Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Estimation of Tricin in ETZL
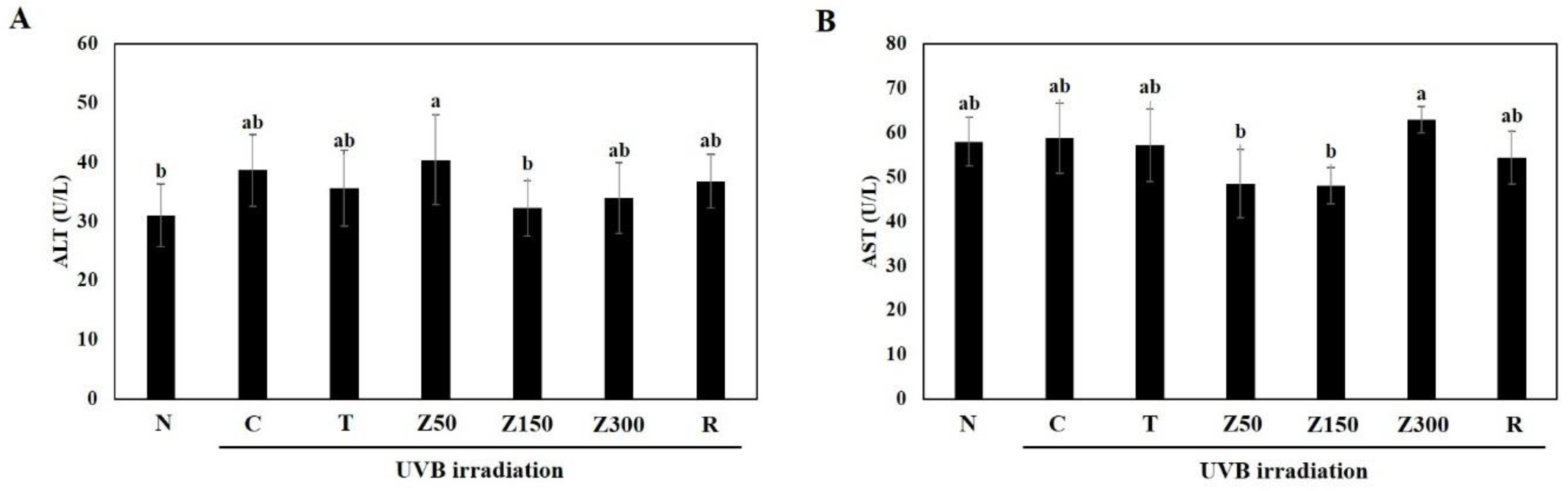
2.2. Effects on Body Weight and Serum Biochemical Indicators
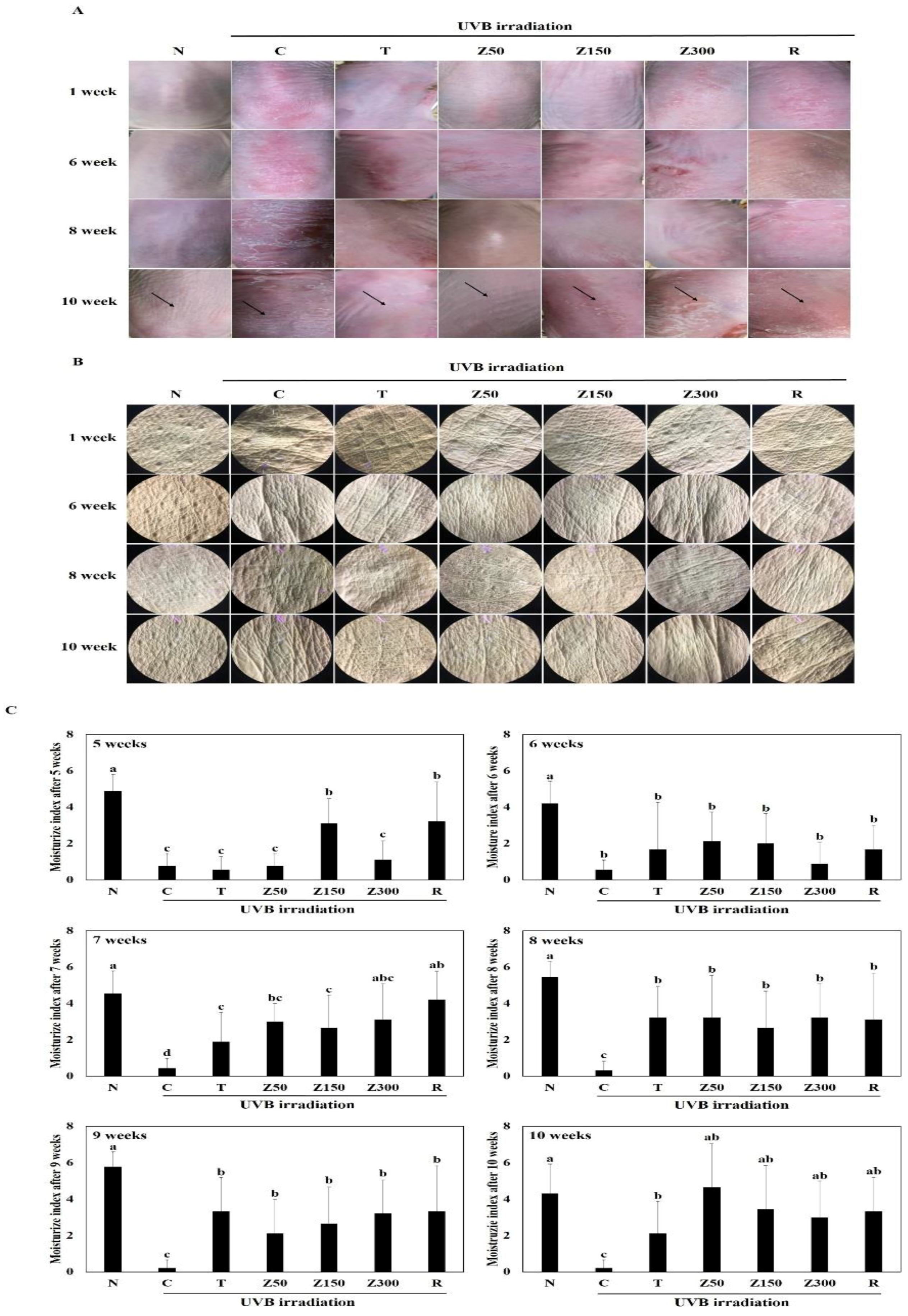
2.3. Effects on Wrinkle Formation
2.4. Effects on Changes in the Moisture Content of the Dorsal Skin
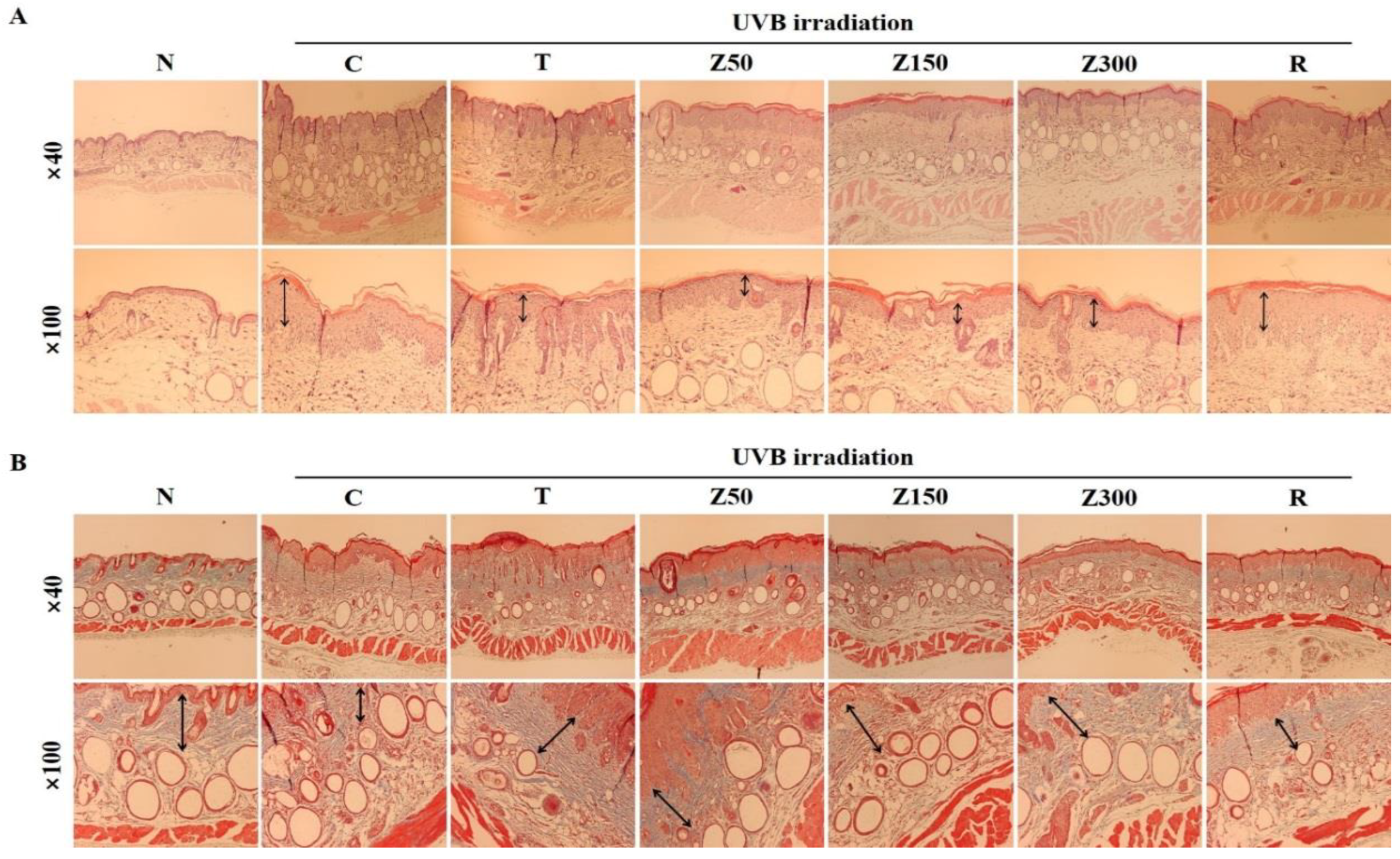
2.5. Effects on Histological Difference of the Dosal Skin
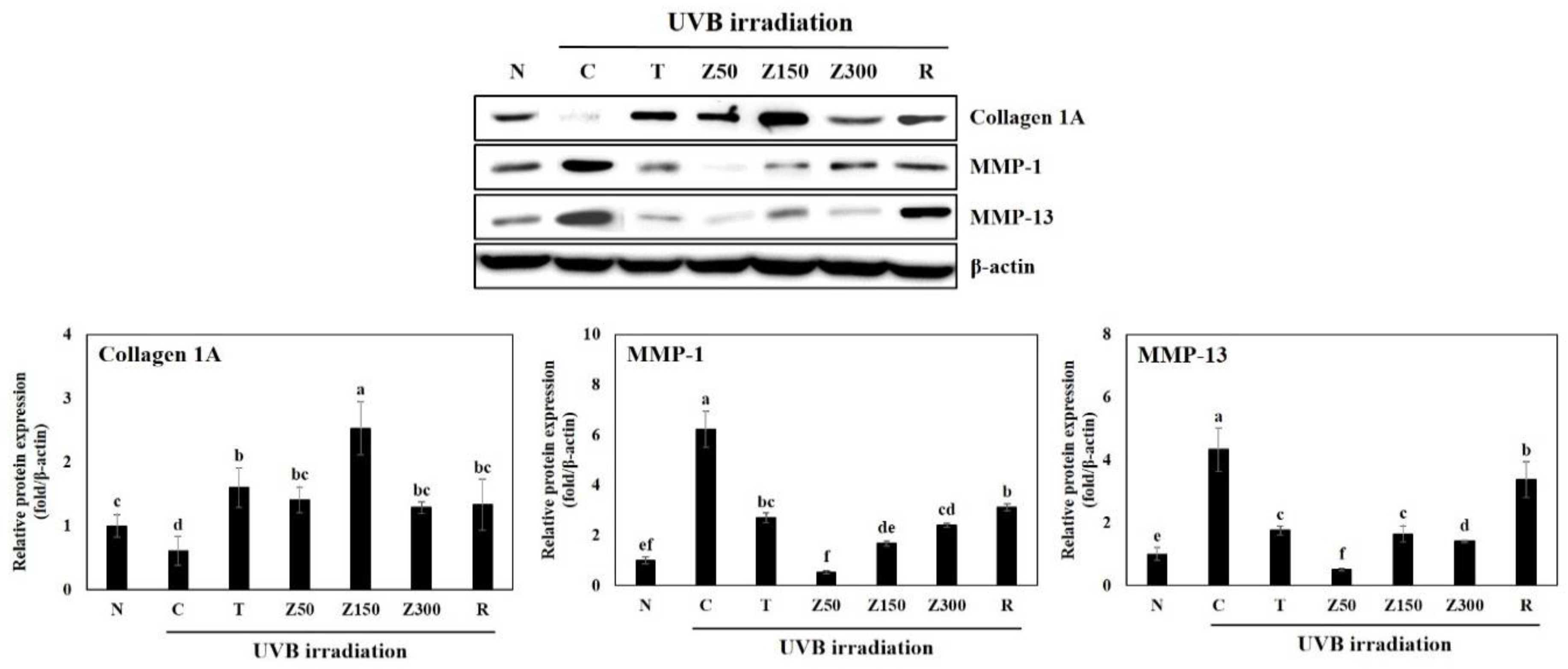
2.6. Effects on Collagen and MMPs Proteins Expression
3. Materials and Methods
3.1. Plant Material and Preparation of ETZL
3.2. HPLC Analysis
3.3. Experimental Animals and UV Irradiation
3.4. Serum Biochemical Analysis
3.5. Morphological and Histological Observation
3.6. Measurement of Skin Moisture
3.7. Western Blot
3.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Malvicini, M.; Gutierrez-Moraga, A.; Rodriguez, M.M.; Gomez-Bustillo, S.; Salazar, L.; Sunkel, C.; Nozal, L.; Salgado, A.; Hidalgo, M.; Lopez-Casas, P.P.; et al. A tricin derivative from deschampsia antarctica Desv. Inhibits colorectal carcinoma growth and liver metastasis through the induction of a specific immune response. Mol. Cancer Ther. 2018, 17, 966–976. [Google Scholar] [CrossRef]
- Cai, H.; Hudson, E.A.; Mann, P.; Verschoyle, R.D.; Greaves, P.; Manson, M.M.; Steward, W.P.; Gescher, A.J. Growth-inhibitory and cell cycle-arresting properties of the rice bran constituent tricin in human-derived breast cancer cells in vitro and in nude mice in vivo. Br. J. Cancer 2004, 91, 1364. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Kurokawa, M.; Obuchi, M.; Li, Y.; Yamada, R.; Sadanari, H.; Matsubara, K.; Watanabe, K.; Koketsu, M.; Tuchida, Y.; et al. Anti-influenza virus activity of tricin, 4’,5,7-trihydroxy-3’,5’-dimethoxyflavone. Antivir. Chem. Chemother. 2011, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shalini, V.; Bhaskar, S.; Kumar, K.S.; Mohanlal, S.; Jayalekshmy, A.; Helen, A. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: Possible role in the inflammatory signaling. Int. Immunopharmacol. 2012, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, M.J.; Mohanlal, S.; Suresh, C.H.; Jayalekshmy, A. DPPH radical scavenging activity of tricin and its conjugates isolated from “Njavara” rice bran: A density functional theory study. J. Agric. Food Chem. 2012, 60, 3693–3699. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Baek, Y.S.; Eun, C.S.; Yu, M.H.; Baek, N.I.; Chung, D.K.; Bang, M.H.; Yang, S.A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 79, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Li, S.M.; Peng, J.; Ke, W.D. Zizania latifolia Turcz. cultivated in China. Genet. Resour. Crop. Evol. 2007, 54, 1211–1217. [Google Scholar] [CrossRef]
- Zhai, C.K.; Tang, W.L.; Jang, X.L.; Lorenz, K.J. Studies of the safety of Chinese wild rice. Food Chem. Toxicol. 1996, 34, 347–352. [Google Scholar] [CrossRef]
- Park, W.H.; Cha, Y.Y. Inhibition effect of Zizania latifolia on apoptosis induced by H2O2 in Neuro2A cell. J. Physio. Pathol. Korean Med. 2005, 19, 1062–1067. [Google Scholar]
- Qian, B.; Luo, Y.; Deng, Y.; Cao, L.; Yang, H.; Shen, Y.; Ping, J. Chemical composition, angiotensin-converting enzyme-inhibitory activity and antioxidant activities of few-flower wild rice (Zizania latifolia Turcz.). J. Sci. Food Agric. 2012, 92, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Whang, E.Y.; Whang, K.; Lee, I.S.; Yang, S.A. Anti-allergic effect of Zizania latifolia Turcz extracts. Korean J. Food Sci. Technol. 2009, 41, 717–721. [Google Scholar]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarker, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Chatterjee, R.; Hannon, D.P. Photoprotective effect of superoxide-scavenging antioxidants against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Photodermatol. Photoimmunol. Photomed. 1990, 7, 56–62. [Google Scholar] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-kB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef]
- Lee, S.S.; Baek, N.I.; Baek, Y.S.; Chung, D.K.; Song, M.C.; Bang, M.H. New flavonolignan glycosides from the aerial parts of Zizania latifolia. Molecules 2015, 20, 5616–5624. [Google Scholar] [CrossRef] [PubMed]
- Chaqour, B.; Seité, S.; Coutant, K.; Fourtanier, A.; Borel, J.P.; Bellon, G. Chronic UVB- and all-trans retinoic-acid-induced qualitative and quantitative changes in hairless mouse skin. J. Photochem. Photobiol. B Biol. 1995, 28, 125–135. [Google Scholar] [CrossRef]
- Chen, S.; Kiss, I.; Tramposch, K.M. Effects of all-trans retinoic acid on UVB-irradiated and non-irradiated hairless mouse skin. J. Invest. Dermatol. 1992, 98, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.F.; Feng, X.X.; Li, C.W.; Zhang, X.J.; Yu, X.T.; Zhou, J.Y.; Zhang, X.; Xie, Y.L.; Su, Z.R.; Zhan, J.Y.X. Prevention of UV radiation-induced cutaneous photoaging in mice by topical administration of patchouli oil. J. Ethnopharmacol. 2014, 154, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Skin aging and natural photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Sorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanism of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoaging: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Inomata, S.; Takada, K.; Tsunenaga, M.; Fukuda, M.; Matsunaga, Y.; Amano, S.; Kobayashi, K.; Nishiyama, T.; Kohno, Y. Possible involvement of gelatinase in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Invest. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.J.; Lee, J.Y.; Kim, W.G.; Park, W.S.; Sim, Y.C.; Lee, S.J. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J. Am. Coll. Nutr. 2004, 23, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Enzyme Treatment Time (h) | Extraction Yield (%) | Tricin Content (mg/100 g) * |
|---|---|---|
| 0 | 10.74 | 19.7 |
| 4 | 16.54 | 16.5 |
| 8 | 16.92 | 19.3 |
| 12 | 17.36 | 21.2 |
| 16 | 17.45 | 25.0 |
| 24 | 15.72 | 24.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.-M.; Park, S.-H.; Jhee, K.-H.; Yang, S.-A. Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract. Molecules 2018, 23, 2254. https://doi.org/10.3390/molecules23092254
Moon J-M, Park S-H, Jhee K-H, Yang S-A. Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract. Molecules. 2018; 23(9):2254. https://doi.org/10.3390/molecules23092254
Chicago/Turabian StyleMoon, Joo-Myung, Se-Ho Park, Kwang-Hwan Jhee, and Seun-Ah Yang. 2018. "Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract" Molecules 23, no. 9: 2254. https://doi.org/10.3390/molecules23092254
APA StyleMoon, J.-M., Park, S.-H., Jhee, K.-H., & Yang, S.-A. (2018). Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract. Molecules, 23(9), 2254. https://doi.org/10.3390/molecules23092254





