Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Studies
2.1.1. Essential Oil Yields and Chemical Composition
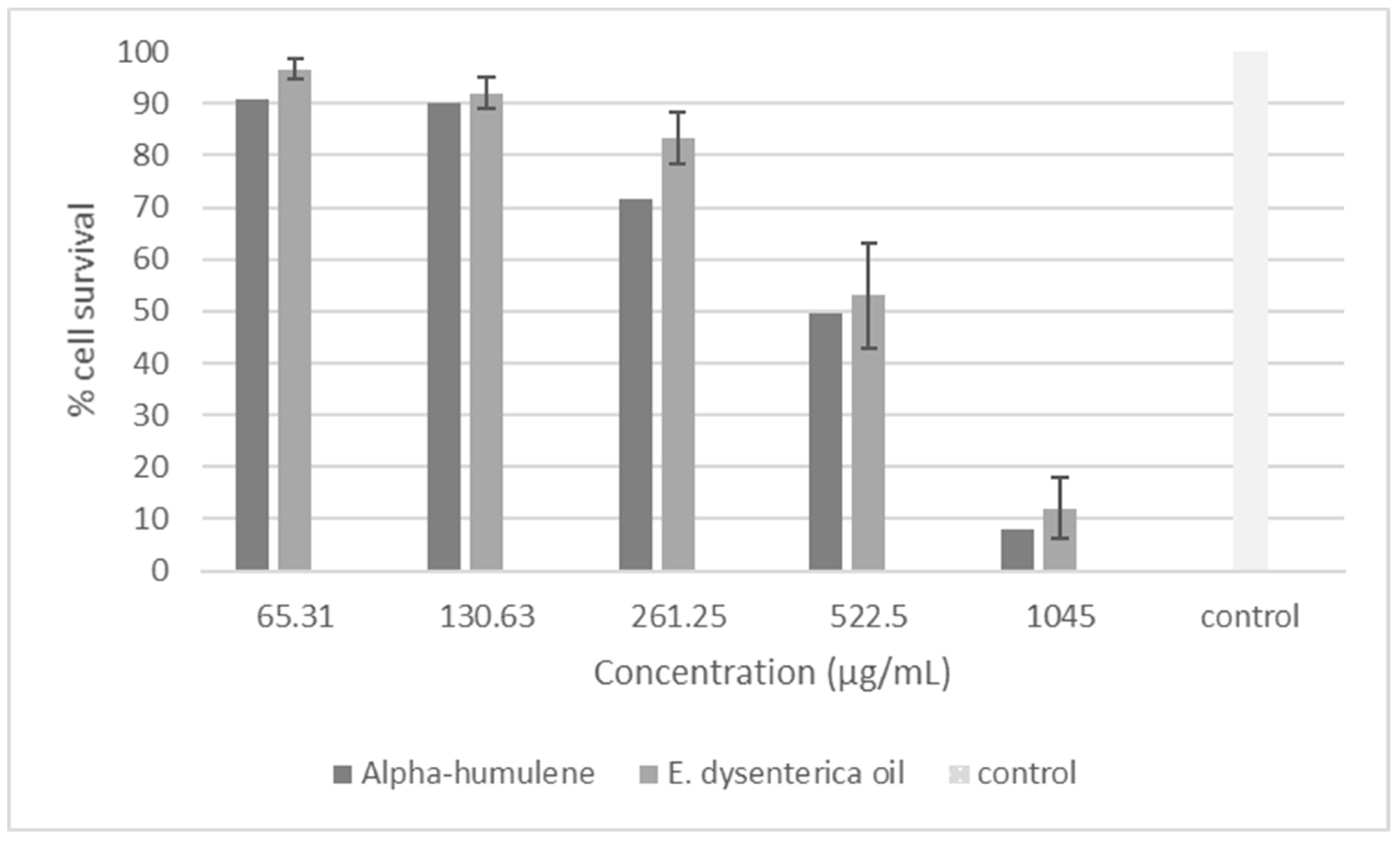
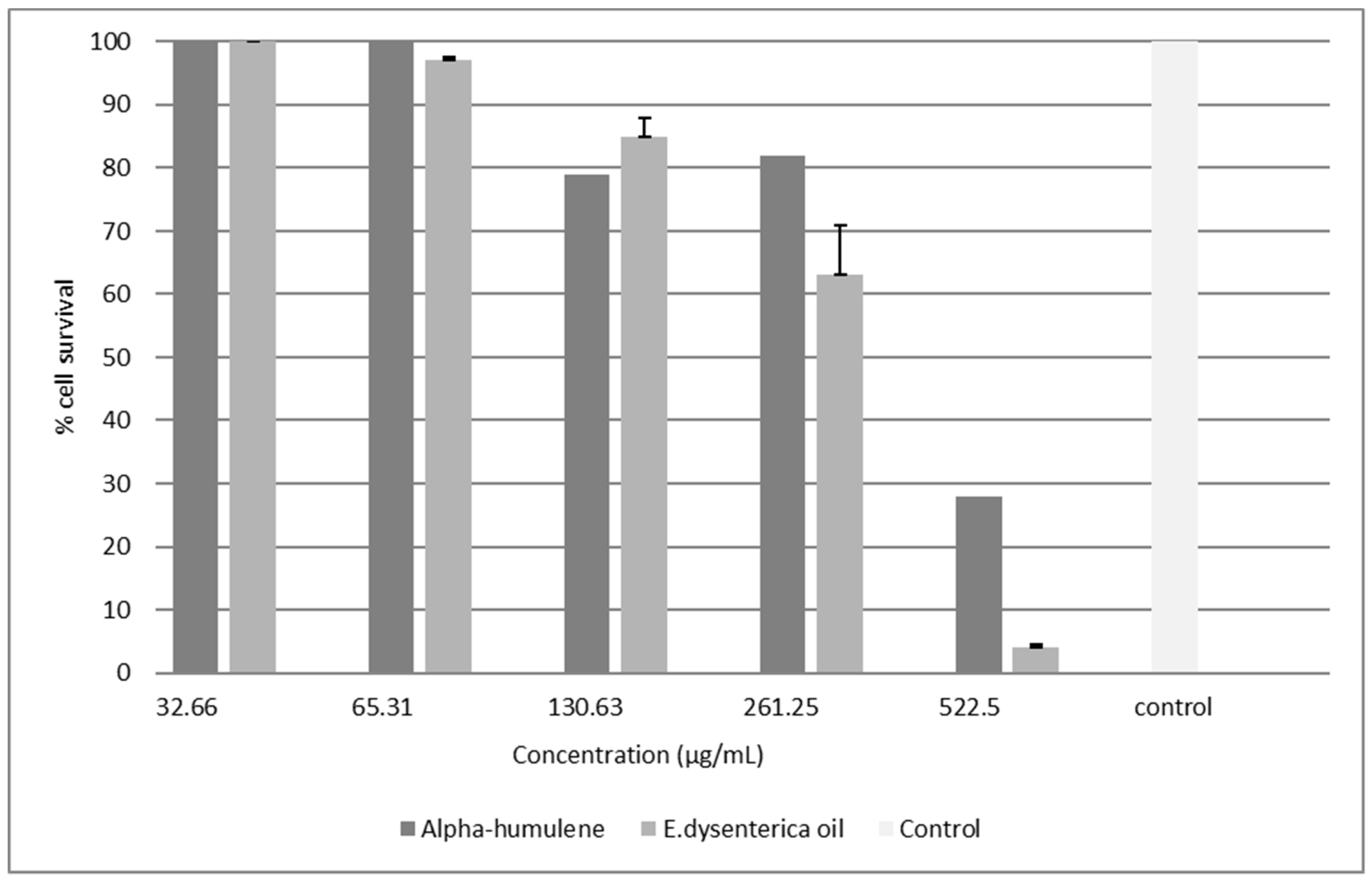
2.1.2. Cytotoxicity-Tetrazolium-Based Colorimetric Assay (MTT)
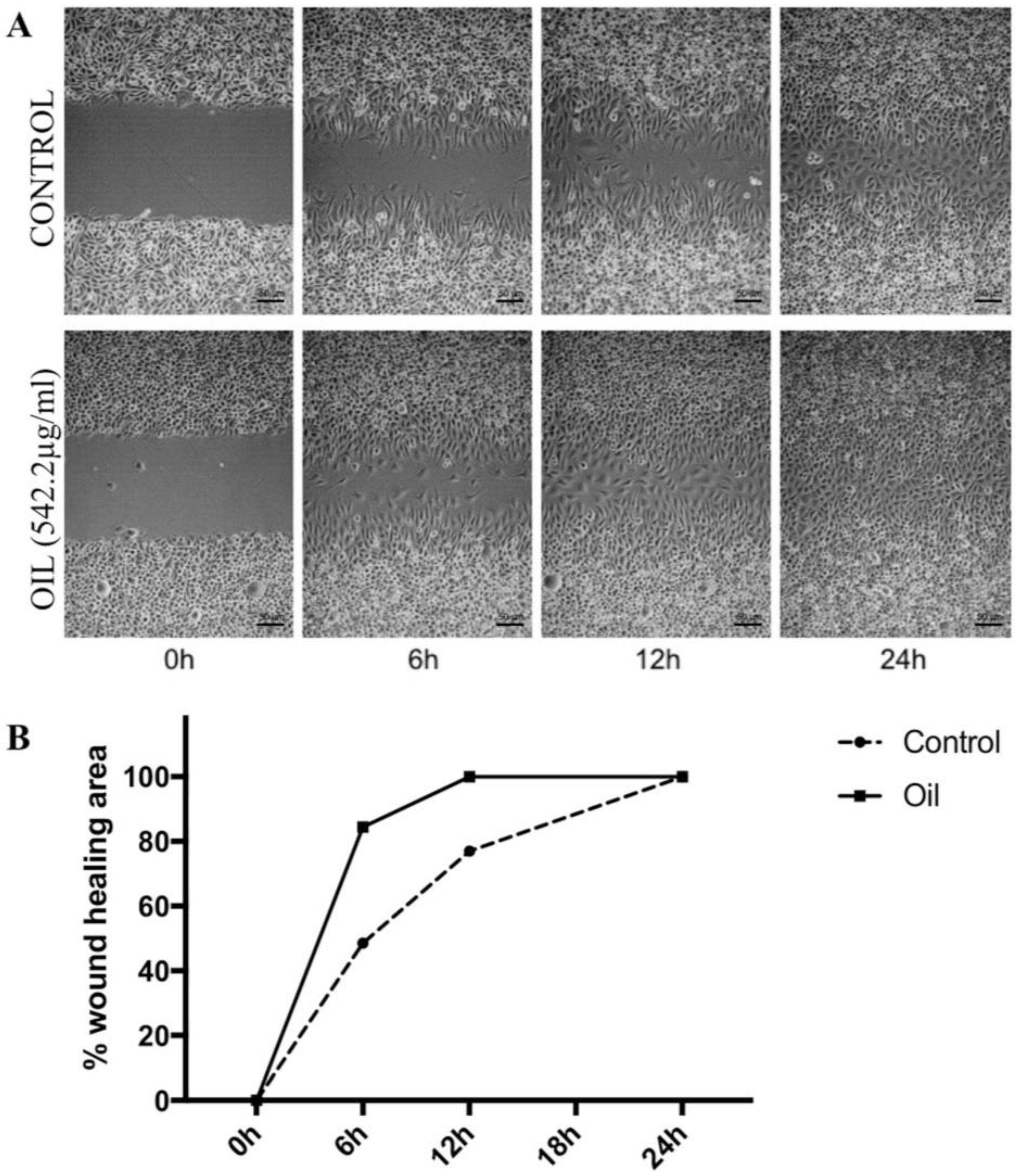
2.1.3. Scratch Wound Healing Assay
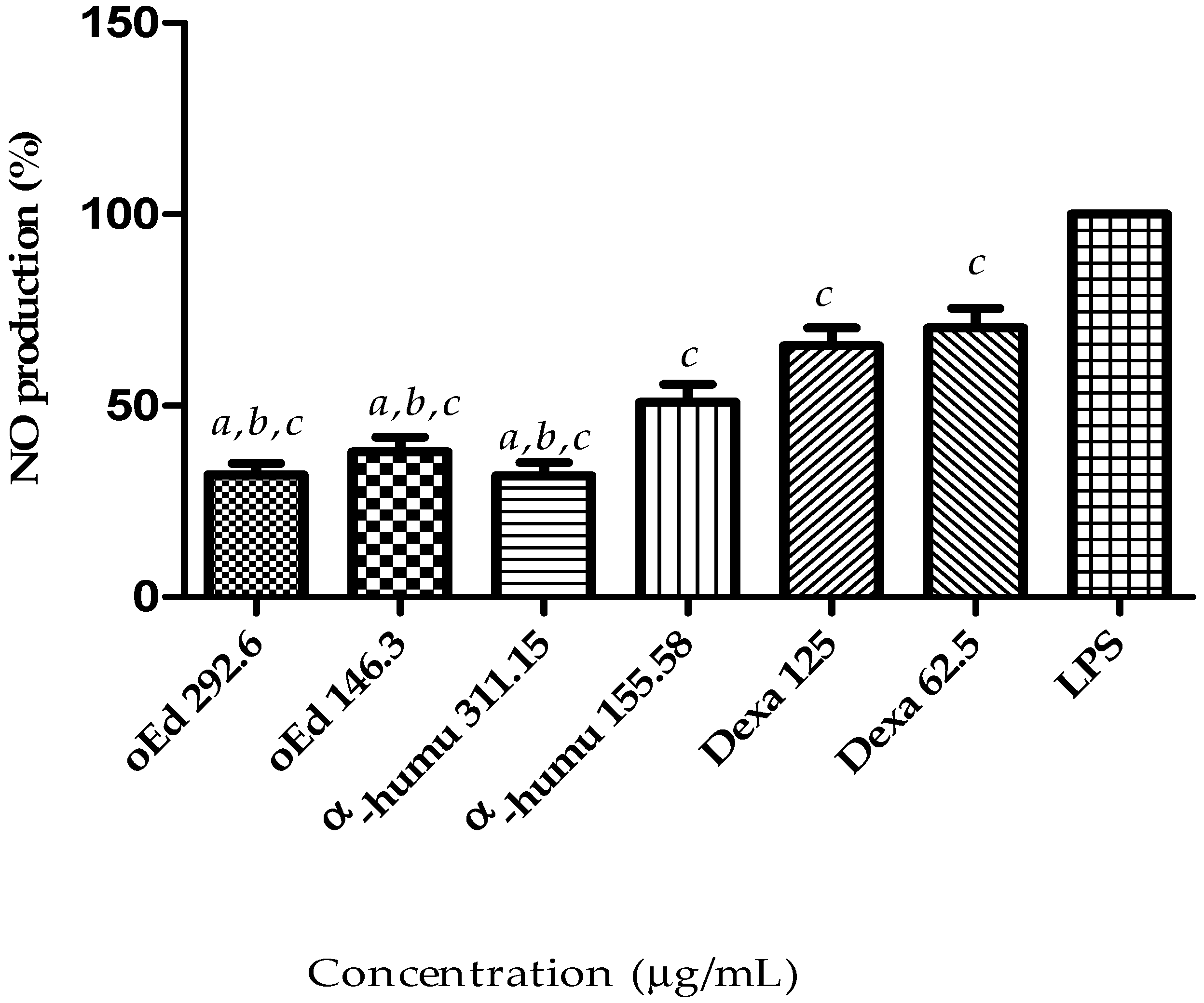
2.1.4. Effect of oEd, α-Humulene and Dexamethasone on NO Production
2.2. In Vivo Studies
2.2.1. Irritation Potential of Essential Oil
2.2.2. Angiogenesis Activity
3. Materials and Methods
3.1. Material
3.2. Plant Material
3.3. Preparation of Essential Oil from E. dysenterica Leaves
3.4. Analysis of Essential Oil from E. dysenterica Leaves Using GC-MS
3.5. Cytotoxicity—Tetrazolium-Based Colorimetric Assay (MTT)
3.6. Scratch Wound Healing Assay
3.7. Nitrite Assay
3.8. HET-CAM Test
3.8.1. Evaluation of the Irritation Potential
3.8.2. Chicken Chorioallantoic Membrane (CAM)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Andrews, K.L.; Houdek, M.T.; Kiemele, L.J. Wound management of chronic diabetic foot ulcers: From the basics to regenerative medicine. Prosthet. Orthot. Int. 2015, 39, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Palhares, D. Caracterização farmacognóstica das folhas de Eugenia dysenterica DC (Myrtaceae Jussieu). Revis. Lecta 2003, 21, 29–36. [Google Scholar]
- Lima, T.; Silva, O.; Silva, L.; Rocha, T.; Grossi-de-Sá, M.; Franco, O.; Leonardecz, E. In Vivo Effects of Cagaita (Eugenia dysenterica, DC.) Leaf Extracts on Diarrhea Treatment. Evid.-Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.; Naves, R.R.; Santos, S.C.; Seraphinc, J.C.; Ferri, P.H. Seasonal Influence on the Essential Oil Variability of Eugenia dysenterica. J. Braz. Chem. Soc. 2009, 20, 967–974. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Guilhon, G.M.S.P.; de Aguiar Andrade, E.H.; Zoghbi, M.G.B.; da Silva Santos, L. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef]
- Cole, R.A.; Haber, W.A.; Setzer, W.N. Chemical composition of essential oils of seven species of Eugenia from Monteverde, Costa Rica. Biochem. Syst. Ecol. 2007, 35, 877–886. [Google Scholar] [CrossRef]
- Duarte, A.R.; Naves, R.R.; Santos, S.C.; Seraphinc, J.C.; Ferri, P.H. Genetic and Environmental Influence on Essential Oil Composition of Eugenia dysenterica. J. Braz. Chem. Soc. 2010, 21, 1459–1467. [Google Scholar] [CrossRef]
- Moreira, L.C.; de Ávila, R.I.; Veloso, D.F.M.C.; Pedrosa, T.N.; Lima, E.S.; do Couto, R.O.; Lima, E.M.; Batista, A.C.; de Paula, J.R.; Valadares, M.C. In vitro safety and efficacy evaluations of a complex botanical mixture of Eugenia dysenterica DC. (Myrtaceae): Prospects for developing a new dermocosmetic product. Toxicol. Vitr. 2017, 45, 397–408. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Furuishi, T.; Katob, Y.; Fukamib, T.; Suzukib, T.; Endoa, T.; Nagasea, H.; Uedaa, H.; Tomonob, K. Effect of Terpenes on the Skin Permeation of Lomerizine Dihydrochloride. J. Pharm. Pharm. Sci. 2013, 16, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.H.; Saliaj, E.; Wettig, S.D.; Dong, C.; Ivanova, M.V.; Huzil, J.T.; Foldvari, M. Effect of chemical permeation enhancers on stratum corneum barrier lipid organizational structure and interferon αpermeability. Mol. Pharm. 2013, 10, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.T.; Gerber, M.; Du Plessis, J.; Hamman, J.H. Transdermal Drug Delivery Enhancement by Compounds of Natural Origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Passos, G.; Medeiros, R.; da Cunha, F.; Ferreira, J.; Campos, M.; Pianowski, L.; Calixto, J. Anti-inflammatory effects of compounds α-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. β-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, F.; Ali, S.S.; Alsofyani, T.; Albar, H. Plectranthus tenuiflorus (Shara) Promotes Wound Healing: In vitro and in vivo Studies. Int. J. Bot. 2010, 6, 69–80. [Google Scholar] [CrossRef]
- Medeiros, R.; Passos, G.; Vitor, C.; Koepp, J.; Mazzuco, T.; Pianowski, L.; Campos, M.; Calixto, J. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural wound healing and bioactive natural products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Singh, S.; Krishna, V.; Mankani, K.; Manjunatha, B.; Vidya, S.; Manohara, Y. Wound healing activity of the leaf extracts and deoxyelephantopin isolated from Elephantopus scaber Linn. Indian J. Pharmacol. 2005, 37, 238–242. [Google Scholar]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13. [Google Scholar] [CrossRef]
- Eming, S.; Wynn, T.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Green, L.C.; De Luzuriaga, K.R.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef]
- Passos, G.F.; Fernandes, E.S.; da Cunha, F.M.; Ferreira, J.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J. Ethnopharmacol. 2007, 110, 323–333. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, J.B. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, H.Y.; Kim, S.-K.; Park, J.H.Y.; Lee, H.J.; Chun, H.S. β-Caryophyllene attenuates dextran sulfate sodium-induced colitis in mice via modulation of gene expression associated mainly with colon inflammation. Toxicol. Rep. 2015, 2, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Siani, A.C.; Souza, M.C.; Henriques, M.G.; Ramos, M.F. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013, 51, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Elias, S.T.; Simeoni, L.A.; de Paula, J.E.; Gomes, S.M.; Guerra, E.N.S.; Fonseca, Y.M.; Silva, E.C.; Silveira, D.; Magalhaes, P.O. Plants from Brazilian Cerrado with Potent Tyrosinase Inhibitory Activity. PLoS ONE 2012, 7, e48589. [Google Scholar] [CrossRef] [PubMed]
- Clementino, S.E.; Garcia, R.S.; Moreira, C.; Pagliarini, B.A.; Cabral, R.B.; Silveira, D.; de Souza, G.E. Voltammetric and spectrophotometric determination of antioxidant activity of Eugenia dysenterica DC leaves extracts. Pak. J. Pharm. Sci. 2016, 29, 535–540. [Google Scholar]
- Gasca, C.A.; Chaves, M.; Fagg, C.W.; Magalhães, P.O.; Fonseca-Bazzo, Y.M.; Silveira, D. Pentacyclic triterpenes from leaves of Cagaita (Eugenia dysenterica). J. Appl. Pharm. Sci. Vol. 2017, 7, 051–054. [Google Scholar]
- Galheigo, M.R.U.; Prado, L.C.d.S.; Mundin, A.M.M.; Gomes, D.O.; Chang, R.; Lima, A.M.C.; Canabrava, H.A.N.; Bispo-da-Silva, L.B. Antidiarrhoeic effect of Eugenia dysenterica DC (Myrtaceae) leaf essential oil. Natl. Prod. Res. 2016, 30, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Marques Costa, C.; Coli Louvisse de Abreu, L.; Pereira dos Santos, E.; Augusto Franca Presgrave, O.; Trindade Rocha Pierucci, A.P.; Rangel Rodrigues, C.; Pereira de Sousa, V.; Nicoli, S.; Ricci Junior, E.; Mendes Cabral, L. Preparation and evaluation of chitosan submicroparticles containing pilocarpine for glaucoma therapy. Curr. Drug Deliv. 2015, 12, 491–503. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Presta, M. The gelatin sponge–chorioallantoic membrane assay. Nat. Protocol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.C.; Shin, V.Y.; Liu, E.S.; Yi, N.Y.; Wu, W.K.; So, W.H.; Chang, F.Y.; Cho, C.H. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur. J. Pharmacol. 2004, 485, 275–281. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Jain, N.; Pathak, R.; Sandhu, S.S. Practices in Wound Healing Studies of Plants. Evid.-Based Complement. Alt. Med. 2011, 17. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Villano, C.M.; Joseph, G.; Schmidt, B.; Shulaev, V.; Shuman, J.L.; Lila, M.A.; Raskin, I. Original Contribution: Wound-healing properties of nut oil from Pouteria lucuma. J. Cosmet. Dermatol. 2010, 9, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Pan, W.; Zhu, G.; Liu, Z.; Lv, D.; Jiang, M. Total flavones of abelmoschus manihot enhances angiogenic ability both in vitro and in vivo. Oncotarget 2017, 8, 69768–69778. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Baek, Y.-H.; Huh, J.-E.; Ko, J.-M.; Woo, H.; Lee, J.-D.; Park, D.-S. Stimulatory effect of Cinnamomum cassia and cinnamic acid on angiogenesis through up-regulation of VEGF and Flk-1/KDR expression. Int. Immunopharmacol. 2009, 9, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.S.; Leal, P.C.; Pianowisky, L.; Calixto, J.B. Pharmacokinetics and tissue distribution of the sesquiterpene α-humulene in mice. Plant. Med. 2008, 74, 1678–1683. [Google Scholar] [CrossRef]
- Perini, J.A.; Angeli-Gamba, T.; Alessandra-Perini, J.; Ferreira, L.C.; Nasciutti, L.E.; Machado, D.E. Topical application of Acheflan on rat skin injury accelerates wound healing: A histopathological, immunohistochemical and biochemical study. BMC Complement. Alt. Med. 2015, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Fernandes, O.F.; Santos, S.C.; Oliveira, C.M.; Liao, L.M.; Ferri, P.H.; Paula, J.R.; Ferreira, H.D.; Sales, B.H.; Maria do Rosário, R.S. Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil. J. Ethnopharmacol. 2000, 72, 111–117. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Luepke, N. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Melo-Reis, P.; Andrade, L.; Silva, C.; Araújo, L.; Pereira, M.; Mrue, F.; Chen-Chen, L. Angiogenic activity of Synadenium umbellatum Pax latex. Braz. J. Biol. 2010, 70, 189–194. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

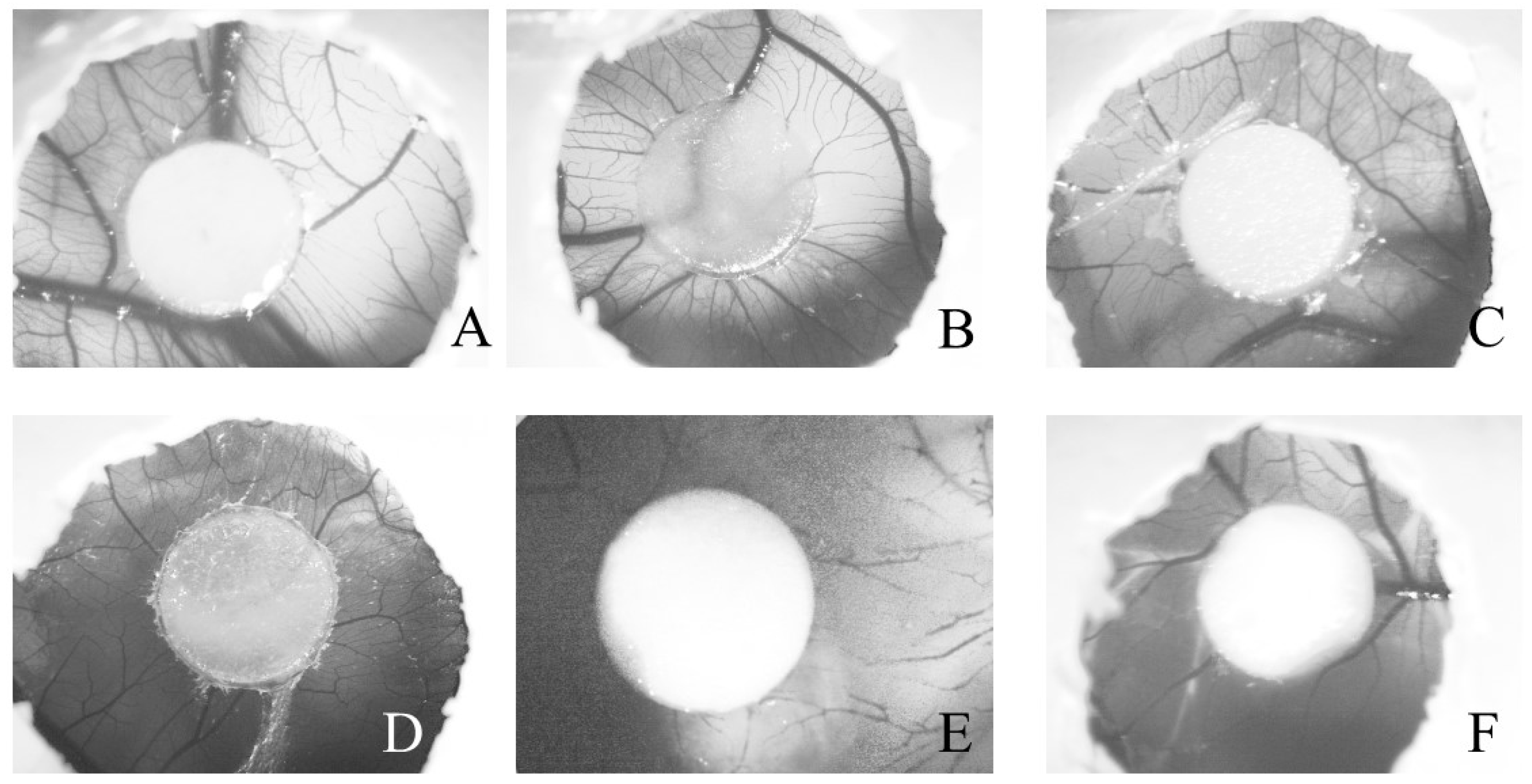
| Samples | Hyperemia | Hemorrhage | Coagulation | IS |
|---|---|---|---|---|
| Olive oil | 0 | 0 | 0 | 0 |
| 0.9% NaCl | 0 | 0 | 0 | 0 |
| 73 µg/mL | 0 | 0 | 0 | 0 |
| 146 µg/mL | 0 | 0 | 0 | 0 |
| 292 µg/mL | 0 | 0 | 0 | 0 |
| 584 µg/mL | 0 | 3 | 0 | 3 |
| 1168 µg/mL | 0 | 5 | 0 | 5 |
| 0.1 M NaOH | 5 | 8 | 8 | 21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazutti da Silva, S.M.; Rezende Costa, C.R.; Martins Gelfuso, G.; Silva Guerra, E.N.; De Medeiros Nóbrega, Y.K.; Gomes, S.M.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; Magalhães, P.d.O. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules 2019, 24, 2. https://doi.org/10.3390/molecules24010002
Mazutti da Silva SM, Rezende Costa CR, Martins Gelfuso G, Silva Guerra EN, De Medeiros Nóbrega YK, Gomes SM, Pic-Taylor A, Fonseca-Bazzo YM, Silveira D, Magalhães PdO. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules. 2019; 24(1):2. https://doi.org/10.3390/molecules24010002
Chicago/Turabian StyleMazutti da Silva, Sandra Márcia, Claudio Rodrigues Rezende Costa, Guilherme Martins Gelfuso, Eliete Neves Silva Guerra, Yanna Karla De Medeiros Nóbrega, Sueli Maria Gomes, Aline Pic-Taylor, Yris Maria Fonseca-Bazzo, Damaris Silveira, and Pérola de Oliveira Magalhães. 2019. "Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves" Molecules 24, no. 1: 2. https://doi.org/10.3390/molecules24010002
APA StyleMazutti da Silva, S. M., Rezende Costa, C. R., Martins Gelfuso, G., Silva Guerra, E. N., De Medeiros Nóbrega, Y. K., Gomes, S. M., Pic-Taylor, A., Fonseca-Bazzo, Y. M., Silveira, D., & Magalhães, P. d. O. (2019). Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules, 24(1), 2. https://doi.org/10.3390/molecules24010002








