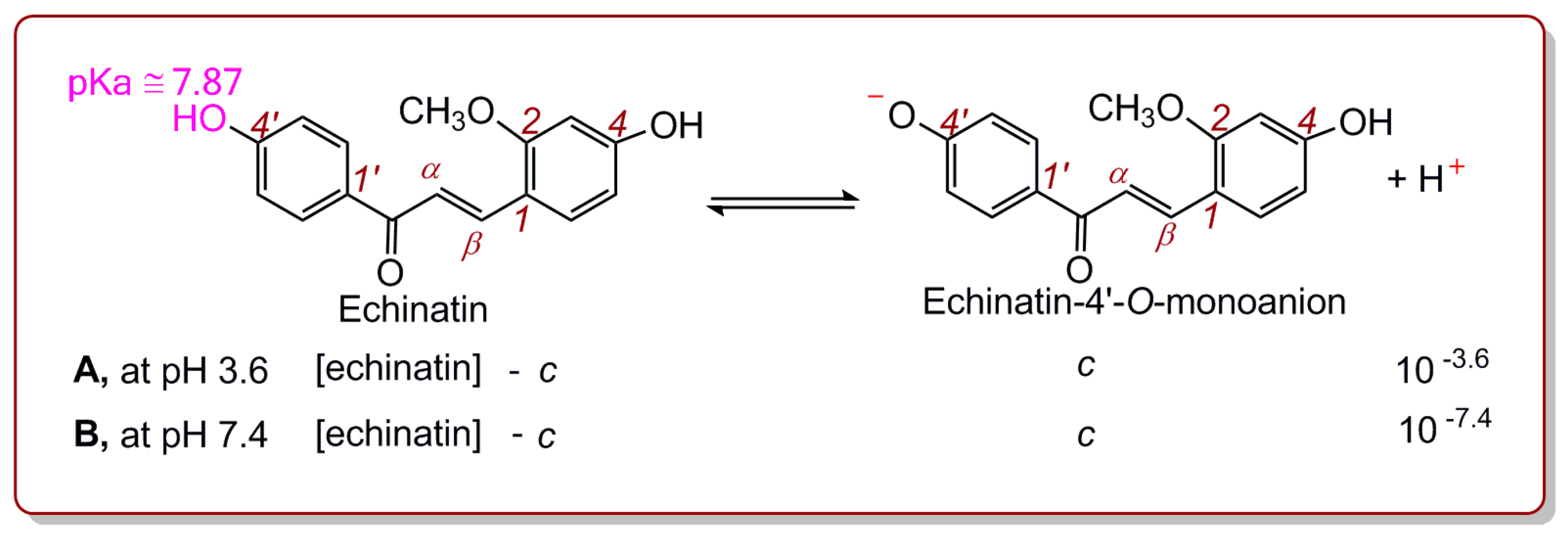
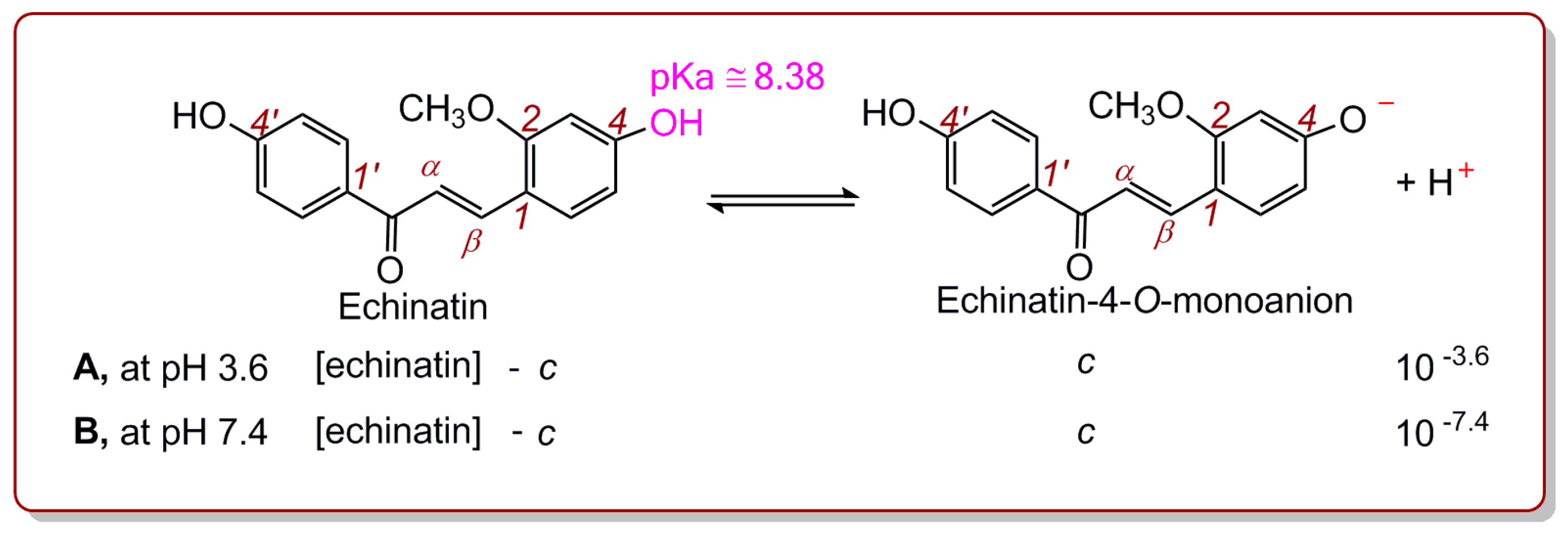
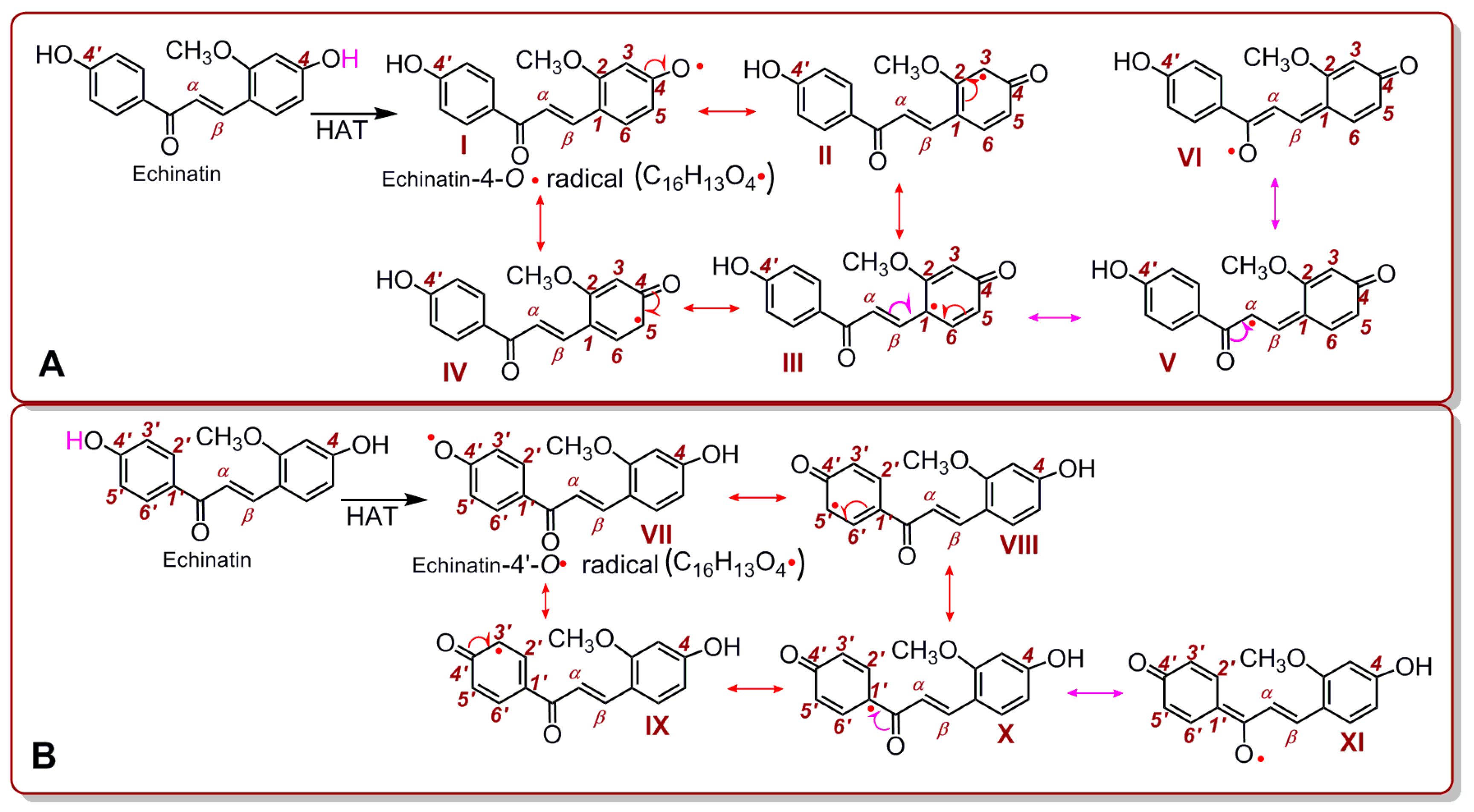
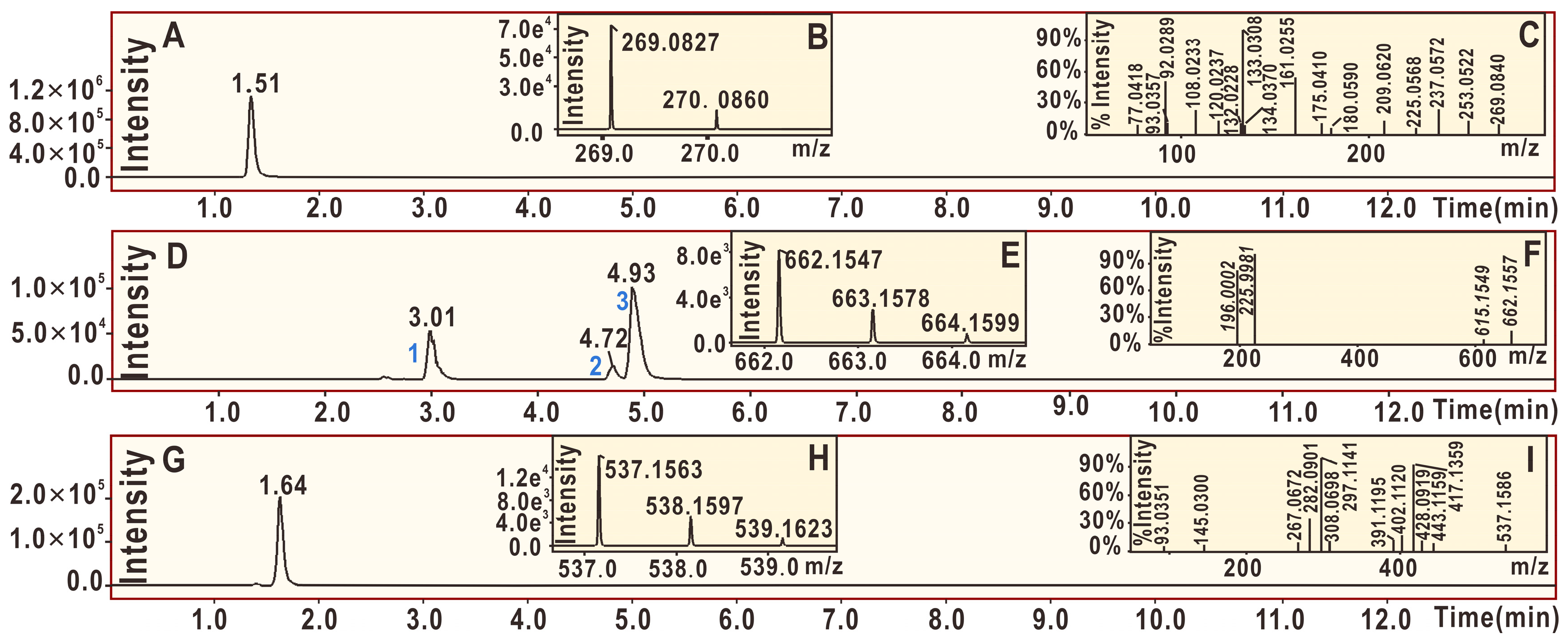
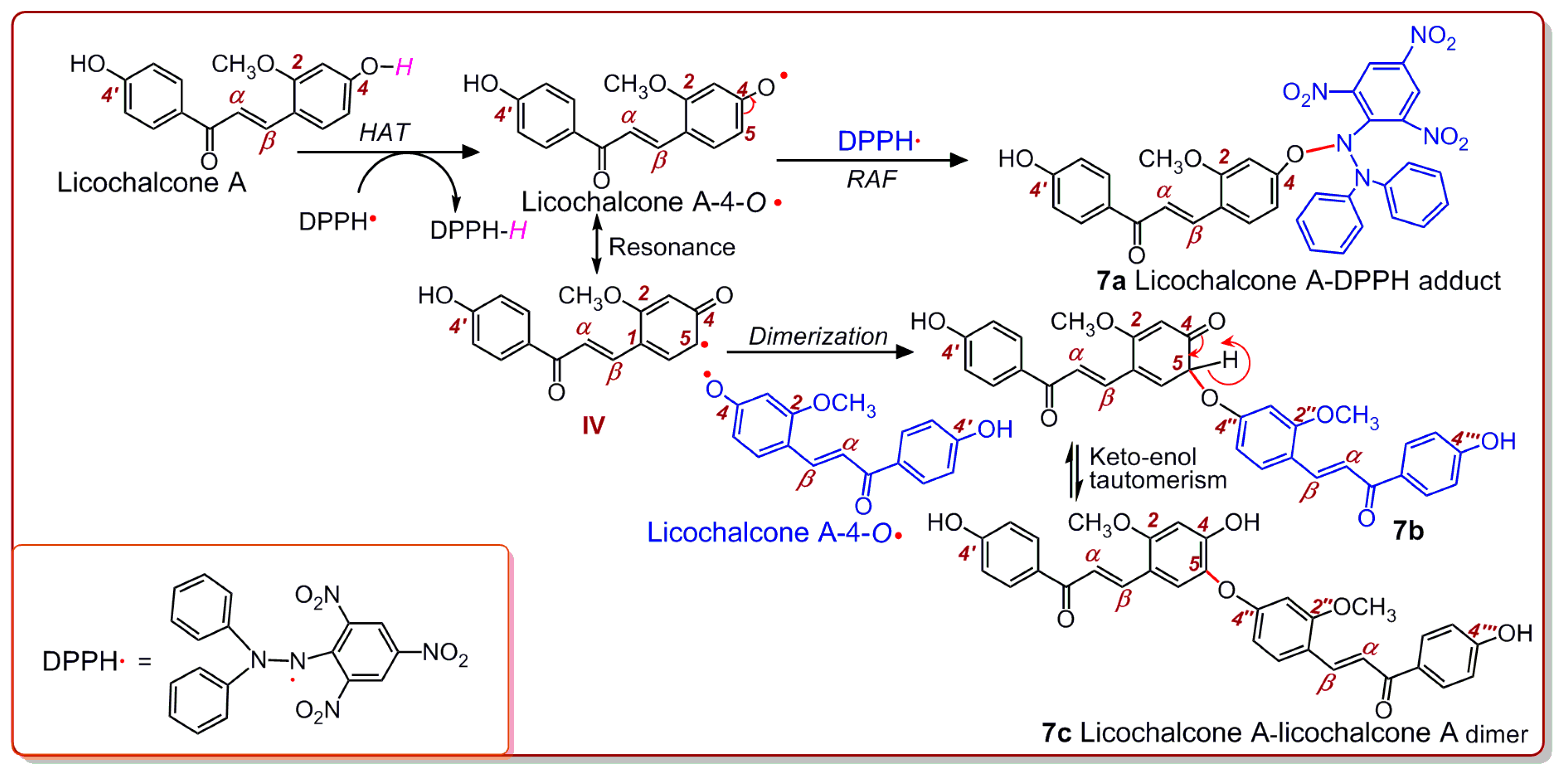
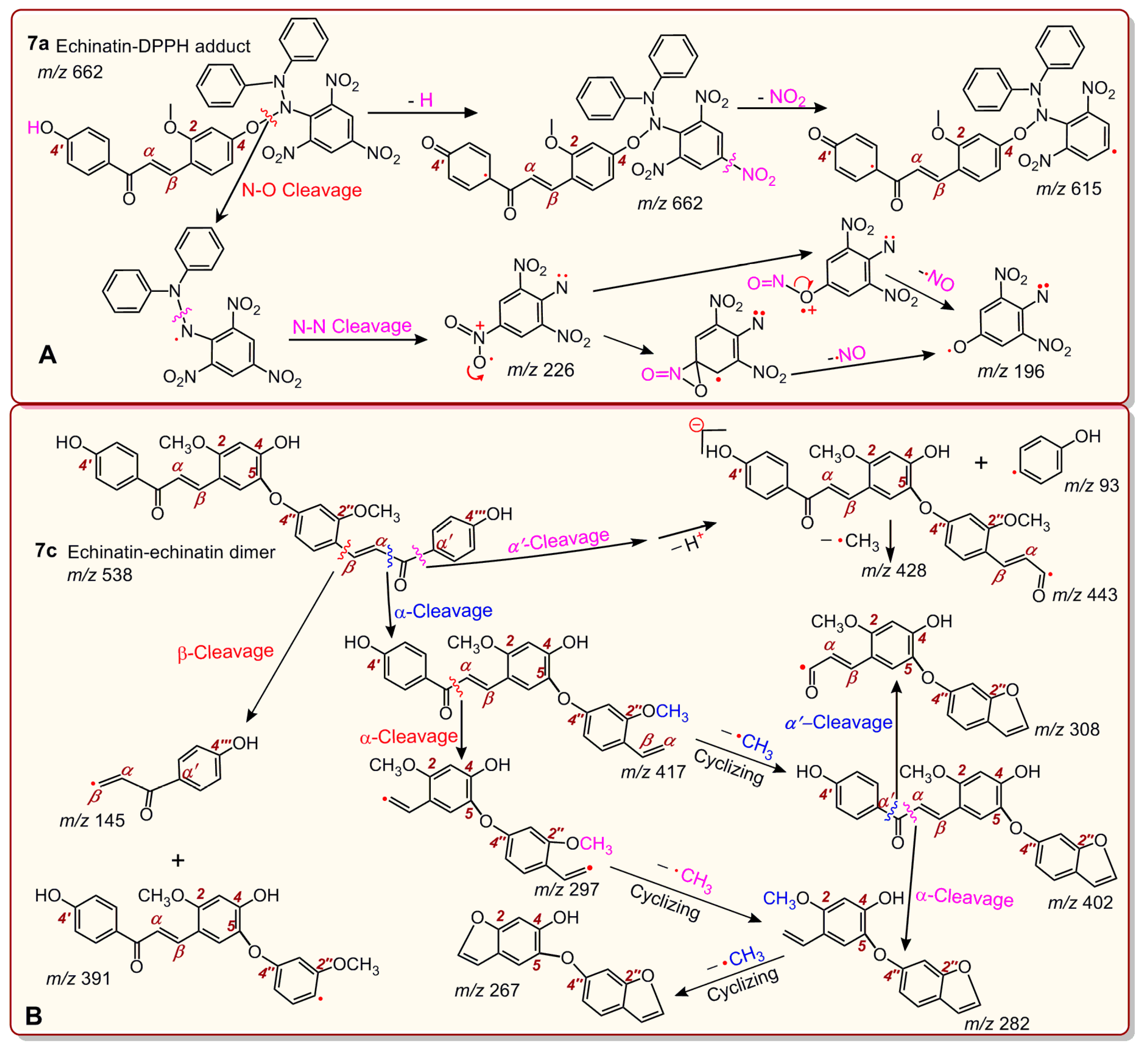
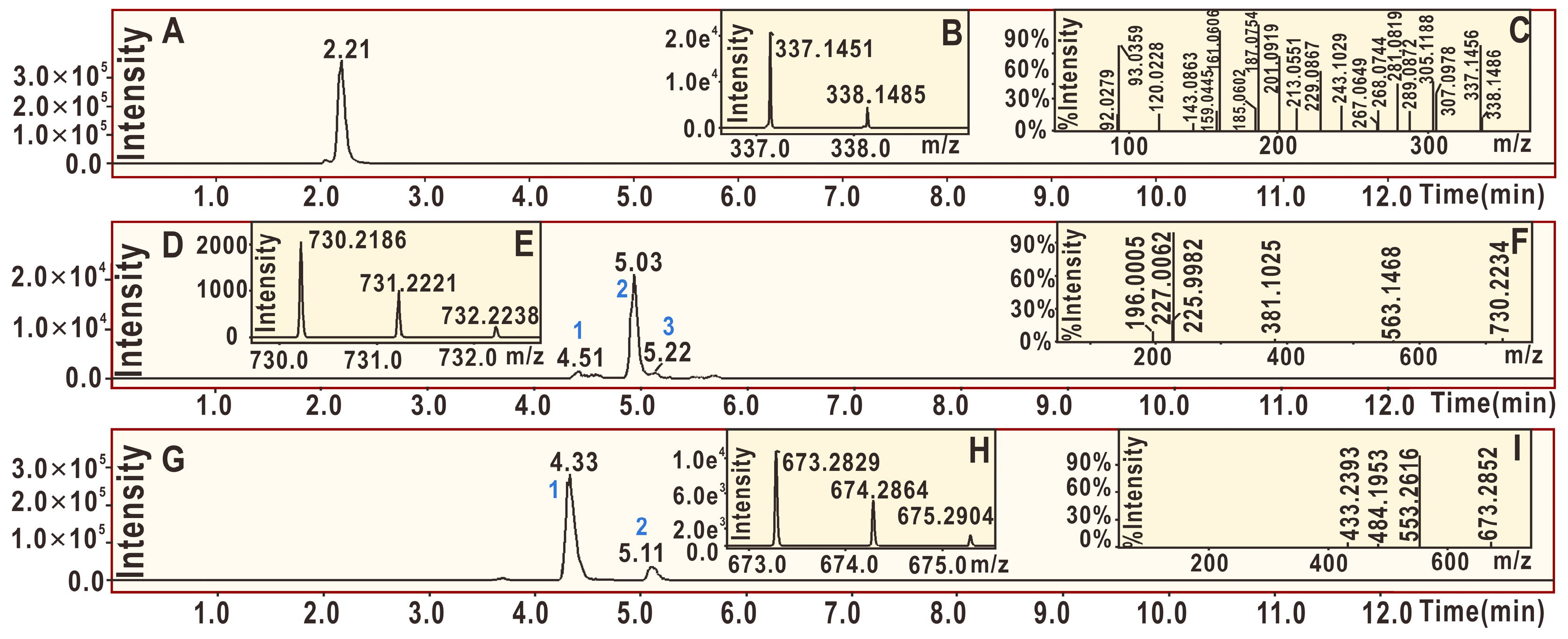
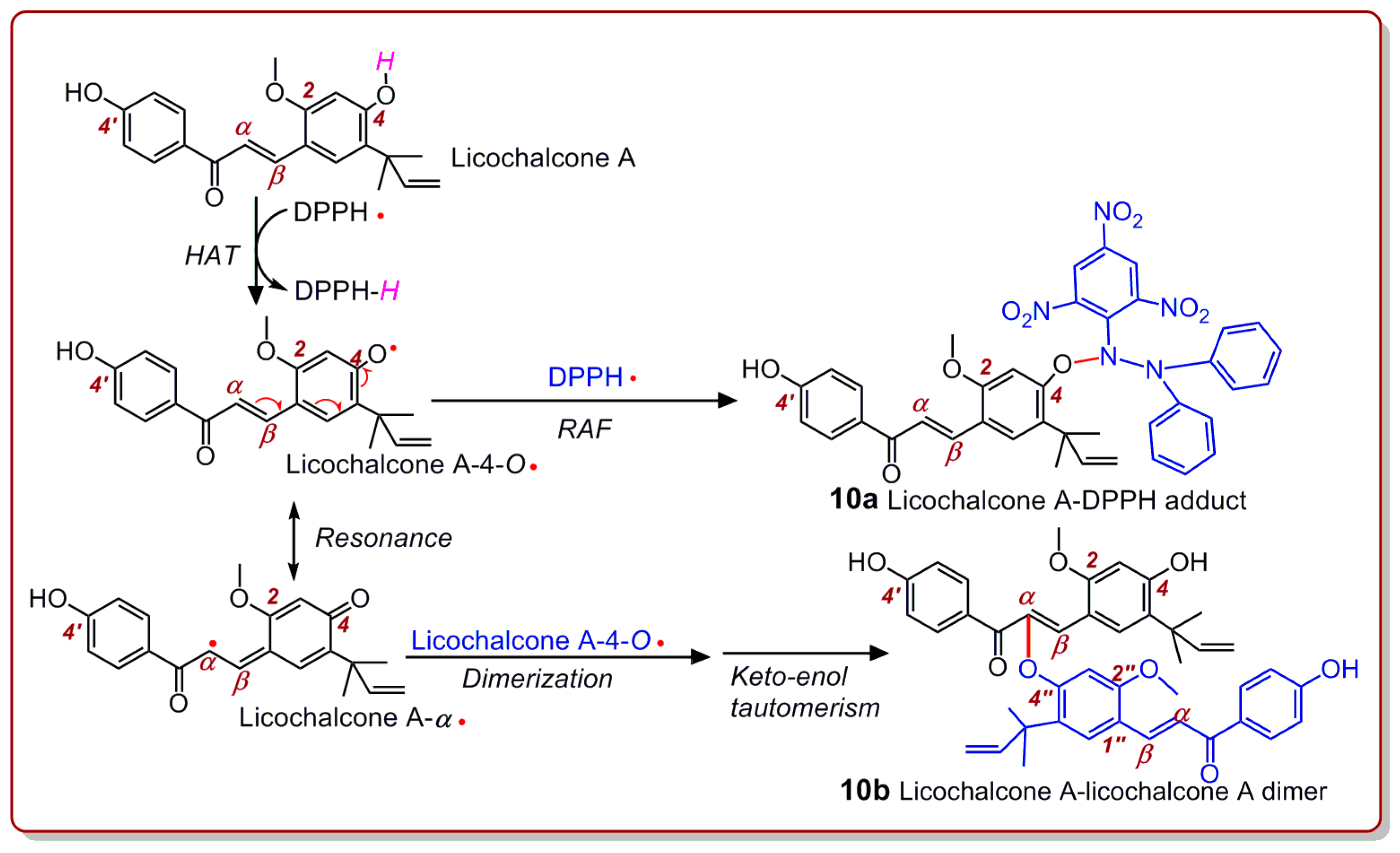
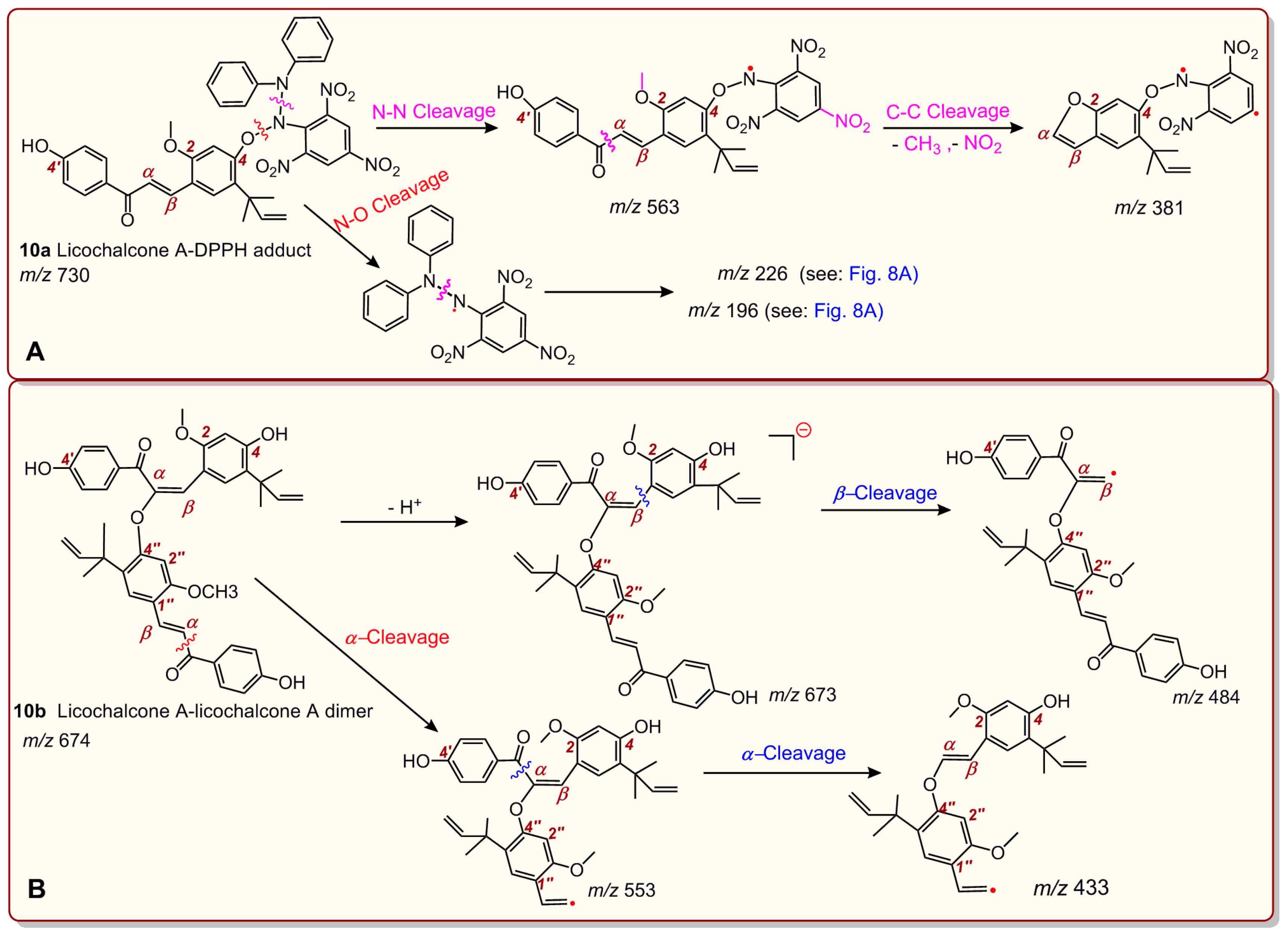
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Fe3+-Reduction Antioxidant Colorimetric Measurement
3.3. Cu2+-Reduction Antioxidant Spectrophotometric Measurement
3.4. PTIO•-Scavenging Colorimetric Measurement
3.5. DPPH•-Scavenging Colorimetric Measurement
3.6. UPLC-ESI-Q-TOF-MS/MS Analysis of DPPH• Reaction Products with Echinatin and Licochalcone A
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CPET | concerted proton electron transfer |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| ET | electron transfer |
| HAT | hydrogen atom transfer |
| PCET | proton-coupled electron transfer |
| PTIO | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide |
| RAF | radical adduct formation |
| SD | standard deviation |
| SEPT | sequential electron proton transfer |
| SPLET | sequential proton loss electron transfer |
| SPSS | Statistical Product and Service Solutions |
| TPTZ | 2,4,6-tripyridyl triazine |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| UPLC-ESI-Q-TOF-MS/MS | ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry |
References
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Meng, R.; Shi, C.; Guo, N. Antioxidative and anticancer properties of Licochalcone A from licorice. J. Ethnopharmacol. 2017, 198, 331–337. [Google Scholar] [CrossRef]
- Wang, X.X.; Liu, G.Y.; Yang, Y.F.; Wu, X.W.; Xu, W.; Yang, X.W. Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 1627. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Ji, S.; Chen, K.; Song, W.; Qiao, X.; Ye, M. Nrf2 activators from Glycyrrhiza inflata and their hepatoprotective activities against CCl4-induced liver injury in mice. Bioorg. Med. Chem. 2017, 25, 5522–5530. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Tanabe, S.; Tago, K.; Itoh, H.; Mashino, T.; Sonoda, Y.; Kasahara, T. Licochalcone A potently inhibits tumor necrosis factor alpha-induced nuclear factor-kappaB activation through the direct inhibition of IkappaB kinase complex activation. Mol. Pharmacol. 2009, 76, 745–753. [Google Scholar] [CrossRef]
- Lee, C.W.; Yen, F.L.; Huang, H.W.; Wu, T.H.; Ko, H.H.; Tzeng, W.S.; Lin, C.C. Resveratrol nanoparticle system improves dissolution properties and enhances the hepatoprotective effect of resveratrol through antioxidant and anti-inflammatory pathways. J. Agric. Food Chem. 2012, 60, 4662–4671. [Google Scholar] [CrossRef]
- Silva, J.B.D.; Mendes, R.F.; Tomasco, V.; Pinto, N.C.C.; de Oliveira, L.G.; Rodrigues, M.N.; Aragao, D.M.O.; Aguiar, J.A.K.; Alves, M.S.; Castanon, M.; et al. New aspects on the hepatoprotective potential associated with the antioxidant, hypocholesterolemic and anti-inflammatory activities of Vernonia condensata Baker. J. Ethnopharmacol. 2017, 198, 399–406. [Google Scholar] [CrossRef]
- Sökmen, M.; Akram, K.M. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology 2016, 24, 81–86. [Google Scholar]
- Kuete, V.; Noumedem, J.A.; Nana, F. Chemistry and pharmacology of 4-hydroxylonchocarpin: A review. Chin. J. Integr. Med. 2013, 19, 475–480. [Google Scholar] [CrossRef]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Hernandez-Marin, E.; Galano, A. Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct. 2012, 3, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; Wen, Q.F.; Li, C.Y.; Su, X.H.; Yuan, Z.P.; Li, Y.F. Two New Naphthoquinone Derivatives from Lysionotus pauciflorus. Helv. Chim. Acta 2013, 96, 1750–1756. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, S.D.; Byres, M.; Gray, A.I. New salicylic acid and isoflavone derivatives from Flemingia paniculata. J. Nat. Prod. 2004, 67, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, X.C.; Ren, Z.X.; Qiu, W.M.; Chen, J.L.; Jiang, Q.; Chen, B.; Chen, D.F. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef]
- Li, X.C. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Daquino, C.; Mackie, I.D.; DiLabio, G.A.; Ingold, K.U. Reaction of phenols with the 2,2-diphenyl-1-picrylhydrazyl radical. Kinetics and DFT calculations applied to determine ArO-H bond dissociation enthalpies and reaction mechanism. J. Org. Chem. 2008, 73, 9270–9282. [Google Scholar] [CrossRef]
- Schrauben, J.N.; Cattaneo, M.; Day, T.C.; Tenderholt, A.L.; Mayer, J.M. Multiple-site concerted proton-electron transfer reactions of hydrogen-bonded phenols are nonadiabatic and well described by semiclassical Marcus theory. J. Am. Chem. Soc. 2012, 134, 16635–16645. [Google Scholar] [CrossRef]
- Li, X.C.; Han, L.; Li, Y.R.; Zhang, J.; Chen, J.M.; Lu, W.B.; Zhao, X.J.; Lai, Y.Y.; Chen, D.F.; Wei, G. Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem. Pharm. Bull. 2016, 64, 319–325. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, K.; Wu, A.; Xie, H.; Guo, Q.; Xue, P.; Maleshibek, Y.; Zhao, W.; Guo, J.; et al. Antioxidation and Cytoprotection of Acteoside and Its Derivatives: Comparison and Mechanistic Chemistry. Molecules 2018, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Jiang, Q.; Wang, T.T.; Liu, J.J.; Chen, D.F. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Munguia, A.; Hernandez-Romero, Y.; Pedraza-Chaverri, J.; Miranda-Molina, A.; Regla, I.; Martinez, A.; Castillo, E. Phenylpropanoid glycoside analogues: Enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PLoS ONE 2011, 6, e20115. [Google Scholar] [CrossRef]
- Fourre, I.; Di Meo, F.; Podloucka, P.; Otyepka, M.; Trouillas, P. Dimerization of quercetin, Diels-Alder vs. radical-coupling approach: A joint thermodynamics, kinetics, and topological study. J. Mol. Model. 2016, 22, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure-Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- Navas Diaz, A.; Ramos Peinado, M.C. Fluorometric determination of curcumin in yogurt and mustard. J. Agric. Food Chem. 1992, 40, 56–59. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rozmer, Z.; Berki, T.; Maasz, G.; Perjesi, P. Different effects of two cyclic chalcone analogues on redox status of Jurkat T cells. Toxicol. Vitro 2014, 28, 1359–1365. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Bektas Oglu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for food antioxidants: Vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol. Biol. 2008, 477, 163–193. [Google Scholar]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and •O2−. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Liu, Z.W.; Chen, C.P. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine 2000, 6, 439–445. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, X.C.; Tian, Y.G.; Lin, Q.Q.; Xie, H.; Lu, W.B.; Chi, Y.G.; Chen, D.F. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect Mesenchymal stem cells from •OH-treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.; Almeida, S.; da Silva, S.; de Souza, G. Examining the reaction between antioxidant compounds and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) through a computational investigation. J. Mol. Model. 2018, 24. [Google Scholar] [CrossRef]
- Boudier, A.; Tournebize, J.; Bartosz, G.; El Hani, S.; Bengueddour, R.; Sapin-Minet, A.; Leroy, P. High-performance liquid chromatographic method to evaluate the hydrogen atom transfer during reaction between 1,1-diphenyl-2-picryl-hydrazyl radical and antioxidants. Anal. Chim. Acta 2012, 711, 97–106. [Google Scholar] [CrossRef]
- Shang, Y.J.; Qian, Y.P.; Liu, X.D.; Dai, F.; Shang, X.L.; Jia, W.Q.; Liu, Q.; Fang, J.G.; Zhou, B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical, and substitution. J. Org. Chem. 2009, 74, 5025–5031. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Xie, H.; Yang, J.; Chen, D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules 2018, 23, 694. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Zhan, R.; Chen, D. Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules 2018, 23, 754. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.C.; Chen, L.; Lu, W.Z.; Chen, X.W.; Han, L.; Chen, D.F. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A Chemical Study. B. Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, W.; Chen, D. Chemical Study on Protective Effect Against Hydroxyl-induced DNA Damage and Antioxidant Mechanism of Myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–390. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Chen, J.; Deng, Y.; Lu, W.; Chen, D. pH Effect and Chemical Mechanisms of Antioxidant Higenamine. Molecules 2018, 23, 2176. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound echinatin is available from the authors. |

| Measurement | Echinatin | Licochalcone A | Trolox |
|---|---|---|---|
| Fe3+-reduction | 338.0 ± 8.6 c | 133.2 ± 0.9 b | 87.5 ± 3.4 a |
| Cu2+-reduction | 228.1 ± 10.6 c | 129.1 ± 2.1 b | 67.5 ± 0.9 a |
| PTIO•-scavenging | 1276.5 ± 149.9 c | 617.4 ± 22.9 b | 270.9 ± 10.8 a |
| DPPH•-scavenging | 394.2 ± 67.5 c | 102.3 ± 3.6 b | 47.8 ± 2.6 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Li, X.; Ouyang, X.; Xie, H.; Chen, D. Antioxidant Mechanisms of Echinatin and Licochalcone A. Molecules 2019, 24, 3. https://doi.org/10.3390/molecules24010003
Liang M, Li X, Ouyang X, Xie H, Chen D. Antioxidant Mechanisms of Echinatin and Licochalcone A. Molecules. 2019; 24(1):3. https://doi.org/10.3390/molecules24010003
Chicago/Turabian StyleLiang, Minshi, Xican Li, Xiaojian Ouyang, Hong Xie, and Dongfeng Chen. 2019. "Antioxidant Mechanisms of Echinatin and Licochalcone A" Molecules 24, no. 1: 3. https://doi.org/10.3390/molecules24010003
APA StyleLiang, M., Li, X., Ouyang, X., Xie, H., & Chen, D. (2019). Antioxidant Mechanisms of Echinatin and Licochalcone A. Molecules, 24(1), 3. https://doi.org/10.3390/molecules24010003






